Abstract
Bovine lens cytoplasmic aldehyde dehydrogenase exhibits Michaelis-Menten kinetics with acetaldehyde, glyceraldehyde 3-phosphate, p-nitrobenzaldehyde, propionaldehyde, glycolaldehyde, glyceraldehyde, phenylacetylaldehyde and succinic semialdehyde as substrates. The enzyme was also active with malondialdehyde, and exhibited an esterase activity. Steady-state kinetic analyses show that the enzyme exhibits a compulsory-ordered ternary-complex mechanism with NAD+ binding before acetaldehyde. The enzyme was inhibited by disulfiram and by p-chloromercuribenzoate, and studies with with mercaptans indicated the involvement of thiol groups in catalysis.
Full text
PDF

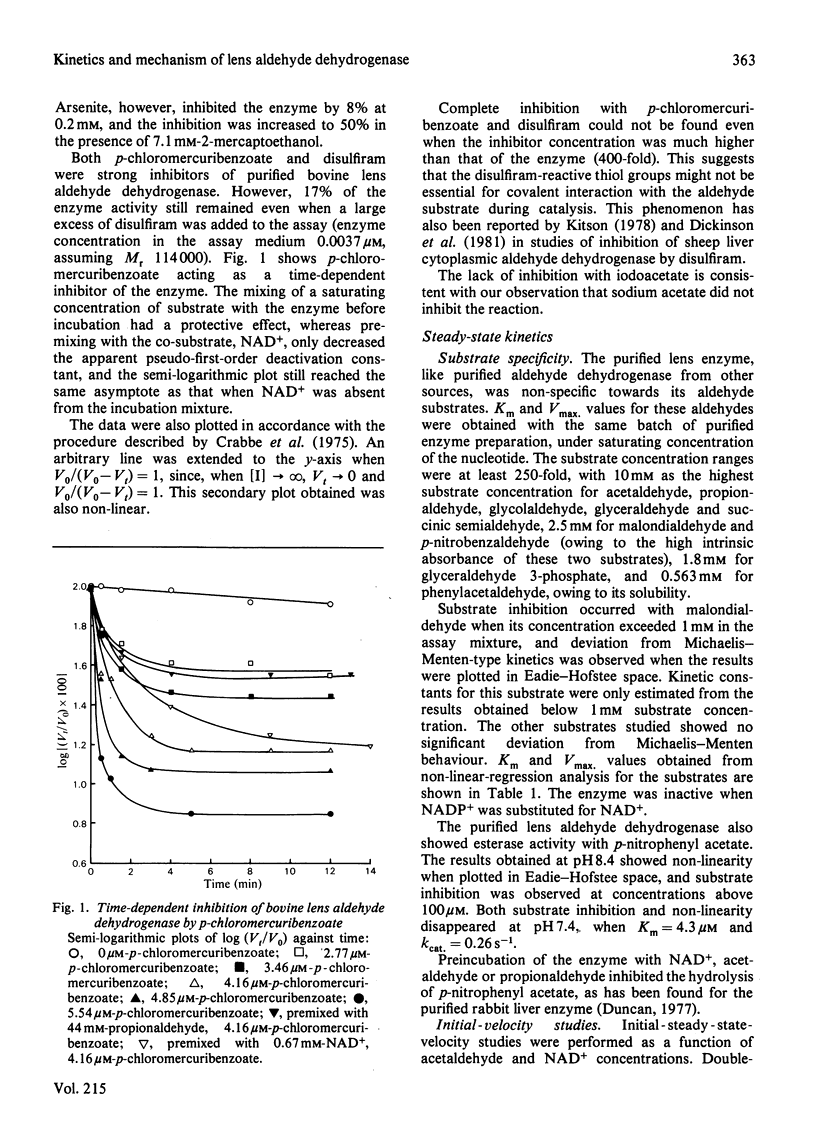
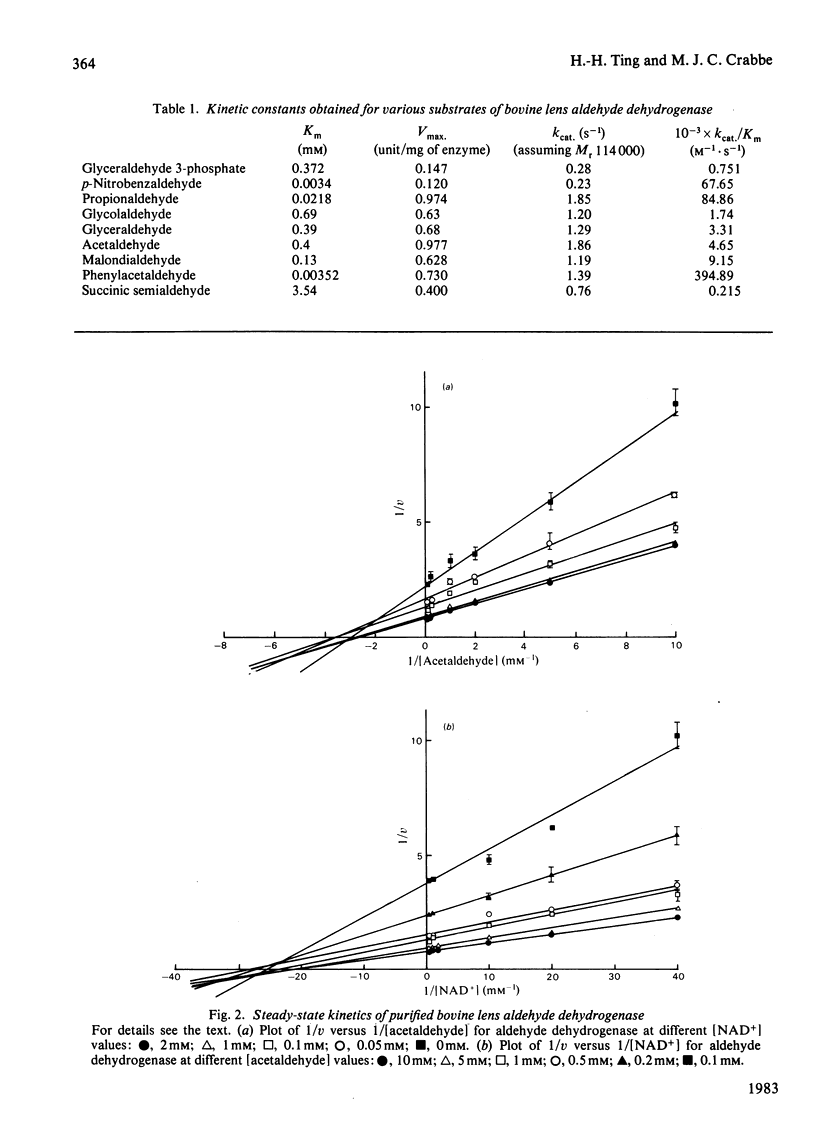

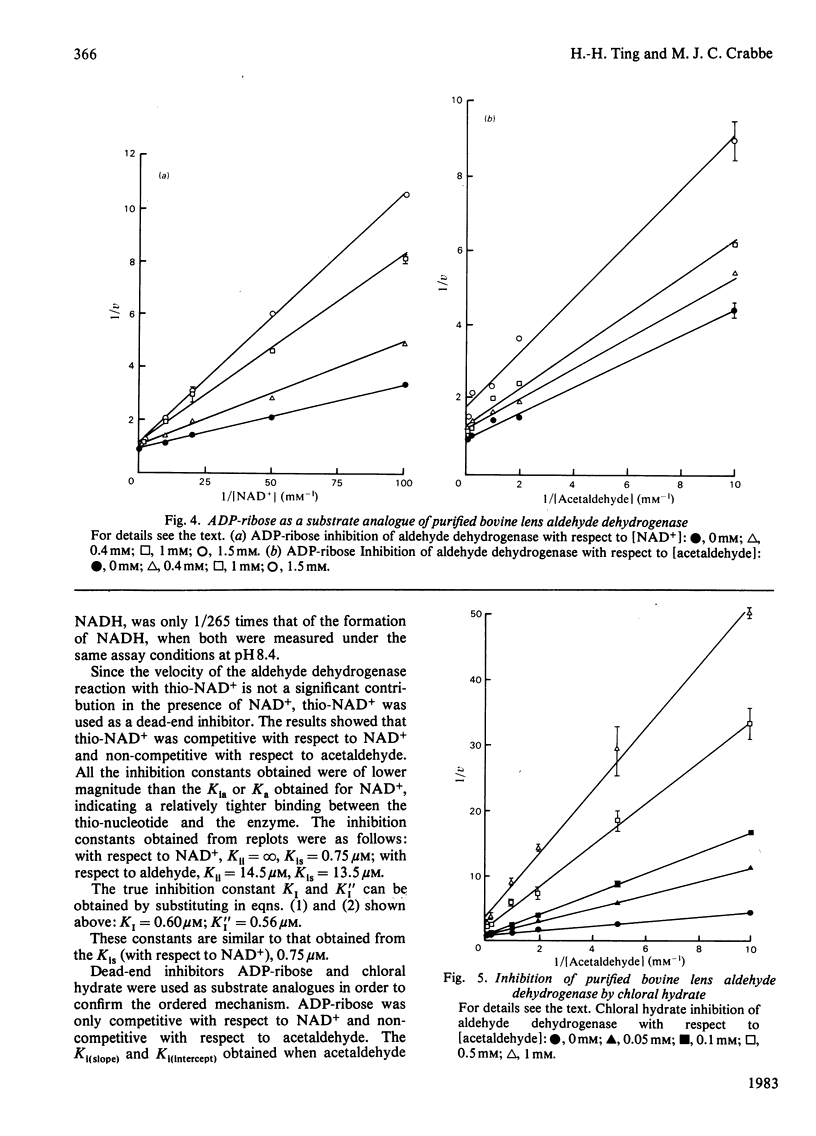
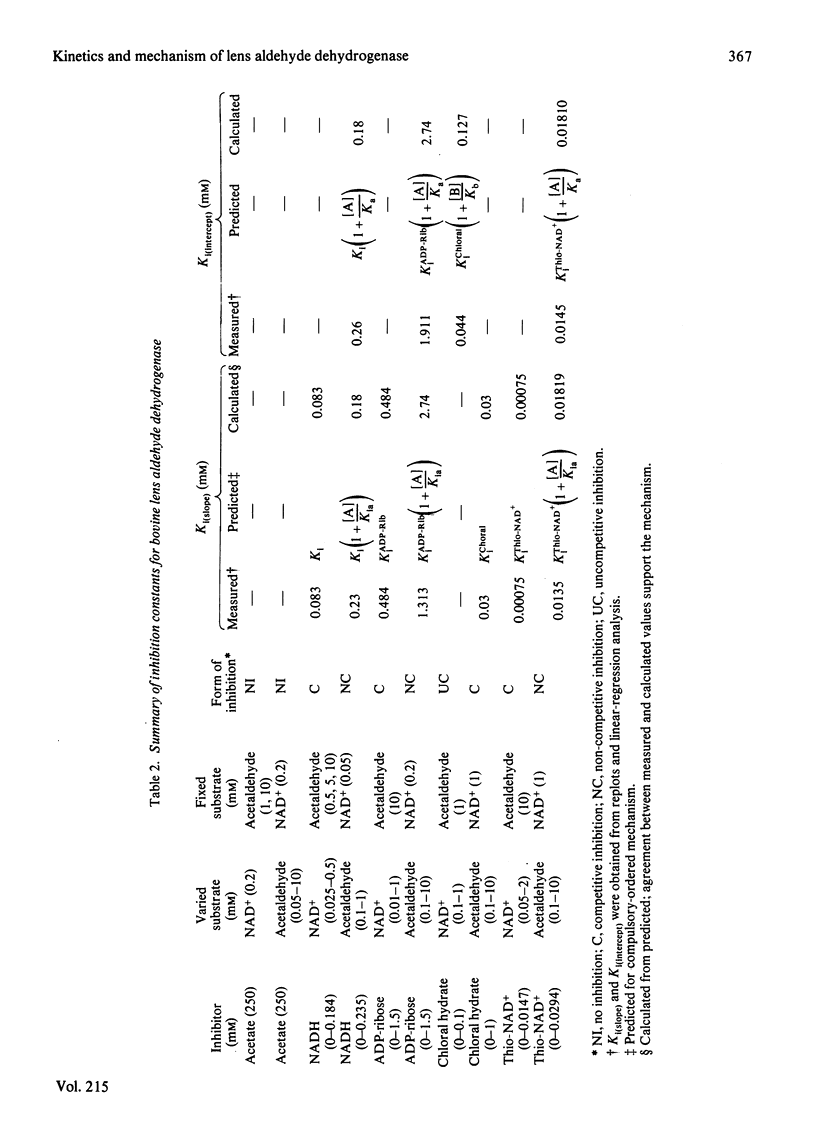

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbury S. L., Jakoby W. B. Ordered binding of substrates to yeast aldehyde dehydrogenase. J Biol Chem. 1971 Mar 25;246(6):1834–1840. [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Clark J. F., Jakoby W. B. Yeast aldehyde dehydrogenase. 3. Preparation of three homogeneous species. J Biol Chem. 1970 Nov 25;245(22):6065–6071. [PubMed] [Google Scholar]
- Crabbe M. J. An enzyme kinetics program for desk-top computers. Comput Biol Med. 1982;12(4):263–283. doi: 10.1016/0010-4825(82)90031-2. [DOI] [PubMed] [Google Scholar]
- Crabbe M. J., Childs R. E., Bardsley W. G. Time-dependent inhibition of diamine oxidase by carbonyl-group reagents and urea. Eur J Biochem. 1975 Dec 15;60(2):325–333. doi: 10.1111/j.1432-1033.1975.tb21007.x. [DOI] [PubMed] [Google Scholar]
- Crabbe M. J., Peckar C. O., Halder A. B., Cheng H. Erythrocyte glyceraldehyde-reductase levels in diabetics with retinopathy and cataract. Lancet. 1980 Dec 13;2(8207):1268–1270. doi: 10.1016/s0140-6736(80)92336-3. [DOI] [PubMed] [Google Scholar]
- Dickinson F. M., Hart G. J., Kitson T. M. The use of pH-gradient ion-exchange chromatography to separate sheep liver cytoplasmic aldehyde dehydrogenase from mitochondrial enzyme contamination, and observations on the interaction between the pure cytoplasmic enzyme and disulfiram. Biochem J. 1981 Dec 1;199(3):573–579. doi: 10.1042/bj1990573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckfeldt J. H., Yonetani T. Subcellular localization of the F1 and F2 isozymes of horse liver aldehyde dehydrogenase. Arch Biochem Biophys. 1976 Aug;175(2):717–722. doi: 10.1016/0003-9861(76)90564-6. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian R., Duncan S. The action of progesterone and diethylstilboestrol on the dehydrogenase and esterase activities of a purified aldehyde dehydrogenase from rabbit liver. Biochem J. 1977 Jan 1;161(1):123–130. doi: 10.1042/bj1610123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEZDY F. J., BENDER M. L. The kinetics of the alpha-chymotrypsin-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry. 1962 Nov;1:1097–1106. doi: 10.1021/bi00912a021. [DOI] [PubMed] [Google Scholar]
- Kitson T. M. Studies on the interaction between disulfiram and sheep liver cytoplasmic aldehyde dehydrogenase. Biochem J. 1978 Oct 1;175(1):83–90. doi: 10.1042/bj1750083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGibbon A. K., Blackwell L. F., Buckley P. D. Kinetics of sheep-liver cytoplasmic aldehyde dehydrogenase. Eur J Biochem. 1977 Jul 1;77(1):93–100. doi: 10.1111/j.1432-1033.1977.tb11645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu R. S., Blair A. H. Human liver akdehyde dehydrogenase. Kinetics of aldehyde oxidation. J Biol Chem. 1975 Oct 10;250(19):7899–7904. [PubMed] [Google Scholar]
- Ting H. H., Crabbe M. J. Bovine lens aldehyde dehydrogenase. Purification and preliminary characterization. Biochem J. 1983 Nov 1;215(2):351–359. doi: 10.1042/bj2150351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallari R. C., Pietruszko R. Kinetic mechanism of the human cytoplasmic aldehyde dehydrogenase E1. Arch Biochem Biophys. 1981 Nov;212(1):9–19. doi: 10.1016/0003-9861(81)90338-6. [DOI] [PubMed] [Google Scholar]
- WALEY S. G. Acidic peptides of the lens. Biochem J. 1956 Dec;64(4):715–726. doi: 10.1042/bj0640715. [DOI] [PMC free article] [PubMed] [Google Scholar]


