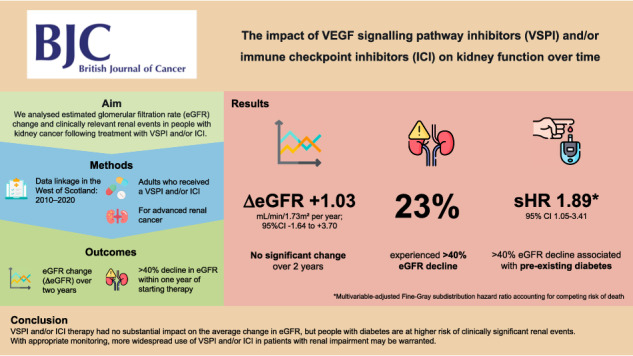
Abstract
Background
Drugs targeting angiogenesis and immunotherapy have transformed outcomes in renal cancer but may contribute to progressive kidney disease.
Methods
We linked healthcare databases in the West of Scotland (spanning 2010–2020) to identify adults with renal cancer who received one or both classes of drugs. Over two years following initiation, estimated glomerular filtration rate (eGFR) slope was modelled using linear mixed-effects models. Additional renal outcomes used competing risk regression considering the competing risk of death.
Results
Amongst 357 adults (62.5% male; median age 63.0 years, IQI 55.0–71.0), there was no significant change in eGFR (annual eGFR change +1.03 mL/min/1.73 m²/year, 95%CI −1.64 to +3.70), nor in subgroups of patients who had nephrectomy, metastatic cancer or an eGFR < 60 mL/min/1.73 m² prior to systemic therapy. A ≥ 40% decline in eGFR occurred in 82 people (23.0%) within one year of starting systemic therapy and was associated with pre-existing diabetes (subhazard ratio 1.89, 95%CI 1.05–3.41).
Discussion
Anti-angiogenic and immune therapy had no substantial impact on the average change in eGFR but people with diabetes are at higher risk of clinically significant renal events. With appropriate monitoring, more widespread use of these agents in patients with renal impairment may be warranted.
Introduction
Long-term survival with well-controlled or cured cancer is rising, with cancer survival doubling in the last 50 years [1], in part due to the successful introduction of new systemic therapies into routine practice. VEGF-signalling pathway inhibitors (VSPI) and/or immune checkpoint inhibitors (ICI) now make up the first-line anti-cancer treatment for a range of cancers [2] and have transformed the outcomes of people with advanced renal cancer [3, 4]. They also carry a significant side effect profile including nephrotoxicity, hypertension and proteinuria [5, 6] which are independent risk factors for chronic kidney disease (CKD) [7, 8], cardiovascular events [9, 10] and mortality [11] in the general population. As cancer survivorship improves, consideration of longer-term consequences of cancer treatment, including renal and cardiometabolic health, becomes increasingly important.
VSPI and ICI therapy have become the mainstay of systemic treatment of advanced renal cancer, either as monotherapy or in combination with other agents [3, 12]. The reported incidence of adverse renal events varies but these are common and, in general, have a good prognosis for renal recovery [13]. Diagnosing renal adverse events from VSPI/ICI is challenging due to the lack of definitive diagnostic criteria and variability in the time of onset, which can be over a year after therapy initiation with some patients remaining on treatment for several years [13–15]. Kidney toxicity may be mediated by VSPI/ICI-induced effects including endothelial dysfunction, podocytopathies, glomerulonephritis, acute interstitial nephritis, thrombotic microangiopathy, hypertension, atherosclerosis and vasculitis [16–18]. Whilst hypertension is commonly observed as a side effect of VSPI therapy [19], documented renal safety events and their association with overall mortality is mainly limited to case reports, including those of irreversible kidney failure and nephrotic syndrome [20]. Systemic absorption of VSPI from intravitreal administration is associated with cases of accelerated hypertension, worsening proteinuria, glomerular disease, thrombotic microangiopathy, and possibly CKD [21].
Data regarding the longer-term risk of renal function decline from VSPI/ICI therapies are inconclusive [5, 15, 22]. Analysis for hard endpoints such as progression to ESKD over shorter follow-up duration, may miss a progressive decline in kidney function that could have a significant impact on quality of life or symptoms in people surviving longer after cancer diagnosis. Estimated glomerular filtration rate (eGFR) slope has been demonstrated as an important marker of kidney function decline and a surrogate marker for future kidney failure [23–25]. eGFR slope over 2–3 years is now routinely used as a critical outcome to delineate differences in the rate of kidney function decline for on- and off-treatment effects in clinical trials [26].
Outside the setting of VSPI/ICI therapy, the relationship between the development of cancer and kidney dysfunction is multifactorial. Prevalent cancer or CKD is associated with a higher risk of developing renal dysfunction than those without, including acute kidney injury (AKI) [27] and proteinuria. People with cancer have a high prevalence of CKD; furthermore, people with CKD have a higher incidence risk of certain cancers [28] and poorer survival [29] than those without CKD. Outcomes from cancer in the context of CKD may be affected by the influence of renal dysfunction upon anti-cancer treatment selection, duration, efficacy and safety [30].
Partial or total nephrectomy has become a pivotal management strategy in people with renal cancer [31, 32]. Nephrectomy, logically, causes a reduction in eGFR, but the elevated risk that this nephron loss poses to future risk of progressive CKD in people with renal cancer is unclear [33]. It appears that there are particular risk factors for developing adverse renal events following nephrectomy in the context of renal cancer, such as proteinuria [34, 35].
This study analyses the eGFR slope as well as clinically significant renal events, after the introduction of VSPI/ICI therapy in advanced renal cancer, and factors associated with a change in eGFR, renal events and all-cause mortality. By doing this, we aim to define the extent of renal risks associated with these therapies. We hypothesised that people treated with VSPI and/or ICI for renal cancer have a significant decline in eGFR over time and that there are higher risk groups of patients who are susceptible to a steeper eGFR decline.
Methods
Study design and data sources
We conducted a retrospective study using integrated data from comprehensive prescribing system, the electronic prescription system for anti-cancer therapies in the West of Scotland, and National Services Scotland SafeHaven databases. These datasets provide comprehensive patient information relating to all patients treated in NHS Greater Glasgow and Clyde (serving a population of 1.4 million), including laboratory data (SCI store), hospital records (SMR01), mortality records (National Records of Scotland – Deaths Data), cancer registry data (SMR06) and renal transplant or dialysis records (Strathclyde Electronic Renal Patient Record, SERPR, Vitalpulse, UK).
We included adults diagnosed with renal cancer who received VSPI or ICI, either as monotherapy or in combination as anti-cancer therapies within the Greater Glasgow and Clyde Health Board between January 2010 and December 2020. Adults with renal cancer were identified using the International Classification of Disease-10 (ICD-10: Version 2019) code C64 from linked cancer registry data from SMR06. People already receiving kidney replacement therapy at cancer incidence were excluded from the analysis. All people received their cancer treatment via the West of Scotland Cancer Network.
Nephrectomy assessment
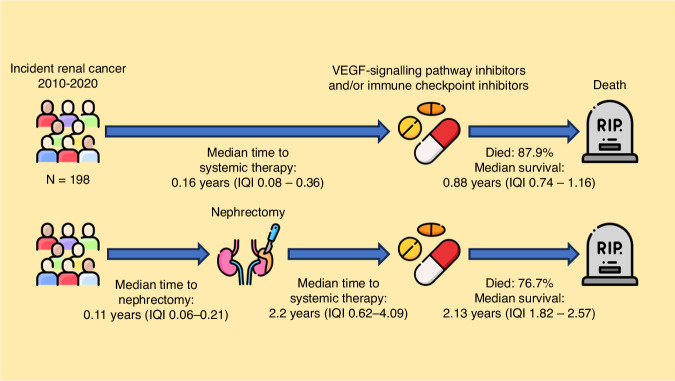
People with nephrectomy or partial nephrectomy prior to systemic therapy were identified using ICD-10 codes and dates from SMR06 and SMR01 data. People with or without nephrectomy prior to systemic therapy were included (Fig. 1).
Fig. 1. Cohort characteristics.
Survival and follow-up duration between renal cancer diagnosis, nephrectomy and/or systemic therapy initiation.
Kidney function assessment
People who had at least one serum creatinine value available at any time before and two recordings after the initiation date of systemic therapy were included. eGFR was calculated using the CKD-EPI equation (2009) without race coefficient [36].
Proteinuria assessment
Available urinary albumin-creatinine ratios (uACR) and protein–creatinine ratios (uPCR) from linked laboratory data were included from any time before and after the initiation of systemic therapy. If uPCR was the only available assessment of proteinuria, this was converted to uACR using a previously published conversion equation [37]. Pre-existing albuminuria before systemic therapy onset was categorised as uACR of > 3 mg/mmol (microscopic) or > 30 mg/mmol (macroscopic) [38].
Metastatic cancer and comorbidity assessment
Metastatic cancer at the point of diagnosis was identified from ‘TNM’ staging data from the cancer registry SMR06 and defined as any patient having with metastatic (‘M’) cancer. People with prevalent diabetes prior to the initiation of systemic therapy were identified using ICD-10 codes E10 and E11 from linked hospitalisation data from SMR01.
Renal outcomes
eGFR slope: defined as change in eGFR within two years following the initiation of VSPI/ICI therapy.
Death from renal cause: defined by ICD-10 codes N00-N19 as the primary cause of death in the death registry and SMR01.
Progression to CKD Stage 5: defined as eGFR < 15 mL/min/1.73m2 at any time during follow-up sustained > 28 days.
ESKD defined as renal transplantation or dialysis from SERPR.
A ≥ 40% decline in eGFR from the baseline average eGFR within the first year of therapy [39].
De-novo proteinuria: defined as normal uACR/uPCR results before treatment followed by elevated levels of proteinuria within one year of therapy [38].
Overall survival
Overall survival was defined by time to death from any cause using death registry and SMR01 data. We reported median, 1-, 2- and 5-year survival. This was additionally analysed in subgroups of patients who did or did not have nephrectomy prior to the onset of systemic therapy.
Statistical analysis
Descriptive statistics at baseline included counts and percentages for binary variables, while continuous variables were expressed as median (interquartile interval, IQI) or mean (standard deviation, SD).
eGFR slope was calculated for people who had two or more serum creatinine measures, within two years following the initiation of VSPI/ICI therapy. A linear mixed-effects model was used to analyse the slope of eGFR from the point of systemic therapy (commonly referred to as the total slope). This model included eGFR as the dependent variable and the time from the start of systemic therapy and the eGFR result (time difference) to allow for calculation of the change in eGFR per year from the point of systemic therapy. We employed random effects for the time difference variable for each patient to model distinct trajectories for each participant over time. We adjusted for age over 60 years at initiation of systemic therapy, sex, nephrectomy before systemic therapy, median eGFR < 60 mL/min/1.73m2 before systemic therapy and diabetes and metastatic cancer at diagnosis. These variables were selected for inclusion in the models due to biological plausibility as factors that might impact eGFR progression. They were included as independent binary variables and models assumed fixed effects. We accounted for individual specific variations by including a random effect for each patient. We included interaction terms: eGFR slope*nephrectomy prior to systemic therapy, eGFR slope*metastatic cancer and eGFR slope*median eGFR < 60 mL/min/1.73m2 before systemic therapy. The inclusion of these interaction terms in the model were tested using likelihood ratio test, using p < 0.05 as a significant improvement in model fit.
Cox proportional hazards models were used to analyse the clinically relevant associated factors of developing a ≥ 40% eGFR decline within the first year of systemic therapy and overall survival. We included univariable and multivariable models, which included the same relevant covariates as eGFR slope analysis. Fine and Gray subdistribution hazards were used to analyse the factors for developing a ≥ 40% eGFR decline within the first year of systemic therapy with a competing risk of death. Sensitivity analyses were conducted by therapy class using consistent analytical methods.
R software (version 4.3.2), employing packages tidyverse, finalfit, survminer, plot and lme4, was used for all analyses. The Model outputs and analysis will be available at publication (https://github.com/benelyan1/eGFR-slope-analysis).
Results
Baseline characteristics
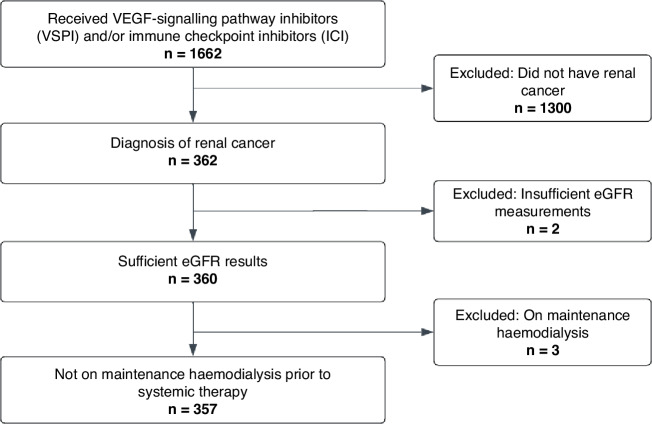
We initially identified 1662 people who received VSPI and/or ICI, 362 of whom had renal cell cancer. We excluded two patients due to insufficient eGFR measurements and three who were on long-term dialysis at the start of systemic therapy. We included 357 patients (62.5% male; median age 63.0 years, IQI 55.0–71.0) with renal cell cancer who had been treated with VSPI and/or ICI (Table 1). (Fig. 2).
Table 1.
Baseline characteristics of the cohort and split by nephrectomy pre and post systemic therapy.
| Characteristic | Nephrectomy before systemic therapy | No nephrectomy before systemic therapy | Overall | p | |
|---|---|---|---|---|---|
| Total: n (%) | 153 (42.9) | 204 (57.1) | 357 (100%) | ||
| Age at systemic therapy: median (IQI) | 62.0 (55.0–71.0) | 64.0 (55.0–70.2) | 63.0 (55.0–71.0) | 0.536 | |
| Sex: n (%) | Male | 100 (65.4) | 127 (62.3) | 227 (63.6) | 0.623 |
| Female | 53 (34.6) | 77 (37.7) | 130 (36.4) | ||
| Median eGFR before systemic therapy: median (IQI) | 70.6 (56.6–84.9) | 79.0 (61.4–96.0) | 74.6 (58.3–91.9) | <0.001 | |
| Microalbuminuria before systemic therapy: n (%) | Yes | 35 (57.4) | 35 (55.6) | 70 (56.5) | 0.981 |
| No | 26 (42.6) | 28 (44.4) | 54 (43.5) | ||
| Macroalbuminuria before systemic therapy: n (%) | Yes | 6 (9.8) | 5 (7.9) | 11 (8.9) | 0.955 |
| No | 55 (90.2) | 58 (92.1) | 113 (91.1) | ||
| Regime class: n (%) | ICI monotherapy | * | 7 (3.4) | * | 0.081 |
| VSPI monotherapy | 138 (90.2) | 164 (80.4) | 302 (84.6) | ||
| Dual ICI | * | 14 (6.9) | * | ||
| VSPI + ICI combination therapy | 8 (5.2) | 19 (9.3) | 27 (7.6) | ||
| Metastatic cancer at diagnosis: n (%) | Yes | 25 (16.3) | 94 (46.1) | 119 (33.3) | <0.001 |
| No | 128 (83.7) | 110 (53.9) | 238 (66.7) | ||
| Diabetes before systemic therapy: n (%) | Yes | 21 (13.7) | 29 (14.2) | 50 (14.0) | 0.999 |
| No | 132 (86.3) | 165 (85.8) | 307 (86.0) |
Proportions of micro- and macroalbuminuria are reported for those that had quantification by urinary albumin or protein–creatinine ratio. n number, IQI Interquartile interval.
*In this table, data points and totals representing fewer than 5 records have been suppressed and replaced with an asterisk to protect patient confidentiality.
Fig. 2. Flow chart.
Patient inclusion flow chart.
Median follow-up from start of systemic therapy to the last eGFR measurement or death was 1.35 years (IQI 0.50–2.61) and each person had a median of 25 eGFR measurements (IQI 13–38) after systemic therapy. VSPI monotherapy was the predominant choice of systemic therapy (86.0%) and was given for a median of five cycles of treatment (IQI 2–9). For the 150 (42.9%) people who had nephrectomy, the median time from nephrectomy to starting systemic therapy was 2.2 years (IQI 0.62–4.09) (Fig. 1).
Prior to systemic therapy, 92 people (25.8%) had an eGFR <60 mL/min/1.73m2 and 4 people (1.1%) had an eGFR < 30 mL/min/1.73m2. People who had nephrectomy (42.9%) prior to systemic therapy had a lower pre-treatment median eGFR (70.4 vs 78.9 mL/min/1.73m2, p < 0.001) and fewer had metastatic cancer at diagnosis (16.3% vs 46.1%, p < 0.001). There was considerable overlap between the people who had one or more of nephrectomy prior to systemic therapy, metastatic cancer at the point of diagnosis and an eGFR < 60 mL/min/1.73m2 (Supplementary fig. S1). Diabetes was present in 50 people (14.0%). uACR or uPCR values were available for 124 people (34.7%). Of these, 70 (56.5%) had evidence of microalbuminuria before commencing therapy and 11 (8.9%) had macroalbuminuria.
eGFR slope analysis
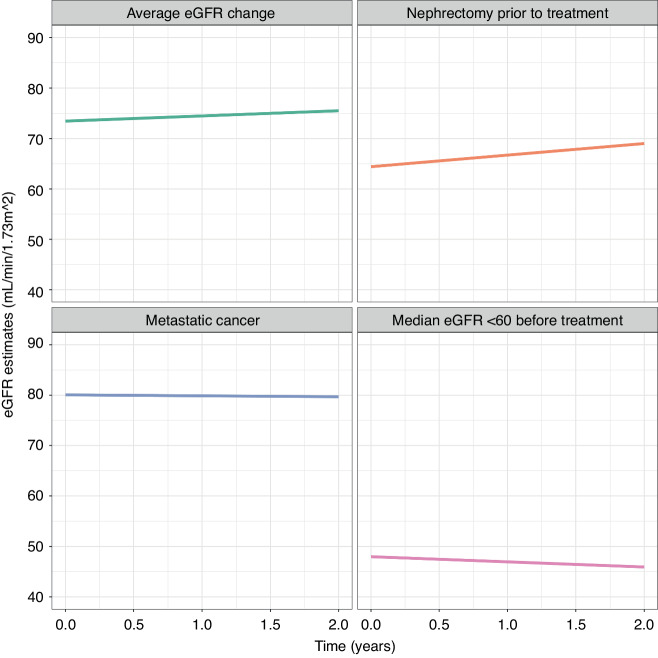
On average, there was no significant change in eGFR per year in people with renal cancer who had VSPI/ICI treatment ( + 1.03 mL/min/1.73m2/year; Table 2), nor in subgroups of people who had nephrectomy prior to systemic therapy ( + 2.30 mL/min/1.73m2/year), with metastatic cancer at the point of diagnosis (−0.18 mL/min/1.73m2/year), or with an eGFR < 60 mL/min/1.73m2 before systemic therapy (−1.02 mL/min/1.73m2/year; Fig. 3). A sensitivity analysis of people who received only VSPI monotherapy revealed similar results. There were insufficient numbers of people who received ICI monotherapy to perform a sensitivity analysis for this group.
Table 2.
Coefficient of change in eGFR per year in the two years following initiation of systemic therapy (average change in eGFR per year and for specific patient groups per year).
| Patient group | Coefficient of eGFR change per year (mL/min/1.73m2) | 95% CI: lower | 95% CI: upper | p-value |
|---|---|---|---|---|
| Whole cohort (unadjusted) | +1.03 | −1.64 | +3.70 | 0.23 |
| Nephrectomy prior to systemic therapy | +2.30 | −1.66 | +6.26 | 0.52 |
| Metastatic cancer at the point of diagnosis | −0.18 | −5.25 | +4.89 | 0.648 |
| Average eGFR < 60 prior to systemic therapy | −1.02 | −6.55 | +4.50 | 0.473 |
Fig. 3. eGFR slope estimates over time of people with renal cancer treated with VEGF-signalling pathway inhibitors and/or immune checkpoint inhibitors using the linear mixed-effects model from the point of systemic therapy to 2 years from follow-up.
Demonstrated as the average eGFR change of the of the group, and specifically for people who had nephrectomy prior to systemic therapy, metastatic cancer at the point of diagnosis and an average eGFR <60 ml/min/1.73m2 before systemic therapy.
Renal events analysis
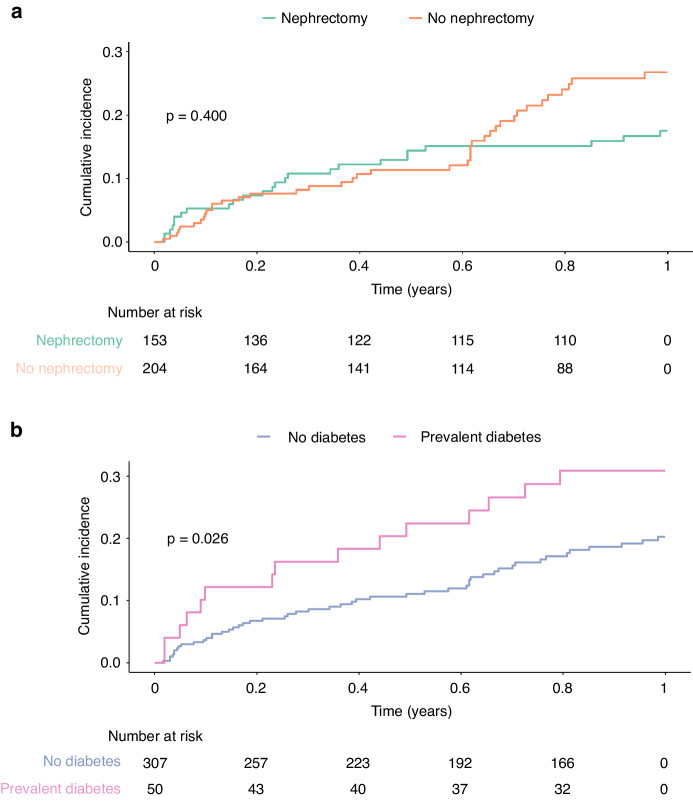
During the follow-up period, 82 (23.0%) people experienced a ≥ 40% decline in eGFR from baseline (Fig. 4). The cumulative incidence of this ≥40% decline in eGFR did not differ significantly between those that did or did not have nephrectomy prior to systemic therapy (p = 0.13).
Fig. 4. Cumulative incidence curves for a ≥ 40% decline of eGFR from the baseline eGFR at the time of initiation of systemic therapy.
The curves are stratified by people who (a) did or did not have nephrectomy prior to systemic therapy and (b) by those who did or did not have prevalent diabetes.
In univariable analysis, diabetes was associated with a 1.72-fold increased risk of ≥40% eGFR decline one year post systemic therapy (95% CI 1.01–2.94, p = 0.046), but this association was attenuated after multivariable adjustment (HR 1.70, 95% CI 0.99–2.92, p = 0.052). Competing risk analysis (Table 3) identified that diabetes diagnosis was the only covariate that demonstrated statistically significant associations of eGFR decline of ≥40% (subhazard ratio 1.89, 95% CI 1.05–3.41, p = 0.035). A sensitivity analysis of the people who received VSPI monotherapy revealed similar results and there were insufficient numbers of people who received ICI monotherapy to perform a sensitivity analysis for this group.
Table 3.
Hazard of eGFR decline of 40% from baseline eGFR within 1 year from the onset of systemic therapy.
| Dependent: 40% eGFR decline within 1 year of systemic therapy | Total (n) | HR (CPH univariable) | HR (CPH multivariable) | HR (Competing risks multivariable) | |
|---|---|---|---|---|---|
| Age at treatment | Median (IQR) | 63.0 (55.0–71.0) | 1.01 (0.99v1.03, p = 0.379) | 1.01 (0.99–1.04, p = 0.228) | 1.01 (0.99–1.03, p = 0.380) |
| Nephrectomy prior to systemic therapy | Yes | 153 | - | - | - |
| No | 204 | 0.87 (0.56–1.34, p = 0.517) | 0.90 (0.57–1.43, p = 0.663) | 1.22 (0.71–2.12, p = 0.470) | |
| Sex | Male | 227 | - | - | - |
| Female | 130 | 0.97 (0.62–1.53, p = 0.896) | 0.99 (0.63–1.56, p = 0.966) | 0.91 (0.54–1.53, p = 0.720) | |
| Average eGFR < 60 prior to systemic therapy | Yes | 96 | - | - | - |
| No | 261 | 1.08 (0.66–1.77, p = 0.762) | 1.28 (0.75–2.16, p = 0.363) | 0.94 (0.54–1.63, p = 0.840) | |
| Diabetes | No | 307 | - | - | - |
| Yes | 50 | 1.77 (1.04–3.01, p = 0.037) | 1.76 (1.03–3.01, p = 0.040) | 2.01 (1.11–3.62, p = 0.021) | |
| Metastatic cancer | No | 238 | - | - | - |
| Yes | 119 | 0.70 (0.43–1.15, p = 0.158) | 0.72 (0.43–1.22, p = 0.225) | 0.87 (0.49–1.55, p = 0.640) |
This is demonstrated as univariable and multivariable cox proportional hazards (CPH) for developing this decline. Fine and Grey subdistribution hazards for developing this decline with a competing risk of death (competing risks multivariable). Multivariable adjustment for all the included covariates.
Four people (1.1%) progressed to a sustained eGFR < 15 mL/min/1.73m2 during follow-up. One of these was established on long-term kidney replacement therapy. None of the people treated with VSPI/ICI who died during follow up had renal failure listed as the primary cause of death.
De-novo proteinuria within 1 year of systemic therapy
Proteinuria quantification was poorly documented. Of the 287 people who did not have proteinuria prior to systemic therapy, proteinuria was quantified within one year of therapy in 75 (27.1%) people. De-novo microscopic albuminuria developed in 23 people (8.3% of those who had quantification), and de-novo macroscopic albuminuria developed 13 people (4.7%).
Patients who had not undergone nephrectomy had higher risk of developing microscopic albuminuria within one year of systemic therapy than those who had not had a nephrectomy (subhazard ratio 4.72, 95% CI 1.14–19.44, p = 0.032). The other covariates did not reach statistical significance. No factors were independently associated with the development of macroalbuminuria.
Overall survival
During follow-up, 297 (83.2%) people died. Median survival was 1.36 years (IQI 1.11–1.75 years). Overall survival at one year was 58.3% (95% CI: 53.4–63.6%), two years 39.6% (95% CI: 34.8–45.0%), and five years 17.2% (95% CI: 13.3–22.2%). For people who had nephrectomy prior to systemic therapy, the median survival was longer: 2.13 years (IQI 1.82–2.57) than people who did not have nephrectomy: 0.88 years (IQI 0.74–1.16). The difference in median survival between the groups was statistically significant (log-rank test: Chi-Square = 19.6, p < 0.001, Supplementary fig. S2).
Metastatic cancer at the point of diagnosis and absence of nephrectomy prior to systemic therapy were associated with increased hazards of death on univariable analysis (p < 0.001). These associations remained significant after adjusting for age, sex, metastatic cancer, nephrectomy and median baseline eGFR < 60 mL/min/1.73m2 (Table 4, Supplementary fig. S3: Adjusted survival HR for no nephrectomy: 1.51 [95% CI: 1.18–1.94, p = 0.001]; Adjusted survival HR for metastatic cancer: 1.77 [95% CI: 1.36–2.29, p < 0.001]). A ≥ 40% acute or chronic decline in eGFR was not associated with increased hazards of death on univariable or multivariable analysis (Adjusted survival HR: 1.11 [0.85–1.46, p = 0.443]).
Table 4.
Hazards of death from the onset of systemic therapy.
| Dependent: Hazards of death | all | Total | HR (CPH univariable) | HR (CPH multivariable) | |
|---|---|---|---|---|---|
| Age > 60 at initiation of systemic therapy | No | 152 (42.6) | 152 (42.6) | - | - |
| Yes | 205 (57.4) | 205 (57.4) | 0.95 (0.75–1.19, p = 0.660) | 0.89 (0.71–1.12, p = 0.317) | |
| Nephrectomy prior to systemic therapy | Yes | 153 (42.9) | 153 (42.9) | - | - |
| No | 204 (57.1) | 204 (57.1) | 1.73 (1.37–2.19, p < 0.001) | 1.53 (1.20–1.96, p = 0.001) | |
| Sex | Male | 227 (63.6) | 227 (63.6) | - | - |
| Female | 130 (36.4) | 130 (36.4) | 1.13 (0.89–1.43, p = 0.311) | 1.14 (0.90–1.44, p = 0.273) | |
| 40% eGFR decline within 1 year of systemic therapy | No | 275 (77.0) | 275 (77.0) | - | - |
| Yes | 82 (23.0) | 82 (23.0) | 1.06 (0.81–1.39, p = 0.676) | 1.11 (0.85–1.46, p = 0.443) | |
| Metastatic cancer | No | 238 (66.7) | 238 (66.7) | - | - |
| Yes | 119 (33.3) | 119 (33.3) | 1.84 (1.45–2.33, p < 0.001) | 1.62 (1.26–2.08, p < 0.001) |
This is demonstrated as univariable, multivariable cox proportional hazards (CPH) for death. Multivariable adjustment for all the included covariates.
Discussion
This study explores the determinants of kidney function in people with renal cancer treated with VSPI and/or ICI. Our findings are generally reassuring for the average change in kidney function after the initiation of VSPI and/or ICI but shed light on several critical aspects related to renal outcomes associated with these important anti-cancer drugs.
The total cohort demonstrated no significant impact in yearly change of eGFR. Notably, this was true for specific subgroups including people with a history of nephrectomy, with metastatic disease at diagnosis or an average eGFR < 60 mL/min/1.73m2 prior to systemic therapy. The impact of these therapies on kidney function in the medium to long term had been unclear despite the justifiable concerns regarding the development of known risk factors for progressive CKD (e.g., hypertension) following their initiation. Whilst our findings are reassuring with regards to kidney function decline at least within the first two years of commencing treatment, they also suggest a complex interplay between cancer progression, survivorship, systemic therapy, management strategies and kidney function measurement in this cohort.
The positive trends in eGFR are likely to represent a fall in serum creatinine due to factors other than kidney function improvement, such as sarcopenia, weight loss and dietary changes. Accurate measurements of kidney function in people with cancer is crucial but often challenging. Inaccurate measurements of eGFR have been shown previously to associate with higher rates of drug-induced toxicity [40] and eligibility for systemic treatments [41]. Cockcroft Gault measurements of creatinine clearance (cg-CrCl) are commonly used in practice for drug dosing, despite a lack of validation in a cancer cohort [42]. Alternative markers of glomerular filtration (such as cystatin C, panel eGFR or measured GFR) [43, 44] and appropriate consideration of the role of tubular creatinine secretion [45] after VSPI/ICI treatment might have yielded different results.
We observed a ≥ 40% decline in eGFR within the first year of systemic therapy in almost a quarter of the participants, and a very small proportion (1.1%) developed a persistent drop in eGFR to < 15 mL/min/1.73m2 or ESKD. The reported rate of nephritis or ‘raised creatinine’ is lower in randomised controlled trials of VSPI [46] and/or ICI [47] as anti-cancer treatments. Whilst it appears that the overall trend of kidney function does not decline, people commonly experience clinically important renal events following treatment initiation and there may be people who are at higher risk of experiencing these events. Diabetes emerged as a key risk factor and, although this association was attenuated after multivariable adjustment, a mechanistically plausible trend to a link between diabetes and worse renal outcomes remained. Whilst a ≥ 40% decline in eGFR within the first year of systemic therapy was not associated with an increased hazard of death, a competing risk multivariable regression analysis censoring for death is an important analysis to consider in this group with a high mortality rate. Diabetes was associated with an increased risk of ≥ 40% decline in eGFR within the first year of systemic therapy, however, diabetes has previously been associated with an overall increased risk of acute kidney injury compared with those without diabetes [48]. The number of people developing eGFR < 15 ml/min/1.732 or ESKD was too small to make further analyses, but further supports the need to study renal-specific implications of these therapies, particularly as their indications broaden to include the treatment of a broader range solid organ tumours and at increasingly early stages [2].
The representation of people with eGFR < 30 mL/min/1.73m2 prior to the initiation of treatment in this cohort was low. This is despite the high prevalence of people with cancer who have co-existing CKD [49], and the large proportion of people in this cohort who had partial or total nephrectomy as part of their cancer management. The under-representation of patients with more advanced CKD suggests that people with CKD may not be treated with these anti-cancer therapies on the basis of reduced kidney function. Our data suggest that exclusion from therapy on this basis may not be justified. People with CKD are under-represented in clinical trials [50] which may also contribute to relative underuse of these drugs in patients with CKD. However, we did note that three people were on maintenance haemodialysis when treated with VSPI/ICI. People with an eGFR < 60 mL/min/1.73m2 prior to systemic therapy did not demonstrate an excessive decline in eGFR slope per year or high rates of a decline of eGFR by ≥40% from baseline within a year. Whilst this population may be prone to selection bias, it does suggest that renal side effects are not an overwhelming issue in this population.
Additionally, 1 in 13 people who had no evidence of proteinuria prior to systemic therapy developed microscopic albuminuria within the first year of systemic therapy although the vast majority did not have proteinuria tested following systemic therapy initiation. The absence of nephrectomy prior to systemic therapy emerged as a significant risk factor for developing de-novo microscopic albuminuria, potentially overlapping with other factors that may be implicated in the decision not to have operative management of the cancer. Initial nephrectomy in patients who present with metastatic disease remains controversial and is generally reserved for those with more favourable prognostic features who may be less likely to suffer renal consequences of advanced cancer. Furthermore, patients who undergo nephrectomy with curative intent but subsequently relapse with metastatic disease have better prognosis and, similarly, may be less likely to suffer renal events on treatment. In general, baseline and subsequent proteinuria was poorly documented in this cohort despite it being well established as a known side effect [51]. However, the long-term implications of developing proteinuria in this setting remain poorly understood.
The clinical significance of the nephron loss following nephrectomy in the context of advanced renal cancer is unclear and complex, due to the impact of cancer on kidney function and some shared risk factors for renal cancer and progressive CKD such as smoking and genetic conditions [30]. There appear to be understandable but important differences in the risk of reduced kidney function between radical nephrectomy and partial nephrectomy [33]. In the context of kidney donation for transplantation, nephrectomy does appear to result in an elevated risk of proteinuria [52] and ESKD [53], despite a significant compensatory rise in eGFR following surgery [54]. Importantly, our data demonstrate that people who had nephrectomy were not a greater risk for average eGFR decline.
While our study provides valuable insights into the renal-specific outcomes of VSPI/ICI therapy in people with renal cancer, it is essential to acknowledge its limitations. First, the high death rate of this cohort may impair the application of these findings to other contemporary cohorts of patients who may have better longer-term survival and longer exposure to treatment. Second, we could not comment on measured kidney function or alternative measures of kidney function as—in keeping with widespread clinical practice—we were limited to creatinine-based measures of eGFR. We have reported eGFR indexed to body surface area (BSA) of 1.73m2, though eGFR non-indexed to BSA may be preferable for drug dosing in situations where BSA is substantially different from the reference value (as may be seen in cancer-associated cachexia). We did not have longitudinal height and weight data to report non-indexed values. Third, the small numbers who had quantification of proteinuria limit the ability to draw conclusions about the entire cohort and analysis of association with outcomes. We did not have data on dipstick urinalysis assessment which further impairs our ability to draw conclusions about the risk of proteinuria. Fourth, we did not have a comparison cohort of patients with renal cancer who did not receive VSPI or ICI, so were unable to dissect the relative contributions of treatment and the cancer itself on the observed renal events. Fifth, we had insufficient numbers to compare combination treatments to monotherapy in our sensitivity analysis. Finally, our cohort was from a single centre and participants were predominantly Caucasian, which may limit generalisability to other populations.
Conclusion
VSPI and ICIs offer people with renal cancer significant improvement in survivorship. Despite case series and prescribing guidelines highlighting adverse impact of VSPI/ICI therapy on renal function, our real-world data on the effect of VSPI/ICI therapy on renal function demonstrate that there is no significant impact on the average change in eGFR but highlights that some groups are at higher risk of clinically significant renal events, such as people with diabetes. Our data suggest that people with prior renal dysfunction may, potentially inappropriately, be denied access to life-prolonging anti-cancer therapy. Further investigation into appropriate renal risk stratification and optimal surveillance strategies is required for people with cancer treated with VSPI and/or ICI.
Supplementary information
Acknowledgements
We thank the patients who contributed to this work. We would like to thank the support of the West of Scotland ChemoCare group and West of Scotland SafeHaven network for supporting the work.
Author contributions
B.M.P.E., J.S.L., P.B.M, N.N.L. and R.J.J. conceived of and designed the study. Data were analysed by B.M.P.E., J.H., N.D.L.M. B.M.P.E. wrote the first draft of the manuscript and prepared figures and tables. All authors contributed to the final manuscript.
Funding
P.B.M. reports lecture honoraria from Astra Zeneca, Pharmacomsos, Bayer, Astellas, GSK and Boehringer Ingelheim outside the submitted work. Outside the submitted work, J.S.L. has received personal lectureship honoraria from Astra Zeneca. NNL reports research grants from Roche Diagnostics, Astra Zeneca and Boehringer Ingelheim as well as consultancy/speaker’s fees from Roche Diagnostics, Myokardia, Pharmacosmos, Akero Therapeutics, CV6 Therapeutics, Jazz Pharma, AstraZeneca and Novartis all outside the submitted work. NNL is supported by a British Heart Foundation Centre of Research Excellence Award (RE/18/6/34217). J.S.L. is personally funded by a Wellcome Trust Early Career Award (301005/Z/23/Z).
Data availability
Data used for this study are available through the West of Scotland ChemoCare and West of Scotland SafeHaven network. The Model outputs and analysis will be made available at the time of publication (https://github.com/benelyan1/eGFR-slope-analysis).
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Ethics approval for access to the data for this study was gained through the Local Privacy Advisory Committee group as part of the West of Scotland SafeHaven Network.
Consent for publication
All outputs from the analysed data (data tables and figures) were reviewed and approved by the West of Scotland SafeHaven support team.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s44276-024-00081-7.
References
- 1.O’Dowd A. Long term cancer survival rates double in England and Wales in past 40 years. BMJ. 2010;341:c3750–c3750. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–300. [DOI] [PubMed] [Google Scholar]
- 4.Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang YH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med. 2021;385:683–94. [DOI] [PubMed] [Google Scholar]
- 5.Estrada CC, Maldonado A, Mallipattu SK. Therapeutic inhibition of VEGF signaling and associated nephrotoxicities. J Am Soc Nephrol. 2019;30:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Short SAP, Sise ME, Prosek JM, Madhavan SM, Soler MJ, et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer. 2021;9:e003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taal MW, Brenner BM. Predicting initiation and progression of chronic kidney disease: developing renal risk scores. Kidney Int. 2006;70:1694–705. [DOI] [PubMed] [Google Scholar]
- 8.McClellan WM, Flanders WD. Risk factors for progressive chronic kidney disease. J Am Soc Nephrol. 2003;14:S65–70. [DOI] [PubMed] [Google Scholar]
- 9.Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. The Lancet. 2010 Jun 12;375:2073–81. 10.1016/s0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed]
- 10.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–9. [DOI] [PubMed] [Google Scholar]
- 12.Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol. 2017;35:591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García-Carro C, Jhaveri KD, Sprangers B. Revisiting the role of acute kidney injury in patients on immune checkpoint inhibitors: a good prognosis renal event with a significant impact on survival. Clin Kidney J. 2023;16:773–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao Ullur A, Côté G, Pelletier K, Kitchlu A. Immunotherapy in oncology and the kidneys: a clinical review of the evaluation and management of kidney immune-related adverse events. Clin Kidney J. 2023;16:939–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izzedine H, Rixe O, Billemont B, Baumelou A, Deray G. Angiogenesis inhibitor therapies: focus on kidney toxicity and hypertension. Am J Kidney Dis. 2007;50:203–18. [DOI] [PubMed] [Google Scholar]
- 16.Hultin S, Nahar K, Menzies AM, Long GV, Fernando SL, Atkinson V, et al. Histological diagnosis of immune checkpoint inhibitor induced acute renal injury in patients with metastatic melanoma: a retrospective case series report. BMC Nephrol. 2020;21:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soria JC, Massard C, Izzedine H. From theoretical synergy to clinical supra-additive toxicity. J Clin Oncol. 2009;27:1359–61. [DOI] [PubMed] [Google Scholar]
- 18.Manohar S, Kompotiatis P, Thongprayoon C, Cheungpasitporn W, Herrmann J, Herrmann SM. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: meta-analysis. Nephrol Dial Transplant. 2019;34:108–17. [DOI] [PubMed] [Google Scholar]
- 19.Lee N, Lee JL, Lee JY. Analysis of anti-angiogenesis-related adverse events associated with vascular endothelial growth factor receptor-tyrosine kinase inhibitors (VEGFR-TKIs) in patients with metastatic renal cell carcinoma. Target Oncol. 2023;18:247–55. [DOI] [PubMed] [Google Scholar]
- 20.Paschke L, Lincke T, Mühlberg KS, Jabs WJ, Lindner TH, Paschke R. Anti VEGF-TKI treatment and new renal adverse events not reported in phase III trials. Eur Thyroid J. 2018;7:308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanna RM, Barsoum M, Arman F, Selamet U, Hasnain H, Kurtz I. Nephrotoxicity induced by intravitreal vascular endothelial growth factor inhibitors: emerging evidence. Kidney Int. 2019;96:572–80. [DOI] [PubMed] [Google Scholar]
- 22.Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, et al. Clinical features and outcomes of immune checkpoint inhibitor–associated AKI: a multicenter study. J Am Soc Nephrol. 2020;31:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heerspink HJL, Tighiouart H, Sang Y, Ballew S, Mondal H, Matsushita K, et al. GFR decline and subsequent risk of established kidney outcomes: a meta-analysis of 37 Randomized Controlled Trials. Am J Kidney Dis. 2014;64:860–6. [DOI] [PubMed] [Google Scholar]
- 24.Inker LA, Collier W, Greene T, Miao S, Chaudhari J, Appel GB, et al. A meta-analysis of GFR slope as a surrogate endpoint for kidney failure. Nat Med. 2023;29: 1867–76. [DOI] [PubMed] [Google Scholar]
- 25.Vonesh E, Tighiouart H, Ying J, Heerspink HL, Lewis J, Staplin N, et al. Mixed-effects models for slope-based endpoints in clinical trials of chronic kidney disease. Stat Med. 2019;38:4218–39. [DOI] [PubMed] [Google Scholar]
- 26.Wanner C. Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA-REG OUTCOME Trial. 29:2755–69. 10.1681/asn.2018010103 [DOI] [PMC free article] [PubMed]
- 27.Rosner MH, Perazella MA. AcuteKidney Injury in Patients with Cancer. Ingelfinger JR, editor. N Engl J Med. 2017 May 4;376:1770–81. 10.1056/nejmra1613984 [DOI] [PubMed]
- 28.Lees JS, Ho F, Parra-Soto S, Celis-Morales C, Welsh P, Sullivan MK, et al. Kidney function and cancer risk: An analysis using creatinine and cystatin C in a cohort study. eClinicalMedicine. 2021;38:101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iff S, Craig JC, Turner R, Chapman JR, Wang JJ, Mitchell P, et al. Reduced estimated GFR and cancer mortality. Am J Kidney Dis. 2014;63:23–30. [DOI] [PubMed] [Google Scholar]
- 30.Lees JS, Elyan BMP, Herrmann SM, Lang NN, Jones RJ, Mark PB. The ‘other’ big complication: how chronic kidney disease impacts on cancer risks and outcomes. Nephrol Dial Transplant. 2023;38:1071–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimm M-O, Oya M, Choueiri TK, Schmidinger M, Quinn DI, Gravis Mescam G, et al. Role of prior nephrectomy for synchronous metastatic renal cell carcinoma (mRCC) on efficacy in patients treated with avelumab + axitinib (A + Ax) or sunitinib (S): Results from JAVELIN Renal 101. Ann Oncol. 2021;32:S690–1. [Google Scholar]
- 32.Levey HR, Scosyrev E, Wu K, Agrawal V, Messing E, Wu G. Overall survival after partial versus radical nephrectomy for a small renal mass: A systematic review and meta-analysis of observational studies. J Urol. 2014;191:e18–9. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Lau WL, Rhee CM, Harley K, Kovesdy CP, Sim JJ, et al. Risk of chronic kidney disease after cancer nephrectomy. Nat Rev Nephrol. 2014;10:135–45. [DOI] [PubMed] [Google Scholar]
- 34.Harasemiw O, Nayak JG, Grubic N, Ferguson TW, Sood MM, Tangri N. A predictive model for kidney failure after nephrectomy for localized kidney cancer: the kidney cancer risk equation. Am J Kidney Dis. 2023;82:656–65. [DOI] [PubMed] [Google Scholar]
- 35.Klarenbach S, Moore RB, Chapman DW, Dong J, Braam B. Adverse renal outcomes in subjects undergoing nephrectomy for renal tumors: a population-based analysis. Eur Urol. 2011;59:333–9. [DOI] [PubMed] [Google Scholar]
- 36.Levey AS, Stevens LA, Schmid CH, Zhang Y(L), Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sumida K, Nadkarni GN, Grams ME, Sang Y, Ballew SH, Coresh J, et al. Conversion of urine protein–creatinine ratio or urine dipstick protein to urine albumin–creatinine ratio for use in chronic kidney disease screening and prognosis. Ann Intern Med. 2020;173:426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NICE. Chronic kidney disease: assessment and management [Internet]. 2021 [cited 2023 Sep 13]. Available from: https://www.nice.org.uk/guidance/ng203/chapter/Update-information
- 39.Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the national kidney foundation and the US food and drug administration. Am J Kidney Dis. 2014;64:821–35. [DOI] [PubMed] [Google Scholar]
- 40.Hanna PE, Wang Q, Strohbehn IA, Moreno D, Harden D, Ouyang T, et al. Medication-related adverse events and discordancies in cystatin C–based vs serum creatinine–based estimated glomerular filtration rate in patients with cancer. JAMA Netw Open. 2023;6:e2321715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsao CK, Moshier E, Seng SM, Godbold J, Grossman S, Winston J, et al. Impact of the CKD-EPI equation for estimating renal function on eligibility for cisplatin-based chemotherapy in patients with urothelial cancer. Clin Genitourin Cancer. 2012;10:15–20. [DOI] [PubMed] [Google Scholar]
- 42.Shepherd STC, Gillen G, Morrison P, Forte C, Macpherson IR, White JD, et al. Performance of formulae based estimates of glomerular filtration rate for carboplatin dosing in stage 1 seminoma. Eur J Cancer. 2014;50:944–52. [DOI] [PubMed] [Google Scholar]
- 43.Fu EL, Levey AS, Coresh J, Elinder CG, Rotmans JI, Dekker FW, et al. Accuracy of GFR estimating equations in patients with discordances between creatinine and cystatin c-based estimations. J Am Soc Nephrol JASN. 2023;34:1241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iversen E, Bengaard AK, Leegaard Andersen A, Tavenier J, Nielsen RL, Juul-Larsen HG, et al. Performance of panel-estimated GFR among hospitalized older adults. Am J Kidney Dis. 2023;82:715–24. [DOI] [PubMed] [Google Scholar]
- 45.Vanhoutte T, Sprangers B. Pseudo-AKI associated with targeted anti-cancer agents-the truth is in the eye of the filtration marker. Clin Kidney J. 2023;16:603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ollero M, Sahali D. Inhibition of the VEGF signalling pathway and glomerular disorders. Nephrol Dial Transplant. 2015;30:1449–55. [DOI] [PubMed] [Google Scholar]
- 47.Izzedine H, Mateus C, Boutros C, Robert C, Rouvier P, Amoura Z, et al. Renal effects of immune checkpoint inhibitors. Nephrol Dial Transplant. 2016;32:936-42 [DOI] [PubMed]
- 48.Kaur A, Sharma GS, Kumbala DR. Acute kidney injury in diabetic patients: a narrative review. Medicine (Baltimore). 2023;102:e33888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Launay-Vacher V, Oudard S, Janus N, Gligorov J, Pourrat X, Rixe O, et al. Prevalence of Renal Insufficiency in cancer patients and implications for anticancer drug management: The renal insufficiency and anticancer medications (IRMA) study. Cancer. 2007;110:1376–84. [DOI] [PubMed] [Google Scholar]
- 50.Elyan BMP, Rankin S, Jones R, Lang NN, Mark PB, Lees JS. Kidney disease patient representation in trials of combination therapy with VEGF-signaling pathway inhibitors and immune checkpoint inhibitors: a systematic review. Kidney Med. 2023;5:100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan AJ, Mo DC, Wu K, Pan HM, Wang DM, Xu XX, et al. Nephrotoxicity of immune checkpoint inhibitor combination therapy in patients with advanced renal cell carcinoma: a meta-analysis. World J Urol. 2023;41:1563–71. [DOI] [PubMed] [Google Scholar]
- 52.Garg AX, Muirhead N, Knoll G, Yang RC, Prasad GVR, Thiessen-Philbrook H, et al. Proteinuria and reduced kidney function in living kidney donors: a systematic review, meta-analysis, and meta-regression. Kidney Int. 2006;70:1801–10. [DOI] [PubMed] [Google Scholar]
- 53.Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311:579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lam NN, Lloyd A, Lentine KL, Quinn RR, Ravani P, Hemmelgarn BR, et al. Changes in kidney function follow living donor nephrectomy. Kidney Int. 2020;98:176–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used for this study are available through the West of Scotland ChemoCare and West of Scotland SafeHaven network. The Model outputs and analysis will be made available at the time of publication (https://github.com/benelyan1/eGFR-slope-analysis).