Abstract
Objectives
In a primary analysis of data from the BRIGHT study (UMIN000035712), photodynamic diagnosis‐assisted transurethral resection of bladder tumor (PDD‐TURBT) using oral 5‐aminolevulinic acid hydrochloride reduced residual tumors in high‐risk non‐muscle invasive bladder cancer (NMIBC). We aimed to evaluate the effectiveness of PDD‐TURBT for intravesical recurrence after a second transurethral resection for high‐risk NMIBC.
Methods
High‐risk NMIBC patients initially treated with PDD‐TURBT (PDD group) were prospectively registered between 2018 and 2020. High‐risk patients with NMIBC who were initially treated with white‐light TURBT (WL group) were retrospectively registered. Intravesical recurrence‐free survival after the second transurethral resection was compared between the PDD and WL groups using propensity score matching analysis.
Results
In total, 177 patients were enrolled in the PDD group, and 306 patients were registered in the WL group. After propensity score matching (146 cases in each group), intravesical recurrence within 1 year was significantly less frequent in the PDD group than in the WL group (p = 0.004; hazard ratio [HR] 0.44, 95% confidence interval [CI]: 0.25–0.77). In subgroup analysis, PDD‐TURBT showed a particularly high efficacy in reducing intravesical recurrence within 1 year, especially in cases of tumors measuring less than 3 cm (p = 0.003; HR 0.31, 95% CI: 0.14–0.67), absence of residual tumor at second transurethral resection (p = 0.020; HR 0.37, 95% CI: 0.16–0.86), and no postoperative intravesical Bacillus Calmette‐Guérin therapy (p < 0.001; HR 0.27, 95% CI: 0.13–0.58).
Conclusions
PDD‐TURBT may reduce short‐term intravesical recurrence in patients with high‐risk NMIBC.
Keywords: 5‐aminolevulinic acid, high‐risk non‐muscle invasive bladder cancer, intravesical recurrence, photodynamic diagnosis, propensity score matching
Abbreviations & Acronyms
- 5‐ALA
5‐aminolevulinic acid hydrochloride
- BCG
Bacillus Calmette‐Guérin
- CI
confidence interval
- CIS
cancer in situ
- HR
hazard ratio
- IQR
interquartile range
- NA
not applicable
- NMIBC
non‐muscle invasive bladder cancer
- PDD
photodynamic diagnosis
- RFS
recurrence‐free survival
- TURBT
transurethral resection of bladder tumor
- UTUC
upper urinary tract urothelial carcinoma
- WL
white light
INTRODUCTION
The standard treatment for non‐muscle invasive bladder cancer (NMIBC) is transurethral resection of bladder tumor (TURBT). However, patients with high‐risk NMIBC, including pT1, high‐grade urothelial carcinoma, and concurrent carcinoma in situ (CIS), exhibit residual tumor detection rates ranging from 33% to 78%, 1 , 2 prompting a strong recommendation for a second transurethral resection (TUR) 3–8 weeks following the initial TURBT. 3 , 4 , 5
Photodynamic diagnosis (PDD) through administration of 5‐aminolevulinic acid hydrochloride (5‐ALA) or hexylaminolevulinate exploits the biological properties of protoporphyrin IX, a fluorescent substance that accumulates in cancer cells. 6 In NMIBC, the use of a fluorescent cystoscope with PDD allows for the observation of cancer lesions through fluorescence emission, garnering attention as a diagnostic method. 7 Moreover, TURBT with PDD (PDD‐TURBT) has been shown to increase the tumor detection rate and reduce the risk of disease recurrence compared to conventional white light TURBT (WL‐TURBT). 8 , 9 , 10 However, conflicting reports from several randomized controlled trials 11 , 12 , 13 have meant that PDD‐TURBT is not strongly recommended, or is recommended only for specific cohorts in major clinical guidelines. 14 , 15 In a study utilizing orally administered 5‐ALA, Nakai et al. reported a significantly higher cancer detection sensitivity in PDD‐TURBT compared with WL‐TURBT in a phase 3 multicenter prospective trial (79.6% vs. 54.1%). 16 Since 2017, oral administration of 5‐ALA has been covered by insurance in Japan as a PDD during TURBT for NMIBC.
While several studies have examined the utility of PDD‐TURBT for NMIBC, research specifically focusing on high‐risk NMIBC, for which a second TUR is performed, is scarce. In a previous multicenter prospective study (BRIGHT study; UMIN ID: UMIN000035712), we compared the residual tumor rates after the second TUR following PDD‐TURBT and WL‐TURBT in cases of high‐risk NMIBC, revealing that PDD‐TURBT significantly reduced the tumor residual rate compared to WL‐TURBT. 17 However, as the second TUR was also performed after WL‐TURBT in high‐risk NMIBC cases, the contribution of PDD‐TURBT to reducing intravesical recurrence remains unclear. In the second analysis of the BRIGHT study, we aimed to evaluate the utility of PDD‐TURBT in high‐risk NMIBC cases by comparing the intravesical recurrence‐free survival (RFS) between both groups.
METHODS
Study design
The design details of the BRIGHT study have been previously published. 17 In this investigation, we conducted a single‐arm, multicenter, prospective study using propensity score‐matching analysis with a retrospective historical control.
Patients who underwent PDD‐TURBT with the oral administration of 5‐ALA between December 2018 and December 2020 were diagnosed with high‐risk NMIBC (high‐grade urothelial carcinoma, pT1, or concurrent CIS), and subsequently underwent a second TUR within 2 months of the initial TURBT, were prospectively enrolled in the PDD group. Patients with incomplete resection or extensive CIS during PDD‐TURBT were excluded. Patients were orally administered 5‐ALA (20 mg/kg) dissolved in distilled water 3 h before insertion of the cystoscope (range: 2–4 h). The primary equipment used in this study was the D‐LIGHT system (KARL STORZ). A dedicated telescope equipped with a charge‐coupled device camera was employed for image capture, and observations were conducted on a monitor screen. In some institutions, the light source utilized was the Aladuck LS‐DLED (SBI Pharma, Japan), with a dedicated cut filter on a standard telescope, and the image monitor was the VISERA ELITE or VISERA ELITE II (Olympus, Japan).
In contrast, patients treated with WL‐TURBT between January 2006 and November 2016 who were diagnosed with high‐risk NMIBC and underwent a second TUR within 2 months after the initial TURBT were retrospectively included as historical controls (WL group).
The sample size of this study was set with a prospective PDD group of 200 cases and a historical data‐based WL group of 300 cases to evaluate the difference in residual tumor rate at the second TUR between the PDD and WL group. 17 Recruitment for the historical cohort was solicited from participating facilities, and registration was closed after exceeding 300 cases.
Endpoints
Follow‐up after the second TUR was scheduled every 3 months for up to 2 years by cystoscopy and urine cytology. The endpoint for this analysis was intravesical RFS assessed at 1 year and throughout the entire observation period, following the second TUR.
Data analysis
The data were locked in March 2023 and analyzed. Propensity score matching was used to compare the intravesical RFS between the PDD and WL groups. Propensity scores were calculated using the background factors of age, sex, tumor diameter, history of NMIBC, number of tumors, concurrent CIS, pathological stage, tumor grade, and postoperative intravesical Bacillus Calmette‐Guérin (BCG) therapy. Pair matching was performed by setting the caliper to 0.2, and background factors and intravesical RFS were compared between the post‐matching groups using Fisher's exact test and the log‐rank test, respectively. The hazard ratios (HRs) of perioperative factors for intravesical recurrence were analyzed using Cox's proportional hazard analysis. The free software, EZR ver. 1.54, was used for the statistical analysis. The significance level of the tests was 5% on both sides, and the confidence interval (CI) estimation was 0.95.
RESULTS
Patient characteristics
As previously reported, 17 a total of 188 patients were enrolled in the PDD group, of which 177 were included in the analysis after exclusion of 11 ineligible cases. In the WL group, 313 patients were registered, and after excluding seven ineligible patients, 306 patients were included in the analysis. The patient backgrounds in each group were consistent with the findings of a previous study. 17 Following propensity score matching (146 cases in each group), no significant differences in clinical background factors were observed between the two groups (Table 1). The residual tumor rates at the second TUR were significantly lower in the PDD group than in the WL group (28% vs. 42%, p = 0.019).
TABLE 1.
Clinicopathological characteristics of photodynamic diagnosis (PDD) and white light (WL) groups after propensity score matching.
| Parameter | PDD group | WL group | p Value |
|---|---|---|---|
| n (%) | n (%) | ||
| All patients | 146 | 146 | |
| Age a , years; median [interquartile range] | 72 [66–78] | 73 [65–79] | 0.73 |
| Sex a | |||
| Male | 125 (85.6) | 129 (88.4) | 0.60 |
| Female | 21 (14.4) | 17 (11.6) | |
| Previous non‐muscle invasive bladder cancer a | |||
| Primary | 130 (89.0) | 128 (87.7) | 0.86 |
| Recurrent | 16 (11.0) | 18 (12.3) | |
| Tumor size a | |||
| <3 cm | 107 (73.3) | 114 (78.1) | 0.41 |
| ≥3 cm | 39 (26.7) | 32 (21.9) | |
| Number of tumors a | |||
| Single | 50 (34.2) | 51 (34.9) | 1.00 |
| Multiple | 96 (65.8) | 95 (65.1) | |
| History of upper urinary tract urothelial carcinoma | |||
| No | 137 (93.8) | 139 (95.2) | 0.80 |
| Yes | 9 (6.2) | 7 (4.8) | |
| Concurrent cancer in situ a | |||
| No | 116 (79.5) | 116 (79.5) | 1.00 |
| Yes | 30 (20.5) | 30 (20.5) | |
| Pathological stage a | |||
| pTa | 31 (21.2) | 29 (19.9) | 0.91 |
| pTis | 1 (0.7) | 2 (1.4) | |
| pT1 | 114 (78.1) | 115 (78.8) | |
| Tumor grade a | |||
| Low | 3 (2.1) | 3 (2.1) | 1.00 |
| High | 143 (97.9) | 143 (97.9) | |
| Residual tumor at second transurethral resection | |||
| Absence | 105 (71.9) | 85 (58.2) | 0.019* |
| Presence | 41 (28.1) | 61 (41.8) | |
| Postoperative intravesical chemotherapy | |||
| No | 125 (85.6) | 116 (79.5) | 0.22 |
| Yes | 21 (14.4) | 30 (20.5) | |
| Postoperative intravesical Bacillus Calmette‐Guérin therapy a | |||
| No | 81 (55.5) | 78 (53.4) | 0.81 |
| Yes | 65 (44.5) | 68 (46.6) | |
Note: Each factor was compared between the PDD and WL group using Fisher's exact test.
Background factors used for propensity score matching.
Statistically significant.
Intravesical RFS
The median follow‐up periods for the PDD and WL groups were 720 days (interquartile range [IQR], 394–750 days) and 730 days (IQR, 341–730 days), respectively. During follow‐up, intravesical recurrence was observed in 35 and 48 patients in the PDD and WL groups, respectively. There was no significant difference in intravesical RFS between the two groups throughout the entire follow‐up period (p = 0.069; HR 0.66, 95% CI: 0.42–1.04; Figure 1a). However, the PDD group demonstrated a significantly favorable intravesical RFS within 1 year (p = 0.004; HR 0.44, 95% CI: 0.25–0.77; Figures 1b and 2). The 1‐year intravesical recurrence‐free rates were 87% (95% CI: 81–92%) and 74% (95% CI: 66–80%) in the PDD and WL groups, respectively.
FIGURE 1.
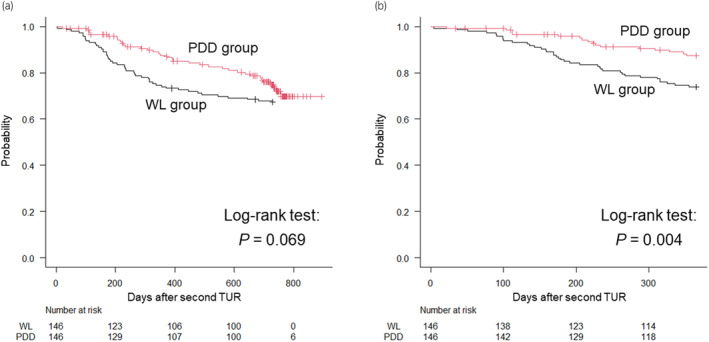
Comparison of the intravesical recurrence‐free survival after second transurethral resection (TUR) between the photodynamic diagnosis (PDD) (n = 146) and white light (WL) (n = 146) groups during the entire observation period (a) and within 1 year (b). Black line = WL group, red line = PDD group.
FIGURE 2.
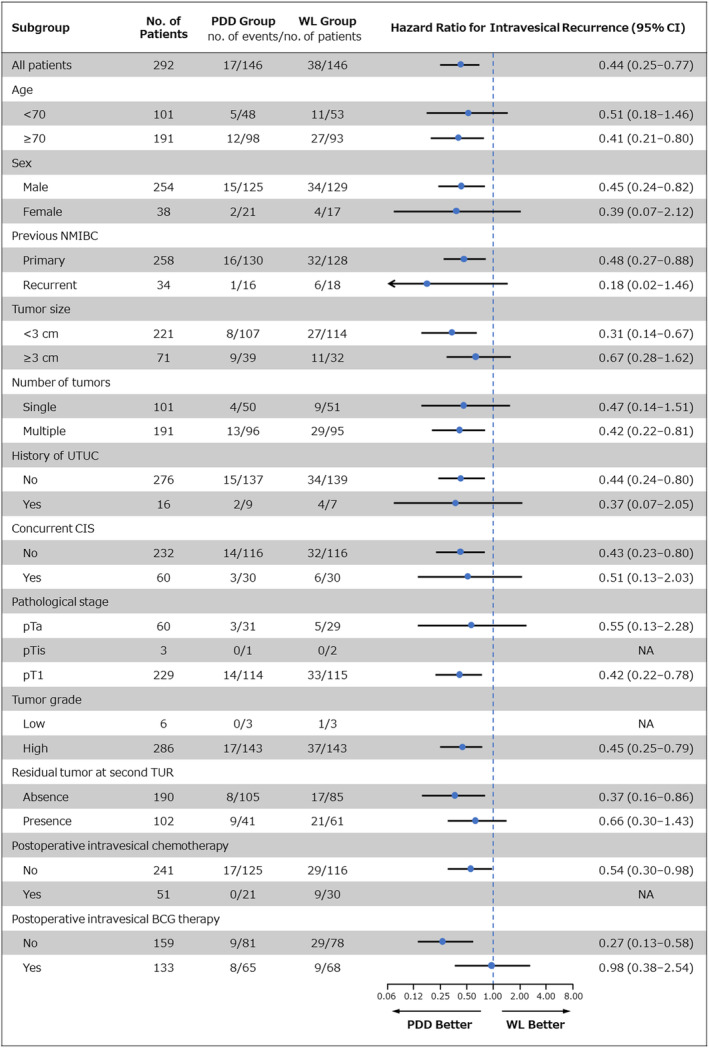
One‐year intravesical recurrence according to subgroups. The hazard ratio for intravesical recurrence was analyzed by Cox's proportional hazard analysis. An arrow indicates that the limits of the confidence interval (CI) are not shown. BCG, Bacillus Calmette‐Guérin; CIS, cancer in situ; NA, not applicable; NMIBC, non‐muscle invasive bladder cancer; PDD, photodynamic diagnosis; TUR, transurethral resection; UTUC, upper urinary tract urothelial carcinoma; WL, white light.
As shown in Figure 2, the perioperative patient and tumor factors, particularly benefiting from PDD‐TURBT in terms of 1‐year intravesical RFS were as follows: tumors measuring less than 3 cm (p = 0.003; HR 0.31, 95% CI: 0.14–0.67), absence of residual tumor at second TUR (p = 0.020; HR 0.37, 95% CI: 0.16–0.86), and no postoperative intravesical BCG therapy (p < 0.001; HR 0.27, 95% CI: 0.13–0.58).
DISCUSSION
This study revealed that PDD‐TURBT with the oral administration of 5‐ALA demonstrated a significantly superior 1‐year intravesical RFS compared to WL‐TURBT in high‐risk NMIBC. In contrast to several randomized controlled trials utilizing the intravesical administration of 5‐ALA where PDD‐TURBT did not show a favorable intravesical RFS compared to WL‐TURBT, 11 , 12 several retrospective studies utilizing the oral administration of 5‐ALA have shown favorable results for PDD‐TURBT compared to WL‐TURBT. 18 , 19 , 20 , 21 Kobayashi et al. and Matsushita et al. compared the PDD group and WL group using propensity score matching, demonstrating a significantly better intravesical RFS in the PDD group than in the WL group. 20 , 21 Interestingly, Kobayashi et al. did not observe any significant difference in intravesical RFS between the two groups over the entire observation period (median for PDD group: 698 days, WL group: 1331 days), but did identify a significant difference within the first 500 days. 20 In our study, similar to Kobayashi et al., no significant difference was observed in intravesical RFS between the two groups over the entire observation period (median for PDD group: 720 days, WL group: 730 days), but a significant difference was noted in the 1‐year intravesical RFS. The most significant clinical benefit of PDD‐TURBT in NMIBC is its ability to reduce the likelihood of overlooking tumors under WL, making it logical to consider that PDD‐TURBT contributes to improvements in short‐term intravesical RFS.
In the present study, we identified three factors that contribute to a favorable 1‐year intravesical RFS in PDD‐TURBT: tumor size less than 3 cm (HR 0.31, 95% CI: 0.14–0.67), absence of residual tumor at second TUR (HR 0.37, 95% CI: 0.16–0.86), and no postoperative intravesical BCG therapy (HR 0.27, 95% CI: 0.13–0.58). Kobayashi et al. reported two factors contributing to a favorable 500‐day intravesical RFS: age less than 75 years and primary occurrence. 20 Matsushita et al. identified tumor size greater than 3 cm as a factor contributing to poor intravesical RFS. 21 Among the three factors elucidated in our study, the absence of residual tumor at second TUR, and no postoperative intravesical BCG therapy are considered to be new candidates that contributes to a favorable intravesical RFS in PDD‐TURBT, whereas tumor size less than 3 cm was already a candidate factor in Matsushita's studies.
Since the primary effect of PDD is to reduce residual tumor, it makes sense that subgroups with no residual tumor would have a greater intravesical RFS advantage in the PDD group. Rather, it should be noted that even in the subgroup with residual tumor, the PDD group showed better intravesical RFS than the WL group (HR 0.66, 95% CI: 0.30–1.43), although this difference was not significant. One reason why the PDD at the initial TURBT affected intravesical RFS after second TUR even in the presence of residual tumor may be that the second TUR is resected mainly around the scar of the main tumor, which may have caused further residual tumor in other lesions.
It was also highlighted that postoperative intravesical BCG therapy is a factor strongly associated with intravesical RFS. In the subgroup that did not receive intravesical BCG therapy, the PDD group had significantly better intravesical RFS than the WL group, whereas in the subgroup that received intravesical BCG therapy, there was little difference in intravesical RFS between the two groups (HR 0.98, 95% CI: 0.38–2.54). This suggests that intravesical BCG therapy has the ability to overcome the disadvantage of WL‐TURBT in intravesical RFS.
This study is novel in that it specifically targets a subset of high‐risk NMIBC patients who require a second TUR. In patients undergoing a second TUR, regardless of the presence of PDD during the initial TURBT, it is anticipated that residual tumors will be reduced by the second TUR, making it challenging to observe a significant difference in intravesical RFS between the PDD and WL groups. Despite this expectation, the PDD group demonstrated a superior 1‐year intravesical RFS, and it could be attributed to several reasons. First, as noted earlier, during the second TUR, the main tumors are primarily excised around the scar, and additional excision is often not performed for other tumors. Second, PDD‐TURBT excels at detecting concurrent CIS and is considered reasonably effective for the resection of CIS lesions. However, this efficacy is challenging with WL‐TURBT, making it more likely that differences will emerge even after a second TUR.
Additionally, one possible reason for the loss of superiority of intravesical RFS in the PDD group in the entire period may be the administration of intravesical BCG therapy in a significant number of patients with high‐risk NMIBC. Intravesical BCG therapy is thought to suppress the emergence of new tumors beyond the second year which are not tumor‐disseminated through the acquisition of innate or acquired immunity.
This study had several limitations. First, it is important to note that this research was not a randomized controlled trial. In the PDD group, patients were prospectively enrolled, whereas in the WL group, historical cases were retrospectively registered, leading to differences in the historical background at the time of TURBT. To mitigate this discrepancy, propensity score matching analysis was employed to approximate patient characteristics between the two groups and compare intravesical RFS. Second, not all cases from all facilities were included in the historical cohort. The registration of the historical cohort was closed after exceeding the goal of 300 cases. Selection bias may have occurred in this regard.
In conclusion, PDD‐TURBT with oral administration of 5‐ALA demonstrated significantly superior 1‐year intravesical RFS compared to WL‐TURBT in high‐risk NMIBC. Additionally, tumor size less than 3 cm, absence of residual tumor at second TUR, and no postoperative intravesical BCG therapy emerged as significant contributors to favorable 1‐year intravesical RFS in the PDD group. These findings suggest potential considerations for clinical decision‐making when opting for PDD‐TURBT with oral administration of 5‐ALA for the management of high‐risk NMIBC.
AUTHOR CONTRIBUTIONS
Taketo Kawai: Data curation; writing—original draft; writing—review and editing. Hideyasu Matsuyama: Conceptualization; project administration; writing—review and editing. Keita Kobayashi: Data curation. Atsushi Ikeda: Data curation. Makito Miyake: Data curation. Koshiro Nishimoto: Data curation. Yuto Matsushita: Dada curation. Hiroyuki Nishiyama: Supervision. Kiyohide Fujimoto: Supervision. Masafumi Oyama: Supervision. Hideaki Miyake: Supervision. Haruhito Azuma: Supervision. Keiji Inoue: Supervision. Takahiko Mitsui: Supervision. Mutsushi Kawakita: Supervision. Chikara Oyama: Supervision. Atsushi Mizokami: Supervision. Takashige Abe: Supervision. Hajime Kuroiwa: Formal analysis; methodology. Haruki Kume: Writing—review and editing; supervision.
CONFLICT OF INTEREST STATEMENT
Hideyasu Matsuyama reports personal fees from SBI Pharmaceuticals Co., Ltd. and Chugai Pharmaceuticals Co. Hiroyuki Nishiyama reports personal fees from Chugai Pharmaceuticals Co. The funding for this study was provided by Chugai Pharmaceutical Co., Ltd. and SBI Pharmaceuticals Co., Ltd. The funding source had no role in the design, practice, or analysis of this study.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
This study was approved by Ethics Committee of Yamaguchi University (H30‐122).
INFORMED CONSENT
Written informed consent was obtained from all participants.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
Registration number of UMIN is 000035712.
ANIMAL STUDIES
Not applicable.
ACKNOWLEDGMENTS
The following investigators also participated in this study, but are not listed as co‐authors: Hideo Fukuhara (Department of Urology, Kochi Medical School, Nankoku, Japan), Kazumasa Komura (Department of Urology, Osaka Medical and Pharmaceutical University Faculty of Medicine, Takatsuki, Japan), Takanori Mochizuki (Department of Urology, University of Yamanashi Graduate School of Medical Sciences, Chuo, Japan), Takahiro Yoneyama (Department of Urology, Hirosaki University Graduate School of Medicine, Hirosaki, Japan), Takahiro Nohara (Department of Integrative Cancer Therapy and Urology, Kanazawa University Graduate School of Medical Science, Kanazawa, Japan), Hidetoshi Kokubun (Department of Urology, Kobe City Medical Center General Hospital, Kobe, Japan), Seiji Yano (Department of Urology, Graduate School of Medicine, Yamaguchi University, Ube, Japan), Yoichi M. Ito (Data Science Center, Promotion Unit, Institute of Health Science Innovation for Medical Care, Hokkaido University Hospital, Sapporo, Japan). This work was supported by the Center for Clinical Research, Yamaguchi University Hospital. We would like to thank Editage (www.editage.com) for English language editing.
REFERENCES
- 1. Miladi M, Peyromaure M, Zerbib M, Saïghi D, Debré B. The value of a second transurethral resection in evaluating patients with bladder tumours. Eur Urol. 2003;43:241–245. [DOI] [PubMed] [Google Scholar]
- 2. Cumberbatch MGK, Foerster B, Catto JWF, Kamat AM, Kassouf W, Jubber I, et al. Repeat transurethral resection in non–muscle‐invasive bladder cancer: a systematic review. Eur Urol. 2018;73:925–933. [DOI] [PubMed] [Google Scholar]
- 3. Matsumoto H, Shiraishi K, Azuma H, Inoue K, Uemura H, Eto M, et al. Clinical practice guidelines for bladder cancer 2019 update by the Japanese Urological Association: summary of the revision. Int J Urol. 2020;27:702–709. [DOI] [PubMed] [Google Scholar]
- 4. Kunieda F, Kitamura H, Niwakawa M, Kuroiwa K, Shinohara N, Tobisu K, et al. Watchful waiting versus intravesical BCG therapy for high‐grade pT1 bladder cancer with pT0 histology after second transurethral resection: Japan clinical oncology group study JCOG1019. Jpn J Clin Oncol. 2012;42:1094–1098. [DOI] [PubMed] [Google Scholar]
- 5. Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and treatment of non‐muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196:1021–1029. [DOI] [PubMed] [Google Scholar]
- 6. Iinuma S, Farshi SS, Ortel B, Hasan T. A mechanistic study of cellular photodestruction with 5‐aminolaevulinic acid‐induced porphyrin. Br J Cancer. 1994;70:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inoue K, Matsuyama H, Fujimoto K, Hirao Y, Watanabe H, Ozono S, et al. The clinical trial on the safety and effectiveness of the photodynamic diagnosis of non‐muscle‐invasive bladder cancer using fluorescent light‐guided cystoscopy after oral administration of 5‐aminolevulinic acid (5‐ALA). Photodiagn Photodyn Ther. 2016;13:91–96. [DOI] [PubMed] [Google Scholar]
- 8. Rink M, Babjuk M, Catto JW, Jichlinski P, Shariat SF, Stenzl A, et al. Hexyl aminolevulinate‐guided fluorescence cystoscopy in the diagnosis and follow‐up of patients with non‐muscle‐invasive bladder cancer: a critical review of the current literature. Eur Urol. 2013;64:624–638. [DOI] [PubMed] [Google Scholar]
- 9. Veeratterapillay R, Gravestock P, Nambiar A, Gupta A, Aboumarzouk O, Rai B, et al. Time to turn on the blue lights: a systematic review and meta‐analysis of photodynamic diagnosis for bladder cancer. Eur Urol Open Sci. 2021;31:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maisch P, Koziarz A, Vajgrt J, Narayan V, Kim MH, Dahm P. Blue vs white light for transurethral resection of non‐muscle‐invasive bladder cancer: an abridged Cochrane review. BJU Int. 2022;130:730–740. [DOI] [PubMed] [Google Scholar]
- 11. Schumacher MC, Holmang S, Davidsson T, Friedrich B, Pedersen J, Wiklund NP. Transurethral resection of non‐muscle‐invasive bladder transitional cell cancers with or without 5‐aminolevulinic acid under visible and fluorescent light: results of a prospective, randomised, multicentre study. Eur Urol. 2010;57:293–299. [DOI] [PubMed] [Google Scholar]
- 12. Stenzl A, Penkoff H, Dajc‐Sommerer E, Zumbraegel A, Hoeltl L, Scholz M, et al. Detection and clinical outcome of urinary bladder cancer with 5‐aminolevulinic acid‐induced fluorescence cystoscopy: a multicenter randomized, double‐blind, placebo‐controlled trial. Cancer. 2011;117:938–947. [DOI] [PubMed] [Google Scholar]
- 13. O'Brien T, Ray E, Chatterton K, Khan MS, Chandra A, Thomas K. Prospective randomized trial of hexylaminolevulinate photodynamic‐assisted transurethral resection of bladder tumour (TURBT) plus single‐shot intravesical mitomycin C vs conventional white‐light TURBT plus mitomycin C in newly presenting non‐muscle‐invasive bladder cancer. BJU Int. 2013;112:1096–1104. [DOI] [PubMed] [Google Scholar]
- 14. Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al. European Association of Urology guidelines on non‐muscle‐invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol. 2022;81:75–94. [DOI] [PubMed] [Google Scholar]
- 15. Flaig TW, Spiess PE, Abern M, Agarwal N, Bangs R, Buyyounouski MK, et al. NCCN clinical practice guidelines in oncology (NCCN guidelines®) for bladder cancer Version 3.2024. National Comprehensive Cancer Network, Inc. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed 29 Apr 2024.
- 16. Nakai Y, Inoue K, Tsuzuki T, Shimamoto T, Shuin T, Nagao K, et al. Oral 5‐aminolevulinic acid‐mediated photodynamic diagnosis using fluorescence cystoscopy for non‐muscle‐invasive bladder cancer: a multicenter phase III study. Int J Urol. 2018;25:723–729. [DOI] [PubMed] [Google Scholar]
- 17. Kobayashi K, Matsuyama H, Kawai T, Ikeda A, Miyake M, Nishimoto K, et al. Bladder cancer prospective cohort study on high‐risk non‐muscle invasive bladder cancer after photodynamic diagnosis‐assisted transurethral resection of the bladder tumor (BRIGHT study). Int J Urol. 2022;29:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fukuhara H, Yamamoto S, Lai HW, Karashima T, Kurabayashi A, Furihata M, et al. Real‐world experience with 5‐aminolevulinic acid for photodynamic diagnosis of bladder cancer (2nd report): reduced bladder recurrence after PDD‐TURBT. Photodiagn Photodyn Ther. 2022;38:102757. [DOI] [PubMed] [Google Scholar]
- 19. Taoka R, Matsuoka Y, Yamasaki M, Kani N, Honda T, Harada S, et al. Photodynamic diagnosis‐assisted transurethral resection using oral 5‐aminolevulinic acid decreases residual cancer and improves recurrence‐free survival in patients with non‐muscle‐invasive bladder cancer. Photodiagn Photodyn Ther. 2022;38:102838. [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi K, Matsuyama H, Oka S, Nakamura K, Misumi T, Hiroyoshi T, et al. Risks and benefits of transurethral resection of the bladder tumor using photodynamic diagnosis with oral 5‐aminolevulinic acid hydrochloride according to age and history of recurrence in patients with non‐muscle invasive bladder cancer. Photodiagn Photodyn Ther. 2023;41:103294. [DOI] [PubMed] [Google Scholar]
- 21. Matsushita Y, Miyake M, Nishimura N, Nishimoto K, Fukuhara H, Kobayashi K, et al. Comparative assessment of disease recurrence after transurethral resection of non‐muscle‐invasive bladder cancer with and without a photodynamic diagnosis using 5‐aminolevulinic acid: a propensity score‐matching analysis. Int J Clin Oncol. 2024;29:205–212. [DOI] [PubMed] [Google Scholar]


