Abstract
Objectives
The JAVELIN Bladder 100 phase 3 trial showed that avelumab first‐line maintenance + best supportive care significantly prolonged overall survival and progression‐free survival versus best supportive care alone in patients with advanced urothelial carcinoma who were progression‐free following first‐line platinum‐based chemotherapy. We report findings from J‐AVENUE (NCT05431777), a real‐world study of avelumab first‐line maintenance therapy in Japan.
Methods
Medical charts of patients with advanced urothelial carcinoma without disease progression following first‐line platinum‐based chemotherapy, who received avelumab maintenance between February and November 2021, were reviewed. Patients were followed until June 2022. The primary endpoint was patient characteristics; secondary endpoints included time to treatment failure and progression‐free survival.
Results
In 79 patients analyzed, median age was 72 years (range, 44–86). Primary tumor site was upper tract in 45.6% and bladder in 54.4%. The most common first‐line chemotherapy regimen was cisplatin + gemcitabine (63.3%). Median number of chemotherapy cycles received was four. Best response to chemotherapy was complete response in 10.1%, partial response in 58.2%, and stable disease in 31.6%. Median treatment‐free interval before avelumab was 4.9 weeks. With avelumab first‐line maintenance therapy, the disease control rate was 58.2%, median time to treatment failure was 4.6 months (95% CI, 3.3–6.4), and median progression‐free survival was 6.1 months (95% CI, 3.6–9.7).
Conclusions
Findings from J‐AVENUE show the effectiveness of avelumab first‐line maintenance in patients with advanced urothelial carcinoma in Japan in clinical practice, with similar progression‐free survival to JAVELIN Bladder 100 and previous real‐world studies, supporting its use as a standard of care.
Keywords: avelumab, immunotherapy, real world clinical trials, treatment outcome, urothelial carcinoma
Abbreviations & Acronyms
- 1L
first‐line
- 2L
second‐line
- aUC
advanced urothelial carcinoma
- BSC
best supportive care
- CR
complete response
- DCR
disease control rate
- ECOG PS
Eastern Cooperative Oncology Group performance status
- HR
hazard ratio
- IQR
interquartile range
- irAE
immune‐related adverse event
- IRR
infusion‐related reaction
- ORR
objective response rate
- OS
overall survival
- PD
progressive disease
- PFS
progression‐free survival
- PR
partial response
- SD
stable disease
- TFI
treatment‐free interval
- TTF
time to treatment failure
- UC
urothelial carcinoma
INTRODUCTION
Bladder cancer is a common cancer associated with considerable morbidity and mortality. 1 The National Cancer Center Japan estimated that in 2022, 24 700 cases of bladder cancer would be diagnosed in Japan and 9900 deaths would be attributed to bladder cancer. 2 Urothelial carcinoma (UC) accounts for approximately 90% of cases of bladder cancer. 3 Patients diagnosed with unresectable locally advanced or metastatic UC (advanced UC; aUC) have a poor prognosis, with a 5‐year overall survival rate of approximately 8%. 4 The prevalence of prognostic factors varies between Asian and non‐Asian patients, including higher frequencies of upper urinary tract primary tumors, bone metastases, and renal dysfunction prior to first‐line (1L) treatment in Asian patients. 5 , 6 In Japan, the proportion of patients with aUC receiving 1L treatment who have upper or lower tract tumors is comparable (upper tract, 40%–45%; lower tract, 50%–55%; both, 5%–10%). 7 , 8 , 9
International treatment guidelines, including Japanese Urological Association guidelines, recommend 1L platinum‐based (cisplatin‐ or carboplatin‐based) chemotherapy for eligible patients with aUC, followed by avelumab 1L maintenance therapy in patients without disease progression. 3 , 10 , 11 , 12 , 13 Although most patients (approximately 80%) have a response or disease control with 1L platinum‐based chemotherapy, durations of progression‐free survival (PFS; median, 6.3–7.1 months) and overall survival (OS; median, 13.1–14.3 months) are limited. 14 , 15 In the JAVELIN Bladder 100 randomized phase 3 trial, 1L maintenance therapy with avelumab (anti–PD‐L1) + best supportive care (BSC) significantly prolonged OS compared with BSC alone in patients with aUC that had not progressed with 1L platinum‐based chemotherapy. 16 After ≥2 years of follow‐up, median OS was 23.8 months (95% CI, 19.9–28.8) in the avelumab + BSC arm versus 15.0 months (95% CI, 13.5–18.2) in the BSC alone arm (hazard ratio [HR], 0.76 [95% CI, 0.63–0.91]; 2‐sided p = 0.0036). PFS was also prolonged with avelumab + BSC versus BSC alone (median, 5.5 vs. 2.1 months; HR, 0.54 [95% CI, 0.46–0.64]; 2‐sided p < 0.0001). 17 Results led to the approval of avelumab 1L maintenance therapy in patients with aUC that has not progressed with platinum‐based chemotherapy in various countries, and provided level 1 evidence to support its incorporation into international treatment guidelines as a standard of care. 3 , 12 , 13 In subgroup analyses of JAVELIN Bladder 100 in patients enrolled in Asian countries (21.0% of patients) or Japan (10.4% of patients), safety and efficacy results were generally consistent with overall trial findings. 18 , 19 These analyses supported the regulatory approval of avelumab in Japan in February 2021 as maintenance therapy following chemotherapy for curatively unresectable UC.
Alongside clinical trials, real‐world studies are needed to confirm effectiveness and safety in day‐to‐day clinical practice. Real‐world data for avelumab 1L maintenance reported to date include studies performed in Europe and the United States. 20 , 21 , 22 Preliminary real‐world data have been reported from Japan in a small population (N = 27). 23 Additionally, an interim analysis of post‐marketing surveillance data for avelumab maintenance therapy (n = 361) showed that the safety profile (primary endpoint) was comparable to that observed in previous clinical trials. 24 To provide further real‐world data for avelumab 1L maintenance in Japan, we report findings from the J‐AVENUE study.
METHODS
Patients
J‐AVENUE (NCT05431777) was a multicenter, noninterventional, retrospective, medical chart review of patients with aUC in Japan who did not have disease progression following 1L platinum‐based chemotherapy, and who started avelumab 1L maintenance therapy between 24 February 2021 (date of regulatory approval in Japan) and 30 November 2021. Patients were followed until 30 June 2022. Study investigators completed one electronic case report form per patient using medical charts, and deidentified/pseudonymized data were transferred to a central database. This study was conducted in accordance with the Declaration of Helsinki and the “Ethical Guidelines for Life Science and Medical Research Involving Human Subjects” issued by the Ministry of Education, Culture, Sports, Science and Technology, the Ministry of Health, Labor and Welfare, and the Ministry of Economy, Trade and Industry in Japan. Informed consent was obtained from all patients, and patients or legal representatives of deceased patients were given the opportunity to opt out of data collection. The study protocol, amendments, and other relevant documents were prospectively approved by an institutional review board or independent ethics committee at each site.
Study outcomes
The primary endpoint was baseline patient characteristics, including data collected at initiation of 1L chemotherapy and avelumab 1L maintenance therapy. Secondary endpoints included: time to treatment failure (TTF) of avelumab (time from start of avelumab to the end of treatment for any cause, including death; if end of treatment was not recorded, TTF was based on the last observation date during the study period); PFS (time from start of avelumab to date of first progressive disease (PD) or death, whichever occurred first; if PD or death was not recorded, patients were censored at initiation of next‐line of therapy or last observation date); best overall response and objective response rate (ORR; defined as best overall response of complete response [CR] or partial response [PR]); safety, including corticosteroid therapy for immune‐related adverse events (irAEs), premedication or treatment for infusion‐related reactions (IRRs), and clinical laboratory test abnormalities; and subsequent therapy received after avelumab. PFS and response were determined by investigators with reference to RECIST 1.1. OS was not assessed because of the short observation period.
Statistical analysis
Continuous variables were summarized using descriptive statistics. Categorical variables were summarized by frequency counts and percentages. Analysis populations for effectiveness and safety included all patients who were enrolled, met eligibility criteria, and were treated with avelumab 1L maintenance therapy. TTF and PFS were estimated using the Kaplan–Meier method, including median values and 95% CIs; TTF and PFS rates with two‐sided 95% CIs were estimated using the Greenwood formula. Duration of follow‐up was calculated using the reverse Kaplan–Meier method (reversing censoring and event indicators). For ORR, 95% CIs were calculated using the Clopper‐Pearson method. An exploratory multivariate logistic regression analysis was performed using Cox regression, which employed a stepwise method for variable input. All analyses were conducted using SAS version 9.4.
RESULTS
Patient characteristics
The study population comprised 79 patients with aUC, who started avelumab 1L maintenance therapy at 15 sites in Japan between February and November 2021, and were observed until 30 June 2022 (data cutoff). All 79 patients were included in effectiveness and safety analyses. Table 1 summarizes baseline characteristics. 25 Median age at the start of avelumab was 72 years (range, 44–86). The location of primary tumor at diagnosis was bladder in 43 patients (54.4%) and upper urinary tract in 36 (45.6%). Most patients (n = 64 [81.0%]) had tumors with pure UC histology; other histologies were UC variant subtypes in 13 patients (16.5%), and pure squamous or small cell carcinoma in one patient (1.3%) each. Eastern Cooperative Oncology Group performance status (ECOG PS) at the start of 1L chemotherapy was 0 in 56 patients (70.9%), 1 in 19 (24.1%), and ≥2 in four (5.1%). The most common 1L chemotherapy regimen was cisplatin + gemcitabine (n = 50, 63.3%). The median duration of 1L chemotherapy was 15.7 weeks (interquartile range [IQR], 10.6–23.6) and the median number of cycles received was four (IQR, 4–6). Best overall response to 1L chemotherapy was CR in eight patients (10.1%), PR in 46 (58.2%), and stable disease (SD) in 25 (31.6%). The median treatment‐free interval (TFI) from end of chemotherapy to start of avelumab was 4.9 weeks (IQR, 3.1–8.0), and the TFI was <4 weeks in 25 patients (32.1%), 4–10 weeks in 39 (50.0%), and >10 weeks in 14 (17.9%). Median follow‐up for PFS from start of avelumab treatment was 12.0 months (95% CI, 10.8–12.8) and from start of 1L chemotherapy was 19.2 months (95% CI, 16.9–24.2).
TABLE 1.
Baseline patient characteristics.
| Characteristic | N = 79 |
|---|---|
| Age at start of avelumab treatment, median (range), years | 72 (44–86) |
| Sex, n (%) | |
| Male | 60 (75.9) |
| Female | 19 (24.1) |
| Primary tumor location, n (%) | |
| Bladder | 43 (54.4) |
| Upper tract (renal pelvis or ureter) | 36 (45.6) |
| Primary tumor histology, n (%) | |
| Pure urothelial carcinoma | 64 (81.0) |
| Urothelial carcinoma subtype | 13 (16.5) |
| Nested | 5 (6.3) |
| Micropapillary | 2 (2.5) |
| Poorly differentiated | 2 (2.5) |
| Sarcomatoid | 2 (2.5) |
| Plasmacytoid/signet ring cell | 1 (1.3) |
| Other | 1 (1.3) |
| Small cell carcinoma | 1 (1.3) |
| Squamous cell carcinoma | 1 (1.3) |
| ECOG PS at start of 1L chemotherapy, n (%) | |
| 0 | 56 (70.9) |
| 1 | 19 (24.1) |
| 2 | 3 (3.8) |
| 3 | 1 (1.3) |
| KPS at start of 1L chemotherapy, n (%) | |
| ≥80% | 73 (92.4) |
| <80% | 6 (7.6) |
| Presence of visceral metastasis at start of 1L chemotherapy, n (%) | |
| Yes | 24 (30.4) |
| No | 53 (67.1) |
| Not reported | 2 (2.5) |
| Metastasis sites at start of 1L chemotherapy, n (%) | |
| Lung | 19 (24.1) |
| Liver | 7 (8.9) |
| Bone | 11 (13.9) |
| Distant lymph nodes | 28 (35.4) |
| Other | 4 (5.1) |
| Bajorin risk a at start of 1L chemotherapy, n (%) | |
| 0 (no risk factors) | 49 (62.0) |
| 1 (one risk factor) | 26 (32.9) |
| 2 (two risk factors) | 2 (2.5) |
| Not reported | 2 (2.5) |
| 1L chemotherapy regimen, n (%) | |
| Cisplatin + gemcitabine | 50 (63.3) |
| Carboplatin + gemcitabine | 24 (30.4) |
| Dose‐dense MVAC | 2 (2.5) |
| Other | 3 (3.8) |
| Number of 1L chemotherapy cycles | |
| Median (IQR) | 4 (4–6) |
| ≤3 cycles, n (%) | 12 (15.2) |
| 4 to 6 cycles, n (%) | 58 (73.4) |
| ≥7 cycles, n (%) | 9 (11.4) |
| Duration of 1L chemotherapy, median (IQR), weeks | 15.7 (10.6–23.6) |
| Treatment‐free interval b | |
| Median (IQR), weeks | 4.9 (3.1–8.0) |
| <4 weeks, n (%) | 25 (32.1) |
| 4 to <6 weeks, n (%) | 22 (28.2) |
| 6 to <8 weeks, n (%) | 11 (14.1) |
| 8 to 10 weeks, n (%) | 6 (7.7) |
| >10 weeks, n (%) | 14 (17.9) |
| Best response to 1L chemotherapy, n (%) | |
| Complete response | 8 (10.1) |
| Partial response | 46 (58.2) |
| Stable disease | 25 (31.6) |
| Presence of medical history prior to avelumab treatment, n (%) | |
| Yes | 31 (39.2) |
| No | 47 (59.5) |
| Not reported | 1 (1.3) |
| Type of medical history prior to avelumab treatment, n (%) | |
| Cardiovascular disorder | 3 (3.8) |
| COPD | 1 (1.3) |
| Cerebrovascular disorder | 1 (1.3) |
| Diabetes mellitus | 1 (1.3) |
| Other | 27 (34.2) |
| Presence of concomitant disease at start of avelumab treatment, n (%) | |
| No | 30 (38.0) |
| Yes | 49 (62.0) |
| Type of concomitant disease at the start of avelumab treatment, n (%) | |
| Diabetes mellitus | 17 (21.5) |
| Cardiovascular disorder | 4 (5.1) |
| Autoimmune disease c | 2 (2.5) |
| COPD | 1 (1.3) |
| Interstitial pneumonia | 1 (1.3) |
| Other | 39 (49.4) |
| Concurrent use of steroids or immunosuppressants at the start of avelumab treatment, n (%) | |
| Yes d | 2 (2.5) |
| No | 77 (97.5) |
Abbreviations: 1L, first line; COPD, chronic obstructive pulmonary disease; ECOG PS, Eastern Cooperative Oncology Group performance status; IQR, interquartile range; KPS, Karnofsky performance status; MVAC, methotrexate, vinblastine, doxorubicin, and cisplatin.
Bajorin risk category is defined by two risk factors: KPS <80% and visceral metastasis. 25
Not reported in one patient.
Basedow's disease (Graves' disease), Hashimoto thyroiditis, and rheumatoid arthritis.
Hydrocortisone (n = 1) and methotrexate (n = 1).
At data cutoff, avelumab treatment was ongoing in 21 patients (26.6%) and had been interrupted based on investigator discretion in one (1.3%) and discontinued in 57 (72.2%) (Table 2). Reasons for discontinuation were PD in 43 (54.4%), AE in 10 (12.7%), PD and AE in one (1.3%), and planned surgery in three (3.4%).
TABLE 2.
Treatment characteristics and reasons for discontinuation.
| N = 79 | |
|---|---|
| Avelumab treatment ongoing at data cutoff, n (%) | 21 (26.6) |
| Avelumab interrupted based on investigator discretion, n (%) | 1 (1.3) |
| Avelumab discontinued, n (%) | 57 (72.2) |
| Progressive disease | 43 (54.4) |
| Adverse event | 10 (12.7) |
| Progressive disease and adverse event | 1 (1.3) |
| Planned surgery | 3 (3.4) |
| Received subsequent treatment after avelumab, n (%) | 37 (46.8) |
Effectiveness outcomes
Median TTF with avelumab 1L maintenance therapy was 4.6 months (95% CI, 3.3–6.4) and the rate of patients without treatment failure at 1 year was 26.3% (95% CI, 16.8–36.8; Figure 1). Median PFS was 6.1 months (95% CI, 3.6–9.7) and the 1‐year PFS rate was 34.9% (95% CI, 24.0–46.0; Figure 2). The ORR with avelumab 1L maintenance therapy was 25.3% (95% CI, 16.2–36.4), including CR in nine patients (11.4%) and PR in 11 patients (13.9%; Table 3); 26 patients (32.9%) had SD as best response, resulting in a disease control rate (DCR) of 58.2% (post hoc calculation). Table S1 summarizes outcomes by tumor histology.
FIGURE 1.
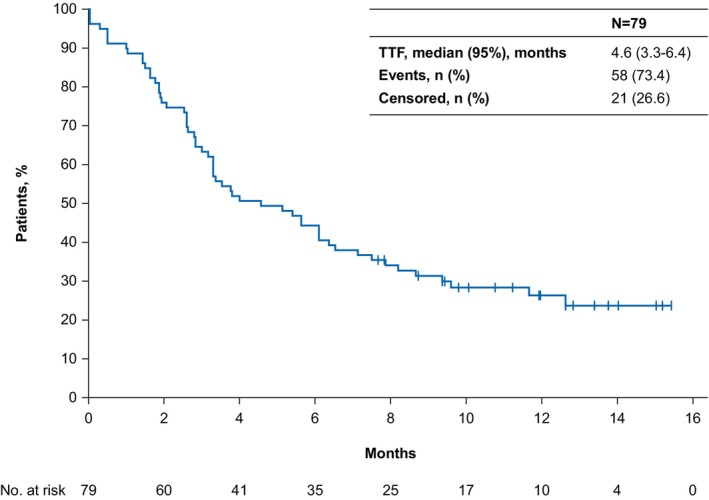
Time to treatment failure (TTF) from start of avelumab first‐line maintenance therapy.
FIGURE 2.

Progression‐free survival (PFS) from start of avelumab first‐line maintenance therapy.
TABLE 3.
Tumor responses with avelumab first‐line maintenance therapy.
| Parameter | N = 79 |
|---|---|
| Confirmed objective response (95% CI), % | 25.3 (16.2–36.4) |
| Confirmed best overall response, n (%) | |
| Complete response | 9 (11.4) |
| Partial response | 11 (13.9) |
| Stable disease | 26 (32.9) |
| Progressive disease | 28 (35.4) |
| Not evaluable a | 5 (6.3) |
| Disease control rate (95% CI), % b | 58.2 (46.6–69.2) |
Patients with no evidence of disease at baseline (start of avelumab treatment, after first‐line chemotherapy).
Best overall response of complete response, partial response, or stable disease.
Safety
Results from selected laboratory tests are shown in Figure 3, along with Japanese reference intervals (where available). 26 Mean levels of hemoglobin, white blood cells, and platelets decreased slightly during chemotherapy and recovered during avelumab treatment to levels similar to those observed at the start of chemotherapy. No major changes were observed in neutrophil‐lymphocyte ratio or C‐reactive protein levels.
FIGURE 3.

Changes in selected laboratory values at different time points compared with JCCLS Japanese reference intervals, 26 including hemoglobin, white blood cells, platelets, CRP, and NLR. 1L, first‐line; CRP, C‐reactive protein; CT, chemotherapy; JCCLS, Japanese Committee for Clinical Laboratory Science; NLR, neutrophil‐lymphocyte ratio.
Nine patients (11.4%) received corticosteroid treatment for an irAE, including high‐dose corticosteroids (starting dose of ≥40 mg of prednisone or equivalent) in five (6.3%). Median duration of corticosteroid treatment was 11.3 weeks (IQR, 3.6–24.7). Nearly all patients (n = 75, 94.9%) received premedication for potential IRRs, consistent with prescribing information. The most common premedication was acetaminophen (n = 68, 86.1%; Table 4). After starting avelumab, five patients (6.3%) received treatment for an IRR.
TABLE 4.
Premedication for potential infusion‐related reactions.
| N = 79 | |
|---|---|
| Premedication received, n (%) | 75 (94.9) |
| Acetaminophen | 68 (86.1) |
| Diphenhydramine hydrochloride | 38 (48.1) |
| Chlorpheniramine maleate | 35 (44.3) |
| Hydrocortisone sodium succinate | 3 (3.8) |
| No premedication received, n (%) | 4 (5.1) |
Subsequent anticancer therapy
After discontinuing avelumab 1L maintenance therapy, 37 patients (46.8% of all patients; 64.9% of patients who discontinued) subsequently received second‐line (2L) treatment, with decreasing proportions receiving later lines (Table 5). Among patients who received 2L treatment, enfortumab vedotin (13/37 [35.1%]) and platinum‐based regimens (20/37 [54.1%]) were commonly administered. Among 20 patients who discontinued avelumab without receiving subsequent treatment, reasons for discontinuation were PD (n = 10), AE (n = 8), surgery (n = 2), and other reason (n = 1), including one patient who discontinued for both PD and AE. Among the eight patients who had discontinued treatment because of an AE, four had received high‐dose corticosteroids.
TABLE 5.
Subsequent anticancer treatment after avelumab first‐line maintenance therapy. Percentages are calculated using the denominator of all patients included in the study (N = 79).
| Line of therapy | Second | Third | Fourth | Fifth |
|---|---|---|---|---|
| n/N (%) | 37/79 (46.8) | 18/79 (22.8) | 6/79 (7.6) | 2/79 (2.5) |
| Treatment, n (%) | ||||
| Enfortumab vedotin | 13 (16.5) | 6 (7.6) | 5 (6.3) | 1 (1.3) |
| Cisplatin + gemcitabine | 12 (15.2) | 0 | 0 | 0 |
| Carboplatin + gemcitabine | 6 (7.6) | 0 | 0 | 0 |
| Pembrolizumab | 3 (3.8) a | 8 (10.1) | 1 (1.3) | 1 (1.3) |
| Paclitaxel + gemcitabine | 1 (1.3) | 1 (1.3) | 0 | 0 |
| Dose‐dense MVAC | 1 (1.3) | 0 | 0 | 0 |
| Nedaplatin + gemcitabine | 1 (1.3) | 1 (1.3) | 0 | 0 |
| Erdafitinib | 0 | 1 (1.3) | 0 | 0 |
| Paclitaxel | 0 | 1 (1.3) | 0 | 0 |
Abbreviation: MVAC, methotrexate, vinblastine, doxorubicin, and cisplatin.
All three patients who received pembrolizumab as second‐line treatment had discontinued avelumab because of disease progression.
Exploratory univariate and multivariate analyses of PFS
In exploratory analyses, the only clinical factor associated with PFS (i.e., 95% CI for the HR not overlapping 1) in both univariate and multivariate analyses was Bajorin risk score. 25 In the multivariate analysis, compared with patients with 0 Bajorin risk factors (reference group), HRs (95% CI) for PFS in patients with 1 or 2 risk factors were 2.65 (1.24–5.65) and 30.47 (6.07–153.06), respectively.
DISCUSSION
The J‐AVENUE retrospective study is the largest real‐world study of the effectiveness of avelumab 1L maintenance in patients with aUC in Japan. Data reported here support the efficacy and safety reported in the JAVELIN Bladder 100 phase 3 trial. 16 , 17 Some patient characteristics in J‐AVENUE differed from those in the avelumab + BSC arm of JAVELIN Bladder 100, 16 including the higher proportion with upper urinary tract primary tumors in J‐AVENUE (45.6% vs. 30.3%), which is consistent with previous studies of aUC in Japan. 7 , 8 , 9 , 19 In addition, the J‐AVENUE population (vs. the avelumab + BSC arm of JAVELIN Bladder 100) had a slightly higher median age (72 vs. 68 years), higher proportion of patients with an ECOG PS of 0 (70.9% vs. 60.9%), lower proportion with visceral metastases (30.4% vs. 54.6%), and lower proportion who had received carboplatin + gemcitabine as 1L regimen (30.4% vs. 42.0%). 16 J‐AVENUE included some patients who would have been ineligible for JAVELIN Bladder 100. 16 , 17 In JAVELIN Bladder 100, all patients had a TFI between 1L chemotherapy and start of avelumab of 4–10 weeks, whereas in J‐AVENUE, the interval was <4 weeks in 32.1% and >10 weeks in 17.9%. Furthermore, in JAVELIN Bladder 100, all patients had received 4–6 cycles of 1L chemotherapy, whereas in J‐AVENUE, 15.2% had received ≤3 cycles and 11.4% had received ≥7 cycles.
In J‐AVENUE, median PFS from the start of avelumab 1L maintenance therapy was 6.1 months (95% CI, 3.6–9.7), similar to the median PFS of 5.5 months (95% CI, 4.2–7.2) observed in the overall avelumab + BSC arm of JAVELIN Bladder 100, 17 and 5.6 months (95% CI, 1.9–9.4) in the Japan subgroup. 19 In real‐world studies of avelumab 1L maintenance in France and Italy, median PFS was 5.7 months (95% CI, 5.3–7.0) and 8.1 months (95% CI, 6.1–10.4), respectively. 20 , 21 Median TTF with avelumab 1L maintenance (time until treatment discontinuation for any reason) was 4.6 months (95% CI, 3.3–6.4); this suggests that few patients discontinued for reasons other than disease progression, consistent with prescribing information, which states that avelumab should be administered until disease progression or unacceptable toxicity. Overall, these results indicate that use of avelumab maintenance treatment in a heterogeneous population, including patients who would have been ineligible for the JAVELIN Bladder 100 trial, did not affect outcomes in clinical practice.
The ORR in J‐AVENUE was 25.3% (95% CI, 16.2–6.4), which is comparable to the ORR of 14.3% (95% CI, 10.8–18.4) reported in JAVELIN Bladder 100. 17 Interpretation of ORR in a maintenance setting is challenging because response indicates tumor reduction compared with assessments after the end of chemotherapy, unlike in the 2L setting where response indicates reduction in tumor burden in patients with disease progression. However, the DCR indicates the proportion of patients who had a clinical benefit with maintenance therapy. In J‐AVENUE, the DCR was 58.2%, which was similar to the DCR in the avelumab + BSC arm of JAVELIN Bladder 100 (50.9%). 17 Thus, it can be inferred that assessments of disease control and tumor progression in clinical practice are performed to the same extent as in clinical trials.
Findings from the J‐AVENUE study suggest that avelumab 1L maintenance therapy has a manageable safety profile. Only 6.3% of patients received high‐dose corticosteroid treatment for an irAE, comparable to JAVELIN Bladder 100 (9.0%). 16 Nearly all patients received premedication to mitigate the risk of potential IRRs, consistent with prescribing information, and only 6.3% of patients required treatment for IRRs after starting avelumab. Furthermore, levels of blood laboratory values that had decreased during 1L chemotherapy showed evidence of recovery during avelumab treatment.
At last follow‐up, 26.6% of patients remained on avelumab treatment and 46.8% had transitioned to 2L therapy, similar to proportions reported in the initial analysis of JAVELIN Bladder 100 in the avelumab arm (24.3% and 42.3%, respectively). 16 Considering only patients who had discontinued avelumab in this study (n = 57), 64.9% received 2L treatment. Drug selection for 2L treatment depends on the patient's condition and drug availability. In the J‐AVENUE population, most patients who discontinued avelumab received either platinum‐based chemotherapy (54.1%) or enfortumab vedotin (35.1%), consistent with treatment guidelines. 3 , 12 , 13 Of eight patients who discontinued avelumab because of AEs and did not receive 2L treatment, four had received high‐dose corticosteroids following an irAE (out of a total of five patients who received high‐dose corticosteroids in the study population), suggesting that irAEs requiring high‐dose corticosteroids is a factor that may limit subsequent treatment.
This study has several limitations. As a noninterventional study focused on a single treatment (with no control arm), it did not gather data on alternative treatments. Because of its retrospective nature, only data included in medical records were available; thus, missing variables may have affected the accuracy of estimates. Bias may have been introduced from site selection and outcome reporting in a limited sample size; therefore, findings may not reflect clinical outcomes across all Japanese patients. Moreover, PFS and response were assessed by investigators and were not standardized across study sites, and the observation period was insufficient to assess OS. Multivariate analyses were limited by low patient numbers in subgroups. Further studies are needed to evaluate different treatment sequences including avelumab 1L maintenance therapy in aUC. Lastly, safety data were limited because post‐marketing surveillance was ongoing. 24
In conclusion, results from the J‐AVENUE study confirm the effectiveness of avelumab 1L maintenance therapy in patients with aUC that had not progressed with 1L platinum‐based chemotherapy in Japan. As a real‐world clinical study, the population was heterogeneous and included some patients with characteristics not included in the JAVELIN Bladder 100 phase 3 trial. However, outcomes were generally similar to those seen in JAVELIN Bladder 100 and other real‐world studies. Findings from J‐AVENUE further support the recommendation of avelumab 1L maintenance as standard of care for patients with aUC that has not progressed with 1L platinum‐based chemotherapy in Japan.
AUTHOR CONTRIBUTIONS
Eiji Kikuchi: supervision (lead); conceptualization (equal); investigation (equal); resources (equal); writing—review & editing (equal). Nozomi Hayakawa: investigation (equal); resources (equal); writing—review & editing (equal). Masashi Nakayama: investigation (equal); resources (equal); writing—review & editing (equal). Masahiro Uno: investigation (equal); resources (equal); writing—review & editing (equal). Hiroomi Nakatsu: investigation (equal); resources (equal); writing—review & editing (equal). Chiyoe Kitagawa: investigation (equal); resources (equal); writing—review & editing (equal). Hideaki Miyake: investigation (equal); resources (equal); writing—review & editing (equal). Takeshi Yamada: investigation (equal); resources (equal); writing—review & editing (equal). Kazutoshi Fujita: investigation (equal); resources (equal); writing—review & editing (equal). Hideaki Shimoyama: investigation (equal); resources (equal); writing—review & editing (equal). Kiyoaki Nishihara: investigation (equal); resources (equal); writing—review & editing (equal). Mizuki Kobayashi: investigation (equal); resources (equal); writing—review & editing (equal). Motonobu Nakamura: investigation (equal); resources (equal); writing—review & editing (equal). Kiyohide Fujimoto: investigation (equal); resources (equal); writing—review & editing (equal). Takeshi Sano: investigation (equal); resources (equal); writing—review & editing (equal). Naotaka Nishiyama: investigation (equal); resources (equal); writing—review & editing (equal). Takayuki Ito: project administration (equal); writing—review & editing (equal). Masahiro Kajita: project administration (equal); writing—review & editing (equal). Takashi Kobayashi: supervision (supporting); conceptualization (equal); investigation (equal); resources (equal); writing—review & editing (equal). Hiroshi Kitamura: supervision (supporting); conceptualization (equal); investigation (equal); resources (equal); writing—review & editing (equal).
FUNDING INFORMATION
This work was supported by Merck Biopharma Co., Ltd., Tokyo, Japan, an affiliate of Merck KGaA (CrossRef Funder ID: https://doi.org/10.13039/100009945) and was previously conducted under an alliance between Merck and Pfizer.
CONFLICT OF INTEREST STATEMENT
E Kikuchi reports consulting or advisory roles for Astellas Pharma, AstraZeneca, Bristol Myers Squibb‐Ono Pharmaceutical, Chugai Pharma, Janssen, Merck, MSD, and Pfizer; speakers' bureau participation for Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb‐Ono Pharmaceutical, Chugai Pharma, Janssen, Kissei Pharmaceutical, Kyorin, Kyowa Kirin International, Merck, MSD, Nippon Kayaku, Nippon Shinyaku, Pfizer, Sanofi, Taiho Pharmaceutical, and Takeda; and institutional research funding from Chugai Pharma, Kissei Pharmaceutical, Kyorin, Kyowa Kirin International, Nihonkayaku, Nippon Shinyaku, Otsuka, Sanofi, Taiho Pharmaceutical, and Takeda. N Hayakawa, M Nakayama, M Uno, H Nakatsu, and C Kitagawa report no conflicts of interest. H Miyake reports payment or honoraria for lectures, speakers' bureau, travel and accommodation expenses, and participation on a data safety monitoring board/advisory board from Janssen. T Yamada, K Fujita, H Shimoyama, K Nishihara, M Kobayashi, M Nakamura, K Fujimoto, T Sano, and N Nishiyama report no conflicts of interest. T Ito and M Kajita report employment with Merck Biopharma Co., Ltd., Tokyo, Japan, an affiliate of Merck KGaA. T Kobayashi reports no conflicts of interest. H Kitamura reports research funding from Astellas, AstraZeneca, Bristol Myers Squibb, and MSD; consulting or advisory roles for AstraZeneca, Janssen, Kissei, and MSD; and honoraria from Astellas, AstraZeneca, Bristol Myers Squibb, Janssen, Merck, Sanofi, and Takeda.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
The protocol for this study has been approved by suitably constituted Ethics Committees of all participating institutions, and it conforms to the provisions of the Declaration of Helsinki and the “Ethical Guidelines for Life Science and Medical Research Involving Human Subjects” issued by the Ministry of Education, Culture, Sports, Science and Technology, the Ministry of Health, Labor and Welfare, and the Ministry of Economy, Trade and Industry in Japan. The approval number for the St. Marianna University School of Medicine Ethics Committee (committee of the institution of the corresponding author) is 5669.
INFORMED CONSENT
Informed consent was obtained from all patients.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
ClinicalTrials.gov: NCT05431777.
ANIMAL STUDIES
Not applicable.
Supporting information
Table S1.
ACKNOWLEDGMENTS
The authors thank the patients and their families, investigators, co‐investigators, and the study teams at each of the participating centers. Medical writing support was provided by Jeremy Gardner on behalf of Nucleus Global, funded by Merck Biopharma Co., Ltd., Tokyo, Japan, an affiliate of Merck KGaA (CrossRef Funder ID: https://doi.org/10.13039/100009945) and Pfizer.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Center Japan . Project cancer statistics: projected cancer incidence in 2022. 2022. Available from: https://ganjoho.jp/reg_stat/statistics/stat/short_pred_en.html. Accessed 19 Mar 2024.
- 3. NCCN clinical practice guidelines in oncology: bladder cancer. v1.2024. Available from: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed 19 Mar 2024.
- 4. National Cancer Institute . SEER Cancer Stat Facts: bladder cancer. Available from: https://seer.cancer.gov/statfacts/html/urinb.html. Accessed 19 Mar 2024.
- 5. Soualhi A, Rammant E, George G, Russell B, Enting D, Nair R, et al. The incidence and prevalence of upper tract urothelial carcinoma: a systematic review. BMC Urol. 2021;21:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin CC, Galsky MD, Krege S, Hahn NM, Ecke T, Sonpavde G, et al. Efficacy of cisplatin‐based chemotherapy as first‐line treatment in Asian patients with metastatic urothelial carcinoma results of an exploratory subgroup analysis of a pool analysis of phase II/III trials. Ann Oncol. 2012;23:xi96–xi97. [Google Scholar]
- 7. Taguchi S, Kawai T, Nakagawa T, Miyakawa J, Kishitani K, Sugimoto K, et al. Improved survival in real‐world patients with advanced urothelial carcinoma: a multicenter propensity score‐matched cohort study comparing a period before the introduction of pembrolizumab (2003–2011) and a more recent period (2016‐2020). Int J Urol. 2022;29:1462–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abe T, Ishizaki J, Kikuchi H, Minami K, Matsumoto R, Harabayashi T, et al. Outcome of metastatic urothelial carcinoma treated by systemic chemotherapy: prognostic factors based on real‐world clinical practice in Japan. Urol Oncol. 2017;35:38.e1–38.e8. [DOI] [PubMed] [Google Scholar]
- 9. Yamamoto S, Kato M, Takeyama Y, Yukimatsu N, Hirayama Y, Otoshi T, et al. A retrospective study on optimal number of cycles of the first‐line platinum‐based chemotherapy for metastatic urothelial carcinoma. Urol Oncol. 2022;40:194.e7–194.e14. [DOI] [PubMed] [Google Scholar]
- 10. Matsumoto H, Shiraishi K, Azuma H, Inoue K, Uemura H, Eto M, et al. Clinical practice guidelines for bladder cancer 2019 update by the Japanese Urological Association: summary of the revision. Int J Urol. 2020;27:702–709. [DOI] [PubMed] [Google Scholar]
- 11. Japanese Urological Association . Supplement to clinical practice guidelines for bladder cancer [2019 update]. 2021. Available from: https://www.urol.or.jp/lib/files/other/guideline/39_bladder_cancer_2019_rev2021_info.pdf. Accessed 19 Mar 2024.
- 12. Powles T, Bellmunt J, Comperat E, de Santis M, Huddart R, Loriot Y, et al. Bladder cancer: ESMO clinical practice guideline for diagnosis, treatment and follow‐up. Ann Oncol. 2022;33:244–258. [DOI] [PubMed] [Google Scholar]
- 13. Cathomas R, Lorch A, Bruins HM, Compérat EM, Cowan NC, Efstathiou JA, et al. The 2021 updated European Association of Urology guidelines on metastatic urothelial carcinoma. Eur Urol. 2022;81:95–103. [DOI] [PubMed] [Google Scholar]
- 14. Powles T, Csoszi T, Ozguroglu M, Matsubara N, Géczi L, Cheng SY, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first‐line therapy for advanced urothelial carcinoma (KEYNOTE‐361): a randomised, open‐label, phase 3 trial. Lancet Oncol. 2021;22:931–945. [DOI] [PubMed] [Google Scholar]
- 15. Galsky MD, Arija JAA, Bamias A, Davis ID, de Santis M, Kikuchi E, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo‐controlled phase 3 trial. Lancet. 2020;395:1547–1557. [DOI] [PubMed] [Google Scholar]
- 16. Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–1230. [DOI] [PubMed] [Google Scholar]
- 17. Powles T, Park SH, Caserta C, Valderrama BP, Gurney H, Ullén A, et al. Avelumab first‐line maintenance for advanced urothelial carcinoma: results from the JAVELIN bladder 100 trial after ≥2 years of follow‐up. J Clin Oncol. 2023;41:3486–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee J‐L, Desai C, Park SH, Tsuchiya N, Su PJ, Chan TTW, et al. Avelumab first‐line maintenance plus best supportive care (BSC) vs. BSC alone for advanced urothelial carcinoma: JAVELIN bladder 100 Asian subgroup analysis. Urol Oncol. 2023;41:256.e17–256.e25. [DOI] [PubMed] [Google Scholar]
- 19. Tomita Y, Yamamoto Y, Tsuchiya N, Kanayama H, Eto M, Miyake H, et al. Avelumab first‐line maintenance plus best supportive care (BSC) vs BSC alone for advanced urothelial carcinoma: JAVELIN bladder 100 Japanese subgroup analysis. Int J Clin Oncol. 2022;27:383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barthelemy P, Loriot Y, Voog E, Eymard JC, Ravaud A, Flechon A, et al. Full analysis from AVENANCE: a real‐world study of avelumab first‐line (1L) maintenance treatment in patients (pts) with advanced urothelial carcinoma (aUC). J Clin Oncol. 2023;41:Abstract 471. [Google Scholar]
- 21. Antonuzzo L, Maruzzo M, De Giorgi U, Santini D, Tambaro R, Buti S, et al. READY: real‐world data from an Italian compassionate use program of avelumab first‐line maintenance (1LM) treatment for locally advanced or metastatic urothelial carcinoma (la/mUC). J Clin Oncol. 2023;41:469. [Google Scholar]
- 22. Bakaloudi DR, Talukder R, Lin GI, Makrakis D, Diamantopoulos LN, Tripathi N, et al. Response and outcomes of maintenance avelumab after platinum‐based chemotherapy (PBC) in patients with advanced urothelial carcinoma (aUC): “real world” experience. Clin Genitourin Cancer. 2023;21:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miyake M, Shimizu T, Oda Y, Tachibana A, Ohmori C, Itami Y, et al. Switch‐maintenance avelumab immunotherapy following first‐line chemotherapy for patients with advanced, unresectable or metastatic urothelial carcinoma: the first Japanese real‐world evidence from a multicenter study. Jpn J Clin Oncol. 2023;53:253–262. [DOI] [PubMed] [Google Scholar]
- 24. Kikuchi E, Nagata M, Sato M, Kambe A, Ogi M, Endo S, et al. Interim analysis of a post‐marketing surveillance (PMS) study of avelumab maintenance therapy in advanced or metastatic urothelial carcinoma (UC) in Japan. Presented at the 110th Annual Meeting of the Japanese Urological Association, Kobe, Japan; April 20‐23. 2023:Abstract OP80‐05.
- 25. Bajorin DF, Dodd PM, Mazumdar M, Fazzari M, McCaffrey JA, Scher HI, et al. Long‐term survival in metastatic transitional‐cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173–3181. [DOI] [PubMed] [Google Scholar]
- 26. Ichihara K, Yomamoto Y, Hotta T, Hosogaya S, Miyachi H, Itoh Y, et al. Collaborative derivation of reference intervals for major clinical laboratory tests in Japan. Ann Clin Biochem. 2016;53:347–356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.


