Abstract
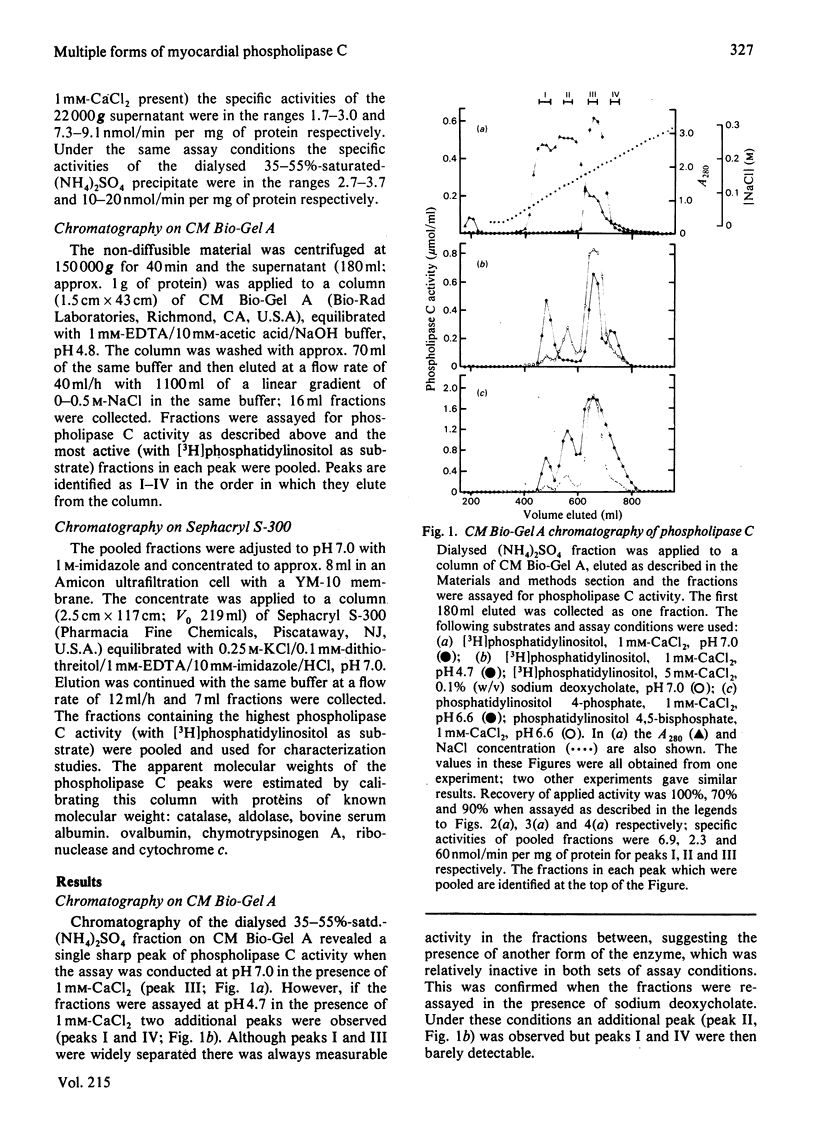
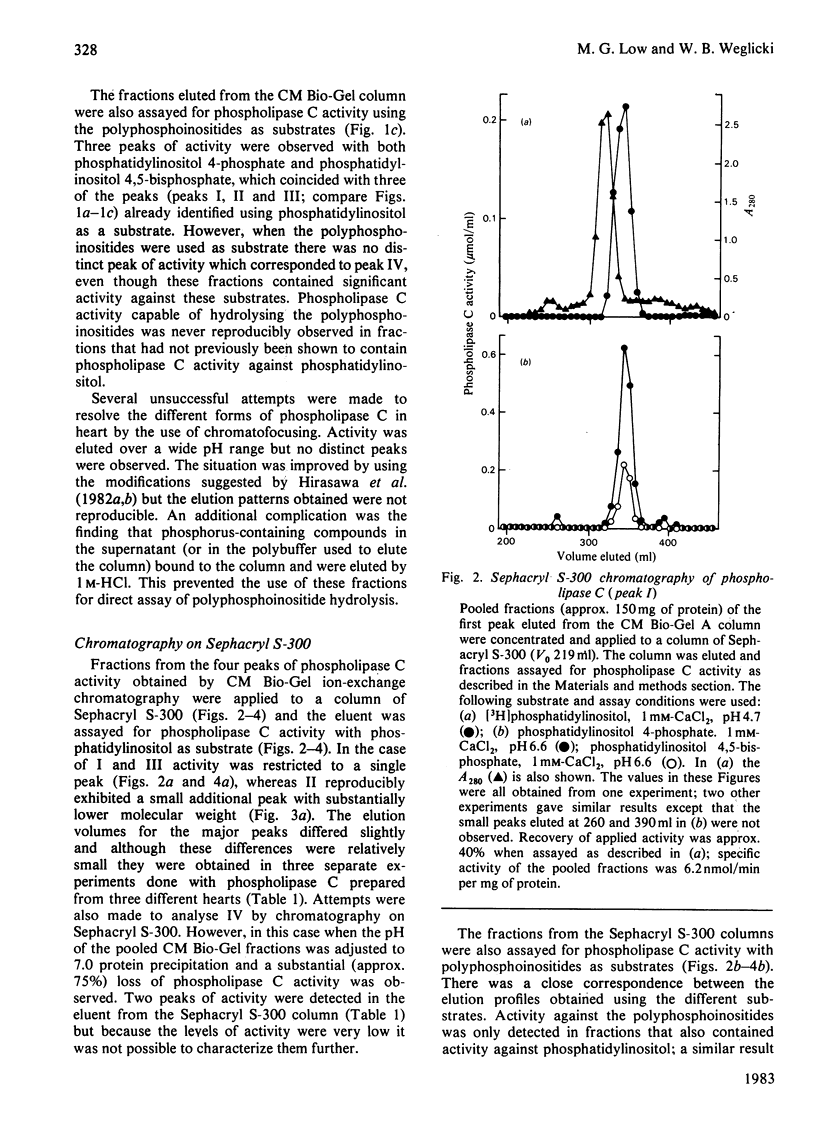
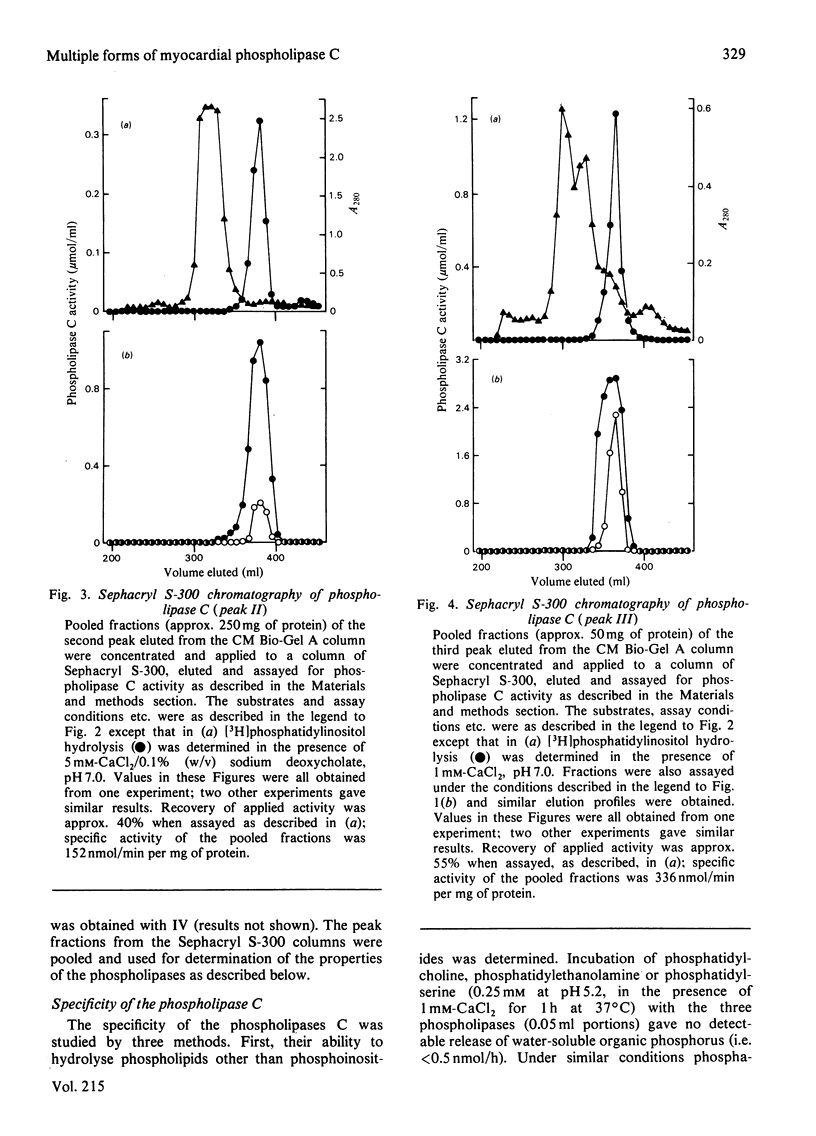
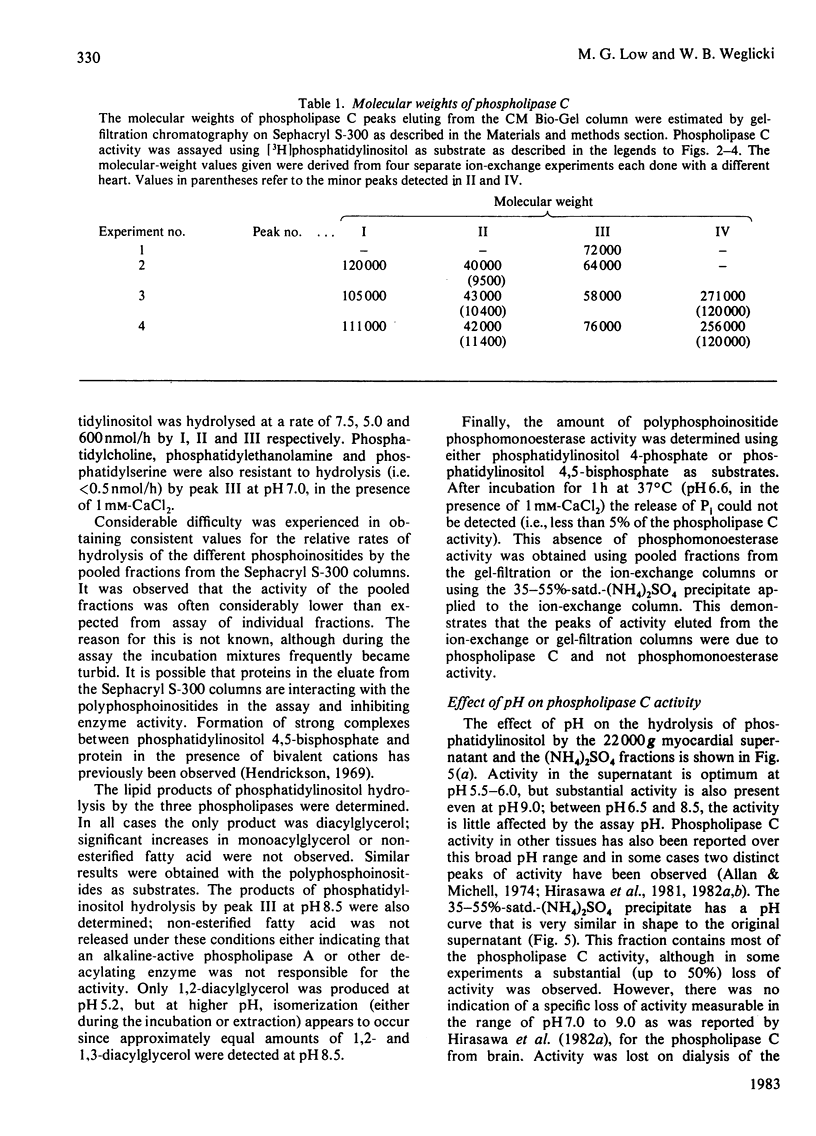
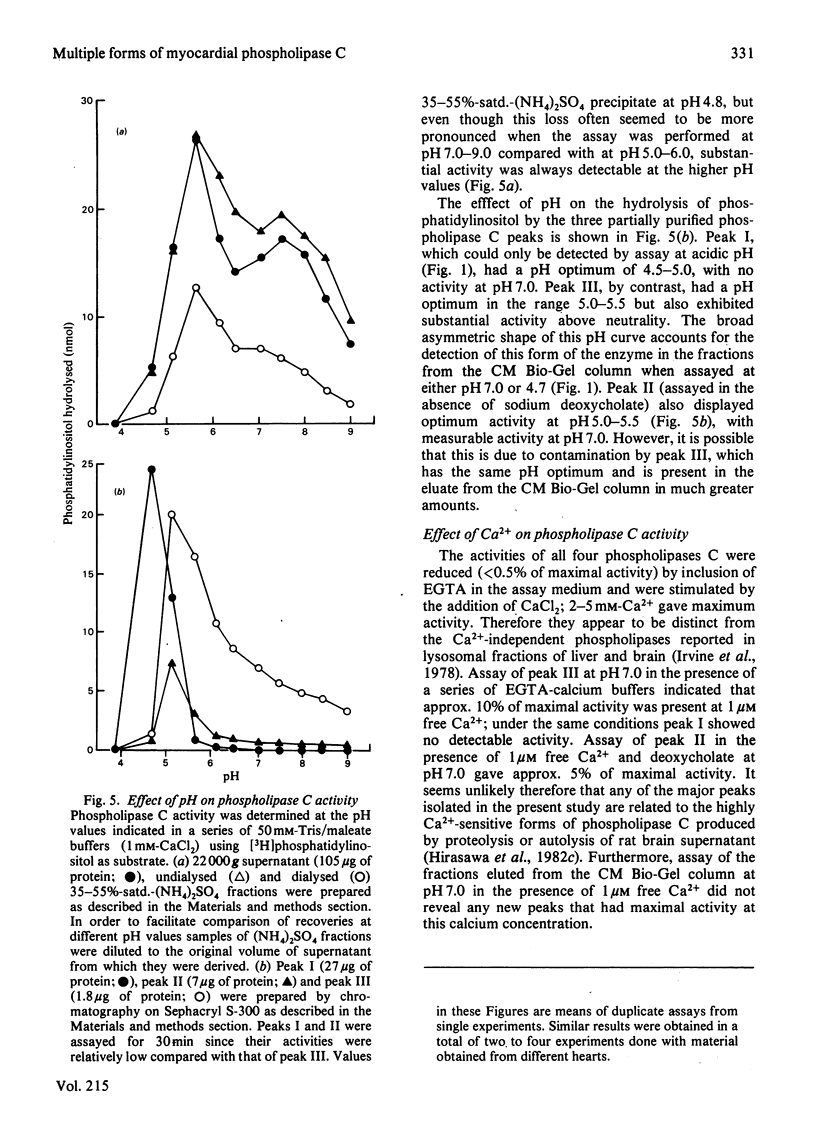
Phospholipase C activity capable of hydrolysing phosphatidylinositol in bovine heart was resolved into four forms (I-IV) by ion-exchange chromatography. Some of these forms could only be detected if the assay was performed at acidic pH (I and IV) or in the presence of deoxycholate (II). Gel-filtration chromatography indicated that the four forms had different molecular weights in the range 40000-120000. I, II and III all had pH optima in the range 4.5-5.5. However, the major form (III) also had substantial activity at pH 7.0 and above. The activities of I, II and III at pH 7.0 were stimulated by deoxycholate; this effect was most marked with I and II, which had very low activity at this pH. All forms of the enzyme were inhibited by EGTA and required 2-5 mM-CaCl2 for maximal activity. When the fractions eluted from the ion-exchange and gel-filtration columns were assayed with polyphosphoinositides as substrates there was a close correspondence to the elution profile obtained with phosphatidylinositol as substrate; there was no evidence for the existence in heart of phospholipase C activities specific for individual phosphoinositides.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A., Luke B., Smith J. P. Studies on the properties of a soluble phosphatidylinositol-phosphodiesterase of rabbit iris smooth muscle. Biochim Biophys Acta. 1980 Aug 7;614(2):425–434. doi: 10.1016/0005-2744(80)90232-6. [DOI] [PubMed] [Google Scholar]
- Akhtar R. A., Abdel-Latif A. A. Studies on the properties of triphosphoinositide phosphomonoesterase and phosphodiesterase of rabbit iris smooth muscle. Biochim Biophys Acta. 1978 Nov 10;527(1):159–170. doi: 10.1016/0005-2744(78)90265-6. [DOI] [PubMed] [Google Scholar]
- Allan D., Michell R. H. A calcium-activated polyphosphoinositide phosphodiesterase in the plasma membrane of human and rabbit erythrocytes. Biochim Biophys Acta. 1978 Apr 4;508(2):277–286. doi: 10.1016/0005-2736(78)90330-9. [DOI] [PubMed] [Google Scholar]
- Allan D., Michell R. H. Phosphatidylinositol cleavage catalysed by the soluble fraction from lymphocytes. Activity at pH5.5 and pH7.0. Biochem J. 1974 Sep;142(3):591–597. doi: 10.1042/bj1420591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton R. S., Hawthorne J. N. The phosphoinositide inositolphosphohydrolase of guinea-pig intestinal mucosa. Eur J Biochem. 1968 Mar;4(1):68–75. doi: 10.1111/j.1432-1033.1968.tb00173.x. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Baginski E. S., Foà P. P., Zak B. Microdetermination of inorganic phosphate, phospholipids, and total phosphate in biologic materials. Clin Chem. 1967 Apr;13(4):326–332. [PubMed] [Google Scholar]
- Chau L. Y., Tai H. H. Resolution into two different forms and study of the properties of phosphatidylinositol-specific phospholipase C from human platelet cytosol. Biochim Biophys Acta. 1982 Nov 12;713(2):344–351. [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The control by Ca2+ of the polyphosphoinositide phosphodiesterase and the Ca2+-pump ATPase in human erythrocytes. Biochem J. 1982 Jan 15;202(1):53–58. doi: 10.1042/bj2020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDRICKSON H. S., BALLOU C. E. ION EXCHANGE CHROMATOGRAPHY OF INTACT BRAIN PHOSPHOINOSITIDES ON DIETHYLAMINOETHYL CELLULOSE BY GRADIENT SALT ELUTION IN A MIXED SOLVENT SYSTEM. J Biol Chem. 1964 May;239:1369–1373. [PubMed] [Google Scholar]
- Hakata H., Kambayashi J., Kosaki G. Purification and characterization of phosphatidylinositol-specific phospholipase C from bovine platelets. J Biochem. 1982 Sep;92(3):929–935. doi: 10.1093/oxfordjournals.jbchem.a134008. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. S. Physical properties and interactions of phosphoinositides. Ann N Y Acad Sci. 1969 Oct 17;165(2):668–676. [PubMed] [Google Scholar]
- Hirasawa K., Irvine R. F., Dawson R. M. Heterogeneity of the calcium-dependent phosphatidylinositol phosphodiesterase in rat brain. Biochem J. 1982 Aug 1;205(2):437–442. doi: 10.1042/bj2050437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa K., Irvine R. F., Dawson R. M. Heterogeneity of the calcium-dependent phosphatidylinositol-phosphodiesterase of rat liver kidney, as revealed by column chromatofocusing. Biochem Biophys Res Commun. 1982 Jul 30;107(2):533–537. doi: 10.1016/0006-291x(82)91524-8. [DOI] [PubMed] [Google Scholar]
- Hirasawa K., Irvine R. F., Dawson R. M. Proteolytic activation can produce a phosphatidylinositol phosphodiesterase highly sensitive to Ca2+. Biochem J. 1982 Sep 15;206(3):675–678. doi: 10.1042/bj2060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Dawson R. M. The distribution of calcium-dependent phosphatidylinositol-specific phosphodiesterase in rat brain. J Neurochem. 1978 Dec;31(6):1427–1434. doi: 10.1111/j.1471-4159.1978.tb06568.x. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Hemington N., Dawson R. M. The hydrolysis of phosphatidylinositol by lysosomal enzymes of rat liver and brain. Biochem J. 1978 Nov 15;176(2):475–484. doi: 10.1042/bj1760475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keough K. M., Thompson W. Soluble and particulate forms of phosphoinositide phosphodiesterase in ox brain. Biochim Biophys Acta. 1972 Jul 7;270(3):324–336. doi: 10.1016/0005-2760(72)90197-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lapetina E. G., Seguin E. B., Agranoff B. W. Preparation of 32P-labeled inositides and their degradation by soluble kidney enzymes. Biochim Biophys Acta. 1975 Jul 22;398(1):118–124. doi: 10.1016/0005-2760(75)90175-7. [DOI] [PubMed] [Google Scholar]
- Low M. G., Zilversmit D. B. Phosphatidylinositol distribution and translocation in sonicated vesicles. A study with exchange protein and phospholipase C. Biochim Biophys Acta. 1980 Feb 28;596(2):223–234. doi: 10.1016/0005-2736(80)90357-0. [DOI] [PubMed] [Google Scholar]
- Luthra M. G., Sheltawy A. The chromatographic separation of phospholipids on alumina with solvents containing ammonium salts. Biochem J. 1972 Jan;126(1):251–253. doi: 10.1042/bj1260251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. J. The association between phosphatidylinositol phosphodiesterase activity and a specific subunit of microtubular protein in rat brain. Biochem J. 1973 Jun;133(2):273–281. doi: 10.1042/bj1330273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S. D. Minireview. Phosphatidylinositol specific phospholipases C. Life Sci. 1982 Apr 19;30(16):1323–1335. doi: 10.1016/0024-3205(82)90016-9. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Nagai Y. Purification of phosphatidylinositol-specific phospholipase C from rat liver. J Biol Chem. 1981 Jul 10;256(13):6769–6775. [PubMed] [Google Scholar]
- Thompson W., Dawson R. M. The triphosphoinositide phosphodiesterase of brain tissue. Biochem J. 1964 May;91(2):237–243. doi: 10.1042/bj0910237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tou J. S., Hurst M. W., Baricos W. H., Huggins C. G. The hydrolysis of triphosphoinositide by a phosphodiesterase in rat kidney cortex. Arch Biochem Biophys. 1973 Feb;154(2):593–600. doi: 10.1016/0003-9861(73)90013-1. [DOI] [PubMed] [Google Scholar]


