Abstract
Prostatic hyperplasia is very common in elderly men and is a typical disease that reduces quality of life. Histologically, hyperplasia of the prostate gland causes obstruction at the bladder outlet, resulting in symptoms such as a weak urine stream. Various factors have been considered to cause histological enlargement of the prostate, but the underlying cause is still unknown. The factors that cause prostate hyperplasia can be broadly classified into intrinsic and extrinsic ones. Extrinsic factors include things that we directly come into contact with such as bacteria and food. On the other hand, intrinsic factors are those that cause changes in functions originally provided in the body due to some cause, including extrinsic factors, such as chronic inflammation and an imbalance of sex hormones. A large number of reports have been made to date regarding the etiology of prostatic hyperplasia, although they have not yet clarified the fundamental cause(s). The various factors currently known should be outlined for future research. Should it be possible to prevent this highly prevalent prostatic hyperplasia which is mainly cause of dcreasing quality of life, there is no doubt that it would be a huge contribution to humanity.
Keywords: benign prostatic hyperplasia, extrinsic factor, intrinsic factor
Abbreviations & Acronyms
- AR‐V7
AR‐variant 7
- BDNF
brain‐derived neurotrophic factor
- BMPs
bone morphogenetic proteins
- BPH
benign prostatic hyperplasia
- DHT
dihydrotestosterone
- EGF
epidermal growth factor
- EGFR
EGF receptor
- EMT
epithelial–mesenchymal transition
- ERK
extracellular signal‐regulated kinase
- FGFs
fibroblast growth factors
- GPR
G protein‐coupled receptor
- HIF
hypoxia inducible factor
- IGF1
Insulin‐like growth factor‐1
- IGFBP‐3
IGF binding protein‐3
- LUTS
lower urinary tract symptoms
- MCP‐1
monocyte chemotactic protein‐1
- MIF
migration inhibitory factor
- NF‐kB
nuclear factor‐kappa B
- PV
prostate volume
- SMCs
smooth muscle cells
- TGF
transforming growth factor
- TRUS
transrectal ultrasound
- TURP
transurethral resection of the prostate
- TZ
transition zone
INTRODUCTION
Benign prostatic hyperplasia (BPH), which is one of the major causes of the decline in the quality of life caused by lower urinary tract symptoms (LUTS), is a common histological finding in elderly men. 1 The gold standard for BPH treatment has been surgical therapy, typified by TURP, but there are concerns about perioperative and postoperative complications especially for the elderly with comorbidities. On the other hand, medical therapy is often more preferred than surgical therapy, but it is less effective than surgical therapy, and in some cases may cause side effects specific to medical therapy. Under these circumstances, a paradigm shift in BPH treatment is occurring due to the rise of minimally invasive surgical treatment in recent years. 2
Enlargement of adenomas, mainly in the transition zone (TZ), is the main histologic finding. 3 , 4 We previously reported that prostate volume (PV) rapidly increased with age in patients with a prostate having a visible TZ with a clear border on transrectal ultrasound (TRUS) compared to that not having one in a community‐based longitudinal study during 15 years (Figures 1 and 2). 5 However, we do not precisely know its etiology, although aging has an influence on it. The fact that the etiology of BPH is not clearly understood has led to a backward step in treatment, resulting in many surgical procedures without prevention or effective early treatment. We would therefore like to enumerate as comprehensively as possible the reports on the etiology of BPH, which consists of intrinsic and extrinsic factors.
FIGURE 1.
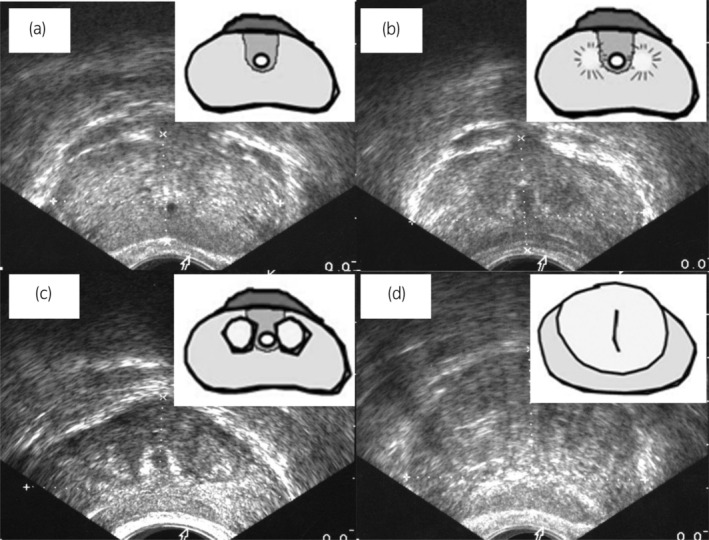
Prostate images on transrectal ultrasonography and schematics of internal prostatic architecture: (a) group 1, prostates with an invisible TZ, (b) group 2, prostates with a visible TZ with an unclear border, (c, d) group 3, prostates with a visible TZ with a clear border. Reprinted with permission from John Wiley & Sons. 4 Order Number: 5678031466051.
FIGURE 2.
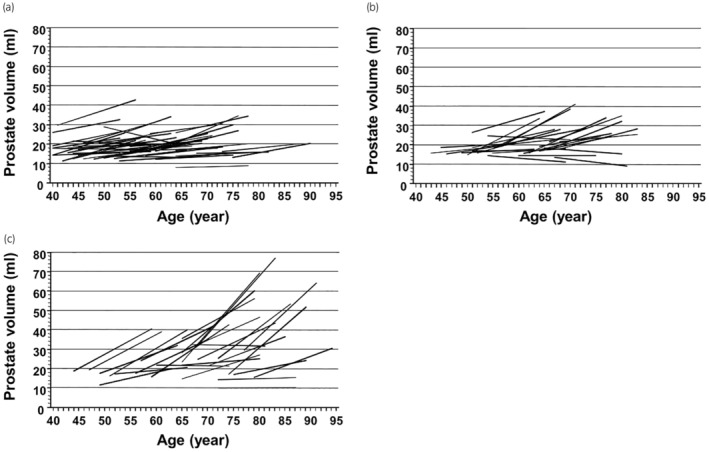
Changes in prostate volume in 15 years by internal prostatic architecture (a) group 1, prostates with an invisible TZ, (b) group 2, prostates with a visible TZ with an unclear border, (c) group 3, prostates with a visible TZ with a clear border. The PV change in group 3 was significantly greater than in groups 1 and 2. Reprinted with permission from John Wiley & Sons. 4 Order Number: 5678031466051.
INTRINSIC FACTORS
Sex hormones
Androgens
There is no doubt that testosterone is an essential factor in prostate growth. Wendel et al. reported that the BPH present in patients with prostatic carcinoma treated with bilateral orchiectomy was of a lower grade than in those patients not having this treatment. 6 Nishi et al. reported that testosterone administration to rats after castration caused regrowth of the prostate. 7 Holmäng et al. reported that eight months of testosterone administration to eugonadal middle‐aged men increased PV by approximately 12%, which was significantly higher than in a placebo group. 8 Furthermore, there are many reports regarding testosterone‐related products. DeKlerk et al. reported that prostatic hyperplasia can be induced in young beagles by treatment with either dihydrotestosterone (DHT) or 5α‐androstane‐3α,17,8‐diol for 4 months. 9
However, a paradox exists in which the prostate is enlarged despite aging and the testosterone level falling. Testosterone alone seems not to be sufficient for developing BPH. Lee et al. reported that total serum testosterone levels were not clearly correlated with their total PV in middle‐aged eugonadal men. 10 Furthermore, testosterone may reduce PV by reducing inflammation within the prostate. Miranda et al. reported that testosterone replacement therapy improved components of metabolic syndrome and decreased prostate inflammation, which is related to the worsening of LUTS in patients with BPH. 11 Marks et al. reported that the effects of 6‐month testosterone replacement therapy in aging hypogonadal men resulted in elevated serum testosterone levels but no change in prostate tissue levels of testosterone and DHT. 12 Testosterone might have a pivotal role in maintaining the gland in a basal immunosuppressive status, thereby reducing the risk of further development of BPH. Quintar et al. reported that the expression of immune‐related proteins in the prostates of rats with orchiectomy gradually increased. 13
The androgen receptor (AR) is essential for normal prostate development and individuals with defective AR signaling do not experience prostate enlargement with age. 14 Bauman et al. reported that AR mRNA expression was increased in stromal cells but decreased in epithelial cells of BPH compared to the normal prostate. 15 This supports the concept that prostatic stromal cells in BPH have become more androgen dependent than in the normal prostate. It is possible that AR signaling within stromal cells alters intercellular signaling and a reawakening of the embryonic mesenchyme, loss of epithelial AR leads to changes in paracrine signaling interactions, aids in stromal or epithelial proliferation evident in BPH. 14
AR‐variant 7 (AR‐V7) mRNA expression were higher in prostate cancer than BPH. 16 However, Austin et al. reported that elevated expression of an AR‐V7 was found in advanced BPH samples and that expression of AR‐V7 significantly correlated with PV. 17 Additionally, a link between inflammatory signaling and AR activity has been observed. Austin et al. reported that activation of nuclear factor‐kappa B (NF‐kB), which regulates the expression of genes involved in inflammatory and immune responses, stimulated the expression of both AR full length and AR‐V7 and elevated the proliferation rates of benign epithelial and stromal cells. 17 , 18 Wu et al. reported that an immune inflammation group had larger prostates and higher AR expression levels in prostatectomy samples than a non‐inflammation group. 19
Estrogens
Although serum androgen levels decline with advancing age, 20 serum estrogens lack an age‐related change. 21 In aging men, paralleling the decrease of testosterone levels, the ratio of estrogens to androgens shows an important increase. Roberts et al. reported that patients with BPH had higher serum estradiol levels or estradiol/bioavailable testosterone ratios. 21 King et al. reported that normal prostate stromal cell proliferation was stimulated by an increasing ratio of estrogen/androgen and proliferation of normal prostate epithelial cells is stimulated as a result of an indirect action of steroids mediated by stromal cells. 22 Popovics et al. reported that steroid hormone imbalance increased macrophage numbers, which is related to prostatic proliferation and fibrosis. 23 Hammarsten et al. reported that total PV correlated significantly with free estradiol and fasting serum insulin. 24 Hyperinsulinemia may be the primary event of BPH according to the insulin and estradiol hypothesis.
Estrogen receptor (ER) subtypes ERα and ERβ are expressed in the prostate. The activation of ER‐α leads to aberrant proliferation, inflammation, and the development of premalignant lesions, whereas the activation of ER‐β is critical in prostatic stromal–epithelial cell signaling and mediating antiproliferative effects that balance the proliferative action of androgens on the epithelia. 25 Nicholson et al. reported that co‐treatment with an ERα antagonist prevented prostate growth in male mice treated with testosterone and estradiol for one month, whereas an ERβ antagonist did not. 26 Krege et al. reported that older ERβ −/− male mice exhibited indications of epithelial hyperplasia in the prostatic collecting ducts. 27 This emphasizes the fact that ERβ inhibits proliferation in the prostate. Nicholson et al. attributed the prostatic hyperplasia observed in ERβ knockout mice to the unopposed action of ERα, suggesting that the ratio ERα/ERβ is an important factor in estrogen‐induced proliferation. 28
G protein‐coupled receptor (GPR) may be one of the key factors for BPH. Estrogens function via GPR/Gαi signaling to modulate epidermal growth factor (EGF) receptor/extracellular signal‐regulated kinase (ERK) and hypoxia inducible factor (HIF)‐1α/transforming growth factor (TGF)‐β1 signaling to increase prostatic stromal cell proliferation and prostatic stromal fibrosis. 29 Additionally, an increase in epithelial cell accumulation caused by the inhibition of GPR30‐mediated apoptosis may contribute to BPH pathogenesis. 30 Estrogens regulate prostate epithelial cell survival via GPR30 and the abundance of GPR30 was shown to be negatively associated with PV.
Aromatase, the enzyme responsible for conversion of testosterone to estradiol, has also been reported to promote BPH. The existence of an androgenic to estrogenic switch in the progression of BPH is facilitated via increased stromal levels of aromatase. 31 The blocking of conversion of androgens to estrogens by aromatase inhibitors seems to prevent prostatic hyperplasia. 32 Habenicht et al. reported that the aromatase inhibitor l‐methyl‐androsta‐1,4‐diene‐3,17‐dion could prevent the hyperplastic change of the prostate in a monkey model. 33 However, in a double‐blind, placebo‐controlled, randomized controlled trial, the aromatase inhibitor atamestane was not found to be effective in BPH patients. 34 It cannot be concluded that aromatase inhibitors are suitable for the treatment of BPH.
Chronic inflammation
BPH may be an immunoinflammatory disease. 35 Chronic inflammation has long been implicated in BPH. Kohnen et al. reported that, of 162 sequential patients undergoing transurethral resection of the prostate (TURP) for BPH with LUTS, 98.1% were observed to have histologic inflammation. 36 Di Silverio et al. reported that inflammation significantly increased with the increase in PV, with 77% of the glands in the largest size category (80–89 cc) demonstrating inflammation, whereas in the smallest size group (30–39 cc) it was present in only 17% of 2981 BPH patients. 37 In an important autopsy series of 167 prostates, chronic inflammation was present in 75% of the glands harboring BPH, compared to only 50% of those without BPH. 38 The REDUCE trial reported the results of prostate biopsy on 8224 patients, with chronic inflammation in 77.6%, and PV was significantly larger in the group with chronic inflammation than in the group without it (46.5 cc vs. 43.4 cc). 39 Robert et al. reported that patients with high‐grade inflammation had higher PV than those with low‐grade inflammation among patients treated with surgery for BPH. 40 The location of inflammation in the prostate might be an important factor affecting PV and the severity of LUTS. Inamura et al. reported that prostatic volume of a group who had a high degree of inflammation in the stroma was significantly larger than in a group in which the dominant site of inflammation was non‐stromal (63.8 vs. 53.8 mL). 41
Immune cells
Lymphocytes and macrophages/monocytes are among the main inflammatory immune cells related to prostatic hyperplasia. In the MTOPS study involving 859 participants who underwent transition zone biopsy, CD4, CD45 and CD68 increased the risk of clinical progression of BPH. 42 Meng et al. reported that the predominant cell type in BPH tissue was T‐lymphocytes, which were identified in 96.1% of all specimens, and the degrees of glandular and stromal inflammation were considered to be the most important factors contributing to increased PV. Stromal hyperplasia was significantly correlated with the degree of stromal inflammation and stromal T‐lymphocyte infiltration. 43 M2a macrophages as a subtype of macrophages could contribute to the development of BPH, possibly via modulating cell apoptosis, the cell cycle, and the epithelial–mesenchymal transition (EMT) process, as well as the fibrotic process. 44 Kakehi and Taoka et al. reported that macrophage inhibitory cytokine‐1 downregulation was an event associated with histological and symptomatic BPH. 45 , 46 Song et al. reported that high levels of macrophage migration inhibitory factor (MIF) were expressed in BPH epithelium and that MIF promoted BPH epithelial cell growth. 47 Lu et al. reported that infiltrating macrophages might promote cell growth of the prostate by inducing paracrine cytokines, promoting EMT in the BPH prostate microenvironment and the crosstalk of macrophages. 48 Furthermore, the primary sources of monocyte chemotactic protein‐1 (MCP‐1) in the prostate may indeed be stromal cells and inflamed epithelial cells. MCP‐1 secreted from stromal cells may stimulate the proliferation of prostatic epithelial cells since both epithelial and stromal cells were found to express the MCP‐1 receptor (CCR2). 49
Inflammatory cytokines
Inflammatory cytokines induced by immune cells greatly influence prostate hyperplasia. Schauer et al. reported that overexpression of IL‐8 inducing fibroblasts in BPH epithelium correlated with a periacinar reactive stroma myofibroblast phenotype fundamentally different from normal prostate fibromuscular stroma. 50 Penna et al. reported that the induction of a BPH cell‐driven autoimmune response, as well as triggering of toll like receptors expressed by BPH cells, upregulates production of IL‐8, IL‐6, and CXCL10, key factors sustaining prostate inflammation, recruiting inflammatory leukocytes, and promoting prostate cell hyperplasia. 51 There is a correlation between serum IL‐6 (sIL‐6) and serum IL‐8 (sIL‐8) in plasma and the severity in patients with BPH. Wu et al. reported in their cross‐sectional studies that high sIL‐6 and sIL‐8 levels in BPH patients were considered to be significantly correlated with acute urinary retention (OR = 9.67/8.85). 52 , 53 Smith et al. reported that IL‐8 and its receptors directly promoted the growth of prostate epithelial cells through the IL‐8 axis in BPH patients. 54 Arivazhagan et al. reported that serum IL‐17, which is a T‐lymphocyte‐derived proinflammatory cytokine, levels in BPH patients were significantly higher than those of a normal control group. 55 Hamakawa et al. reported in their study using a rat model mimicking human BPH that IL‐18 may act directly in BPH pathogenesis by inducing thrombospondin‐1. 56 The complement system is activated in BPH and may contribute to the progression of prostatic proliferation. Hata et al. reported that activation of the complement system is initiated via the classical pathway by formation of antigen–antibody complexes in prostate tissue of a rat BPH model. 57
GROWTH FACTORS
Insulin‐like growth factor‐1
Insulin‐like growth factor‐1 (IGF1) is expressed within the stromal and epithelial cells of the prostate. The increase of IGF1 seems to play a role in the pathogenesis of BPH. IGF1 usually declines with age, although metabolic abnormalities like diabetes and acromegaly lead to an increase in IGF1, which has been shown to have a strong mitogenic and antiapoptotic effect on prostate tissue. 58 Additionally, the overactivity of the growth hormone/IGF1 axis plays an important role in the pathogenesis of BPH in patients with acromegaly. Kumar et al. reported that these patients had a greater PV than healthy controls. 59 IGF‐binding protein‐3 (IGFBP‐3) inhibits the anti‐apoptotic properties of IGF‐1. 60 Sreenivasulu et al. reported that increased insulin and reduced IGFBP‐3/PSA levels predict increased prostate size in patients with BPH. 61
Fibroblast growth factor
Fibroblast growth factors (FGFs) and their tyrosine kinase receptors (FGFRs) have autocrine and paracrine functions in the prostate gland. 62 The role of the FGF/FGFR system in BPH has been intensively investigated. FGF2 (basic FGF, bFGF) is expressed to a large extent in BPH epithelium and stroma. Mori et al. reported that mRNA expression of bFGF in BPH specimens was enhanced twofold compared with normal prostate tissue. 63 bFGF is mitogenic for stromal cells and there is a correlation of bFGF with increased stromal proliferation in the prostate. 64 Saez et al. reported that bFGF expression was not detected in epithelial cells and markedly decreased in stromal cells after finasteride treatment for BPH compared with the control. 65 bFGF can be measured in the urine of BPH patients. O'Brien et al. reported that urinary bFGF was elevated in BPH patients compared with controls. 66 In addition, inflammatory cytokines, including lL‐1a and IL‐8, can upregulate bFGF in the prostate. 67 FGF‐7 (keratinocyte growth factor, KGF) is also overexpressed in BPH. 67 KGF is mitogenic for epithelial cells, but not stromal cells, isolated from BPH tissues, and the KGF‐associated receptor gene is expressed only by epithelial cells. 68 Additionally, KGF plays an important role as a mesenchymal paracrine mediator of androgen‐induced epithelial growth. 69 On the other hand, Planz et al. reported that androgens stimulate cell proliferation as well as KGF protein and gene expression in human prostate stromal cells and that androgen‐induced stromal‐derived KGF stimulates prostate epithelial cell growth. 70 Serum levels of KGF are elevated in BPH and correlate with serum PSA. Mehta et al. reported that serum KGF levels in patients with serum PSA of less than 10 ng/ml were significantly higher in BPH than in a prostate cancer group. 71 In addition, FGF8 is also known as an androgen‐induced growth factor. Wang et al. reported that FGF8 mRNA is expressed in BPH and that the majority of FGF8 expression was by epithelial cells. 72
Epidermal growth factor and transforming growth factor alpha
EGF is linked to the growth and differentiation of epithelial cells in the prostate. In BPH tissue, EGF is highly present in periurethral lesions, and its expression is promoted by exposure to testosterone and DHT. 73 Additionally, inhibition of the EGF receptor (EGFR) is linked to decreased proliferation signals to prostatic epithelial cell lines. Kim et al. reported that the PV and PSA levels of BPH patients were associated with the EGF and EGFR single nucleotide polymorphisms. 74 Frydenberg et al. reported that EGFR expression was present in 81% of BPH specimens in immunohistochemistry. 75 TGF‐α and EGF have many biological effects in common. 76 De Miguel et al. reported that more TGF‐α and EGF appeared in both basal and columnar cells of the epithelium in BPH specimens than in normal prostate tissue. 77
Transforming growth factor beta
TGF‐β is an essential cytokine related to proliferation and apoptosis. There are three different isoforms: TGF‐β1, TGF‐β2, and TGF‐β3. In the prostate, smooth muscles release TGF‐β. While epithelium in the prostate has receptors for TGF‐β, the modulation found there is paracrine. 78 TGF‐β has dual potential; it stimulates cell proliferation at low doses, but at higher doses it has an inhibitory effect on prostatic stromal cells. 79 TGF‐β may play an important role in controlling prostatic growth in aging men. Peehl et al. reported that TGF‐β leads to the differentiation of stromal cells and smooth muscle cells (SMCs) and promotes the aggregation of SMCs in muscular nodules. 80 Additionally, Sampson et al. reported that, in vitro, TGF‐β1 induces the differentiation of fibroblasts into myofibroblasts in the stroma. 81
Vascular endothelial growth factor
Vessel densities were found to be higher in hyperplastic nodules than in adjacent normal tissue in the prostate. 82 Wieszczeczyński et al. reported that a significant increase in the serum VEGF level was observed in a group of dogs affected by BPH and a positive correlation between prostate size and the level of VEGF. 83 Therefore, Hatano et al. observed a reduction in prostate volume during the treatment by sunitinib which is a tyrosine kinase inhibitor of VEGF receptor against metastatic renal cell carcinoma. 84
Bone morphogenetic protein 5
Bone morphogenetic proteins (BMPs) are secreted proteins belonging to the TGF‐β superfamily. Their biological functions were initially elucidated as osteogenic factors. According to Liu et al., BMP5 is localized in both the epithelial and stromal cells of the prostate and upregulated in hyperplastic prostate tissue when compared with controls. 85
Brain‐derived neurotrophic factor
Brain‐derived neurotrophic factor (BDNF) plays an important role in regulating the survival and differentiation of neuronal populations during development, and its receptors are key regulatory proteins in the prostate. Gao et al. reported that BPH patients exhibited higher expression levels of BDNF in serum than normal controls, although no correlation was found between BDNF levels and PV. 86 Wang et al. reported that the urinary BDNF level was significantly higher in patients with BPH than in controls. 87
GENETIC FACTORS
Race is independently associated with BPH. Fowke reported that African‐American men are about half as likely to be diagnosed with BPH compared to Caucasian men (4.1% vs. 9.9%, respectively). 88 On the other hand, they reported that surgical intervention typically reserved for severe BPH was more common among African‐American men. Few studies have been conducted to discover the genetic determinants of BPH risk. Hellwege et al. reported that BPH is likely to be substantially heritable, with consistent point estimates near 60% across two comparable cohorts. 89 Cheng et al. reported that the patients with BPH had significantly shorter leukocyte telomere length, particularly in rs2736100, rs2736098 and rs12696304, compared to healthy controls. 90 They predicted that short telomeres may promote cell proliferation by the senescence‐associated secretory phenotype mechanism, thereby eventually leading to BPH development.
We should also pay attention to the relationship between non‐coding RNA and BPH. Some studies have reported that long non‐coding (lnc) RNAs may be considered as biomarkers for differentiating BPH and prostate cancer. Bayat et al. revealed high expression of lncRNA Cat2184.4 in BPH samples compared to prostate cancer ones. 91 The relationship between microRNAs (miR) and BPH has also been reported in several studies. Greco reported in their meta‐analysis that the potential value of miR‐221 as a potential biomarker for BPH. 92
Although there are no clear reports to date, the possibility that prostate cancer in TZ and BPH are genetically related cannot be denied. Approximately 25% of prostate cancer originated from TZ. 93 Cancerous lesions occurring in TZ and benign lesions such as BPH have similar features on MRI. 94 Furthermore, TZ cancer cells sometimes show a nodular growth pattern similar to hyperplasia. 95
OTHERS
Epithelial–mesenchymal transition
The EMT process plays an important role in BPH. McNeal proposed the embryonic reawakening hypothesis in which the phenomenon of epithelial cell sprouting within the stroma is due to the re‐emergence of the prostate‐inducing effect during the embryonic period in adulthood. 96 Alonso et al. reported that E‐cadherin, an epithelial marker, was downregulated in the epithelial cells of BPH tissue and they observed intense expression of EMT markers. 97 TGF‐β/Smad signaling related to EMT may play an important role in the increased stromal accumulation and epithelial growth. 97 Fraga et al. reported that expression of vimentin, which is a stromal marker, was significantly increased in the epithelial tissues of BPH. 98
Thyroid hormones
Thyroid hormones play an important role in cell differentiation, growth and metabolism. Thyroid hormone receptors are strongly expressed in the human prostate. 99 Eldhose et al. reported that serum free Triiodothyronine (T3) was significantly increased and serum thyroid‐stimulating hormone (TSH) was significantly reduced in BPH patients compared with controls. 100 Additionally, serum‐free T3 correlated positively and serum TSH negatively with PV. 100
Insulin
Several studies have addressed insulin resistance and hyperinsulinemia, components of metabolic syndrome, which are contributing factors in the development of BPH. Insulin is required for prostate growth, and hyperinsulinemia can promote cell proliferation and prostatic enlargement. McKeehan et al. revealed a direct mitogenic effect of insulin on prostate epithelial cells in vitro. 101 Hammarsten et al. reported that the annual prostate growth rate in the top quartile group for the serum insulin level was significantly faster than in the bottom quartile group, and a statistically significant correlation was found between the fasting plasma insulin level and PV. 102 Furthermore, there is a significant correlation between insulin resistance and the PV increase. 103 Chen et al. reported that PV increase with increasing blood glucose levels and body mass index in elderly patients with newly diagnosed type 2 diabetes. 104
Neuroendocrine cells
Neuroendocrine (NE) cells are one type of epithelial cells of the prostate. Their function is to maintain homeostasis in the prostate by producing a variety of neurosecretory products having growth‐promoting activities. 105 , 106 NE cells may play an important role in prostatic hyperplasia during the early stages. There are many NE cells in and around small adenomatous nodules, whereas there are few NE cells in large adenomatous nodules. 107 , 108 Furthermore, we reported that while the rate of NE cells in the ventral prostatic duct tended to decrease with age, BPH model rats exhibited greater preservation of NE cells. 109
Heat shock proteins
Heat shock proteins (HSPs), which are chaperone proteins, are known to be among the key proteins that play a role in maintaining cellular homeostasis. They contribute to the process of proper folding of newly formed proteins and regenerate damaged proteins. 110 In the course of BPH, the overexpression of HSPs is observed. Hata et al. have reported that HSP90 is an autoantigen that binds to the IgG autoantibody and forms an antigen–antibody complex that binds to factor C1q, thus activating the classical complement pathway in the process of promoting prostate hyperplasia. 111 Kim et al. reported that a deficiency of the nitroquinoline 1‐oxide (NQO1) enzyme, which is an flavin adenine dinucleotide dependent flavoprotein that performs various functions in the cellular defense system, increased the expression of HSP90, which increases the affinity of AR for testosterone and can be responsible for enlargement of the prostate gland in NQO1 knockout mice. 112
Cell adhesion molecules
Integrins and cadherins are cell adhesion molecules (CAMs). CAMs enable cells to interact with other cells, influencing tissue remodeling, fibrosis, inflammation, and cell survival. 113 BPH tissue displays a decrease in the number of tight junctions, suggesting increased permeability in BPH tissues. Li et al. reported that E‐cadherin expression was decreased and displayed a discontinuous pattern in BPH specimens compared with normal prostate tissues. 114 BMP5 is upregulated in hyperplastic prostate tissues and localized both in the epithelial and stromal components. 85 Liu et al. used siRNA to knock down BMP5 in prostate stromal cell line (WPMY‐1) and epithelial cell line (BPH‐1) and observed increased E‐cadherin expression, decreased N‐cadherin expression, cell cycle arrest, and cell proliferation inhibition. 85
Oxidative stress
Oxidative DNA damage causes point mutations, deletions, and rearrangements that contribute to alterations in the normal control of programmed cell death, thereby leading to tissue hyperplasia. 115 In BPH, 8‐OH deoxyguanosine (dG) is a marker of oxidative DNA damage. Vital et al. reported that human BPH tissues contained significantly higher levels of 8‐OH dG than control transition zone tissues and the levels of 8‐OH dG were correlated with prostate weight. 116 Additionally, they reported that mice which highly express reactive oxygen species (ROS) specifically in the prostate had increased oxidative DNA damage in the prostate, increased prostate weight, increased epithelial proliferation, and histological changes, including stromal thickening, and fibrosis, when compared to wild‐type controls. 116 Endogenous defenses against ROS include antioxidant enzymes such as superoxide dismutase (SOD). Aydin et al. reported that lipid peroxidation was significantly increased, with decreased SOD activity in BPH tissue compared with controls. 117
Apoptosis
Apoptosis is involved in the development and homeostatic maintenance of tissues and organs, including the prostate, and is regulated by different death‐ or survival‐related genes. 118 The glycoprotein Dickkopf‐related protein 3 (Dkk‐3) is mainly expressed in the prostate epithelial cells, whereas in BPH it is elevated in stromal cells. 119 Zenzmaier et al. reported that overexpression of Dkk‐3 in BPH caused inhibition of the apoptosis machinery through a reduction in anti‐apoptotic protein expression. 120 Apoptosis in BPH is also regulated by the inhibitors of apoptosis proteins (IAPs), which are able to interfere with caspases. Increased expression of IAPs has been shown in BPH tissue. In experimental BPH, cIAP‐1, cIAP‐2, neuronal apoptosis inhibitor protein (NAIP), and survivin have been detected by molecular analysis. 121
EXTRINSIC FACTORS
Microbiota
In recent years, there have been increasing reports of the microbiota contributing to prostatic enlargement. When discussing the microbiota, their localization must be considered. Possible microbiota associated with prostatic hyperplasia include gut microbiota, urine microbiota, and microbiota in the prostate.
Gut microbiota
The gut microbiota might affect prostatic hyperplasia in elderly males. Takezawa et al. found that the Firmicutes/Bacteroidetes ratio of gut microbiota was significantly higher in a prostatic enlargement (PE) group than in a non‐PE group. 122 An et al. reported that decreased abundance of Lactobacillus and increased abundances of Flavonifractor, Acetatifactor, Oscillibacter, Pseudoflavonifractor, Butyricimonas, and Anaerotruncus as gut microbiota were potential indicators for the diagnosis of BPH. 123 Li et al. reported that in gut samples Muribaculaceae, Turicibacteraceae, Turicibacter and Coprococcus significantly decreased in a rat group with BPH, whereas Mollicutes and Prevotella significantly increased compared with a control rat group. 124 Additionally, BPH treatment also alters the gut microbiota. An et al. reported that the abundances of Lactobacillus and Acetatifactor, which are associated with the promotion and inhibition of prostate apoptosis, were normalized after finasteride treatment. 123 Dong et al. reported that sodium butyrate treatment, which inhibits cell cycle progression and induces apoptosis and autophagy, as a postbiotic, and fecal microbiota transplantation provides relief from ulcerative colitis‐induced BPH in an in vivo study. 125 On the other hand, the gut microbiota might affect testosterone metabolism in the elderly male. Several studies reported that Firmicutes were significantly increased in the gut microbiota in elderly men with high testosterone levels compared with those with low testosterone levels. 126 , 127 Li et al. reported that differential metabolites were significantly enriched in steroid hormone biosynthesis, ovarian steroidogenesis, biosynthesis of unsaturated fatty acids and bile secretion when they analyzed gut samples from rats with BPH and healthy control rats. 124
Urinary microbiota
Changes in the urethral and bladder microbiota with age might be associated with increasing LUTS in older males, which is typically due to BPH. A “core” urinary microbiome could potentially exist, as evidenced by the fact that when samples are grouped by age, fluctuations in abundance between age groups and age‐specific genera are found. 128 Tsai et al. reported that the urine microbiota composition of BPH was distinct from that of a control group and the top five microbial genera that showed significant differences between groups were Alcaligenes, Pseudomonas, Lactobacillus, Akkermansia, and Cetobacterium. 129 An association between LUTS and the urinary microbiome has also been noted. Lee et al. reported that ten bacterial genera were present in samples of a BPH group and urine samples, showing the presence of bacterial genera correlated with a high IPSS and severe storage and voiding symptoms. 130 Thomas et al. found a statistically significant association between a diagnosis of LUTS and the presence of JC virus, a common neurotropic human polyomavirus linked to severe neurologic disease in rare cases. 131 On the other hand, we need to be careful about the existence of significant differences in the microbiota among voided urine, seminal fluid, and expressed prostatic secretions. 132
Prostatic microbiota
Microbiota are present in the prostate, although there are still few reports about this. Okada et al. reported that the prostate microbiota are located in the prostatic duct, and Burkholderia was the most abundantly detected operational taxonomic unit, which was different from that of catheterized urine. 133 Jain et al. reported that majority of the isolates in the BPH prostate were coagulase‐positive Staphylococcus, E. coli and Micrococcus spp. The E. coli isolated from one of the tissues was able to activate NF‐κB and induce DNA damage in prostate epithelial cells and also correlated with the severity of inflammation. 134 Reduced diversity of the microbiota in the prostatic duct is associated with BPH. 133 Reduced diversity, called dysbiosis, is considered to be one of the abnormal conditions of the microbiota. More complex and diverse microbiota may have more health benefits for the host, and reduced diversity of microbiota is included in dysbiosis.
Trichomonas vaginalis
Several observational studies have shown a positive association between sexually transmitted infections (STIs) and BPH/LUTS. 135 Trichomonas vaginalis (TV) is one of the typical STIs. Mitteregger et al. reported that the TV infection rate was 34% in BPH. 136 Kim and Kim et al. reported that TV‐infected BPH‐1 cells activated mast cells, which release the inflammatory mediators CXCL8, CCL2, IL‐1β, and IL‐6 through the ROS, mitogen‐activated protein kinase (MAPK), and NF‐κB signaling pathways that induce prostatic stromal cell proliferation. 137 , 138 Additionally, inflamed BPH‐1 cells infected with TV promote prostate cell proliferation through leptin‐leptin receptor signaling. Therefore, it is likely that TV contributes to prostate enlargement in BPH via adipocyte leptin release as a result of prostate inflammation. 139
Diet
There are many mechanisms whereby dietary patterns could affect the risk of symptomatic BPH. The Prostate Cancer Prevention Trial showed that higher consumption of fat and red meat and lower consumption of protein and vegetables may increase the risk of symptomatic BPH. 140 Lagiou et al. reported in their case–control study that butter and margarine may be associated with an increased risk of BPH and that intake of fruits containing β‐carotene, lutein and vitamin C may reduce this risk. 141 Vignozzi et al. reported in an animal study that the proinflammatory effects of a high‐fat diet result in prostate inflammation, hypoxia and tissue remodeling. 142 Zhang et al. reported that vitamin D‐deficient diets induced prostatic inflammation and fibrosis in middle‐aged mice through the activation of an NF‐κB‐mediated pathway and the production of IL‐6, as well as upregulation of the signal transducer and activator of transcription 3‐mediated pathway that stimulates cell proliferation and growth. 143 Li et al. showed that the combination of androgens and high‐fat diet‐induced hyperinsulinemia promoted BPH in rats and that the activation of p‐ERK1/2, which is a member of the MAPK family, could be implicated in this process. 144 Furthermore, maternal nutritional status may influence the prostatic development of offspring. Shibamori et al. reported that maternal consumption of a low protein diet promotes epithelial hyperplasia, while a high fat diet leads to increased stromal growth. 145
SUMMARY
Previous reports on the etiology of hyperplasia of the male prostate in the post‐middle age period divided into two categories, intrinsic and extrinsic, have reminded us of the great variety of etiologies (Figures 3 and 4). In particular, there are many types of intrinsic factors, and the mechanism is very complex. Although we have discussed many intrinsic and extrinsic factors as causes of prostate hyperplasia, it is not clear which factors are most important. However, there is no doubt that various factors related to aging are involved, and it seems that preventing as much as possible the common health problems that occur with aging will suppress the onset and progression of prostatic hyperplasia. It remains to be determined whether exposure to extrinsic factors such as those described here at some point before midlife triggers intrinsic factors that lead to histological enlargement of the prostate, whether intrinsic factors alone cause it, or whether there is some other underlying cause. Thus, further basic and clinical research is necessary to clarify the etiology of this condition.
FIGURE 3.
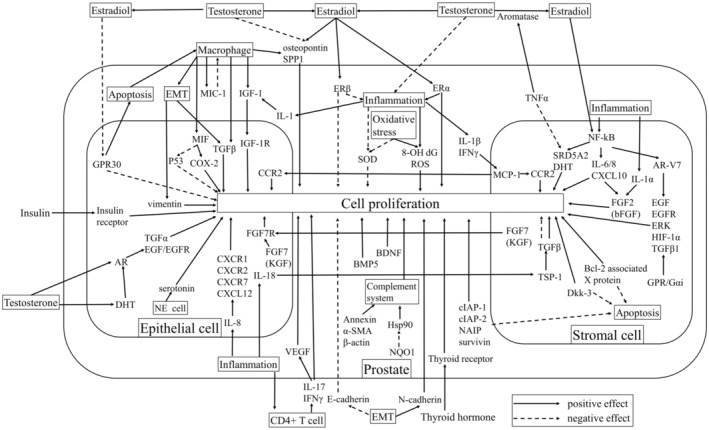
Intrinsic factors that cause prostatic hyperplasia. AR, androgen receptor; BDNF, brain‐derived neurotrophic factor; BMP, bone morphogenetic protein; dG, deoxyguanosine; DHT, dihydrotestosterone; EGF, epidermal growth factor; EMT, epithelial–mesenchymal transition; ER, estrogen receptor; ERK, extracellular signal‐regulated kinase; FGF, fibroblast growth factor; GPR, G protein‐coupled receptor; HIF, hypoxia inducible factor; HSP, heat shock protein; IAP, inhibitors of apoptosis protein; IGF, insulin‐like growth factor; KGF, keratinocyte growth factor; MCP, monocyte chemotactic protein; MIC‐1, macrophage inhibitory cytokine‐1; MIF, macrophage migration inhibitory factor; NAIP, neuronal apoptosis inhibitor protein; NE, neuroendocrine; NF‐kB, nuclear factor‐kappa B; NQO, nitroquinoline 1‐oxide; ROS, reactive oxygen species; SMA, smooth muscle actin; SOD, superoxide dismutase; SPP, secreted phosphoprotein; SRD5A2, steroid 5 alpha‐reductase 2; TGF, transforming growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
FIGURE 4.
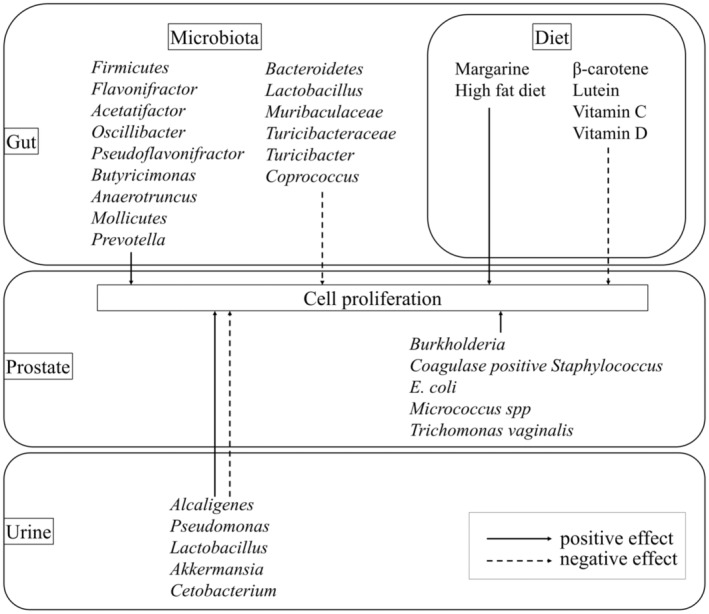
Extrinsic factors that cause prostatic hyperplasia.
AUTHOR CONTRIBUTIONS
Yuki Kyoda: Conceptualization; Methodology; Writing—original draft; Investigation. Kosuke Shibamori: Supervision; Investigation. Tetsuya Shindo: Supervision; Writing—review & editing. Takeshi Maehana: Supervision. Kohei Hashimoto: Supervision. Ko Kobayashi: Supervision. Toshiaki Tanaka: Supervision; Writing—review & editing. Fumimasa Fukuta: Supervision; Investigation; Writing—review & editing. Naoya Masumori: Supervision; Conceptualization; Writing—review & editing; Investigation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
The approval number: N/A.
INFORMED CONSENT
N/A.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
N/A.
ANIMAL STUDIES
N/A.
FUNDING INFORMATION
None.
ACKNOWLEDGMENTS
The authors thank Mr. Kim Barrymore for advice on English usage in this article.
REFERENCES
- 1. Tsukamoto T, Kumamoto Y, Masumori N, Miyake H, Rhodes T, Girman CJ, et al. Prevalence of prostatism in Japanese men in a community‐based study with comparison to a similar American study. J Urol. 1995;154:391–395. [DOI] [PubMed] [Google Scholar]
- 2. Bortnick E, Stock J, Ferrer F. Genito‐urinary rhabdomyosarcoma‐challenges and controversies for the urologist. Transl Androl Urol. 2020;9:2422–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McNeal JE. Origin and evolution of benign prostatic enlargement. Investig Urol. 1978;15:340–345. [PubMed] [Google Scholar]
- 4. Masumori N, Tsukamoto T, Kumamoto Y, Miyake H, Rhodes T, Girman CJ, et al. Age‐related differences in internal prostatic architecture on transrectal ultrasonography: results of a community based survey in Japan. J Urol. 1997;157:1718–1722. [PubMed] [Google Scholar]
- 5. Fukuta F, Masumori N, Mori M, Tsukamoto T. Internal prostatic architecture on transrectal ultrasonography predicts future prostatic growth: natural history of prostatic hyperplasia in a 15‐year longitudinal community‐based study. Prostate. 2011;71:597–603. [DOI] [PubMed] [Google Scholar]
- 6. Wendel EF, Brannen GE, Putong PB, Grayhack JT. The effect of orchiectomy and estrogens of benign prostatic hyperplasia. J Urol. 1972;108:116–119. [DOI] [PubMed] [Google Scholar]
- 7. Nishi N, Oya H, Matsumoto K, Nakamura T, Miyanaka H, Wada F. Changes in gene expression of growth factors and their receptors during castration‐induced involution and androgen‐induced regrowth of rat prostates. Prostate. 1996;28:139–152. [DOI] [PubMed] [Google Scholar]
- 8. Holmang S, Marin P, Lindstedt G, Hedelin H. Effect of long‐term oral testosterone undecanoate treatment on prostate volume and serum prostate‐specific antigen concentration in eugonadal middle‐aged men. Prostate. 1993;23:99–106. [DOI] [PubMed] [Google Scholar]
- 9. DeKlerk DP, Coffey DS, Ewing LL, McDermott IR, Reiner WG, Robinson CH, et al. Comparison of spontaneous and experimentally induced canine prostatic hyperplasia. J Clin Invest. 1979;64:842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee JH, Kim Y, Park YW, Lee DG. Relationship between benign prostatic hyperplasia/lower urinary tract symptoms and total serum testosterone level in healthy middle‐aged eugonadal men. J Sex Med. 2014;11:1309–1315. [DOI] [PubMed] [Google Scholar]
- 11. Miranda EP, Torres LO. Late‐onset hypogonadism: prostate safety. Andrology. 2020;8:1606–1613. [DOI] [PubMed] [Google Scholar]
- 12. Marks LS, Mazer NA, Mostaghel E, Hess DL, Dorey FJ, Epstein JI, et al. Effect of testosterone replacement therapy on prostate tissue in men with late‐onset hypogonadism: a randomized controlled trial. JAMA. 2006;296:2351–2361. [DOI] [PubMed] [Google Scholar]
- 13. Quintar AA, Leimgruber C, Pessah OA, Doll A, Maldonado CA. Androgen depletion augments antibacterial prostate host defences in rats. Int J Androl. 2012;35:845–859. [DOI] [PubMed] [Google Scholar]
- 14. Vickman RE, Franco OE, Moline DC, Vander Griend DJ, Thumbikat P, Hayward SW. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: a review. Asian J Urol. 2020;7:191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bauman DR, Steckelbroeck S, Peehl DM, Penning TM. Transcript profiling of the androgen signal in normal prostate, benign prostatic hyperplasia, and prostate cancer. Endocrinology. 2006;147:5806–5816. [DOI] [PubMed] [Google Scholar]
- 16. Hillebrand AC, Pizzolato LS, Neto BS, Branchini G, Brum IS. Androgen receptor isoforms expression in benign prostatic hyperplasia and primary prostate cancer. PLoS One. 2018;13:e0200613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Austin DC, Strand DW, Love HL, Franco OE, Jang A, Grabowska MM, et al. NF‐kappaB and androgen receptor variant expression correlate with human BPH progression. Prostate. 2016;76:491–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Austin DC, Strand DW, Love HL, Franco OE, Grabowska MM, Miller NL, et al. NF‐kappaB and androgen receptor variant 7 induce expression of SRD5A isoforms and confer 5ARI resistance. Prostate. 2016;76:1004–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu ZL, Yuan Y, Geng H, Xia SJ. Influence of immune inflammation on androgen receptor expression in benign prostatic hyperplasia tissue. Asian J Androl. 2012;14:316–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belanger A, Candas B, Dupont A, Cusan L, Diamond P, Gomez JL, et al. Changes in serum concentrations of conjugated and unconjugated steroids in 40‐ to 80‐year‐old men. J Clin Endocrinol Metab. 1994;79:1086–1090. [DOI] [PubMed] [Google Scholar]
- 21. Roberts RO, Jacobson DJ, Rhodes T, Klee GG, Leiber MM, Jacobsen SJ. Serum sex hormones and measures of benign prostatic hyperplasia. Prostate. 2004;61:124–131. [DOI] [PubMed] [Google Scholar]
- 22. King KJ, Nicholson HD, Assinder SJ. Effect of increasing ratio of estrogen: androgen on proliferation of normal human prostate stromal and epithelial cells, and the malignant cell line LNCaP. Prostate. 2006;66:105–114. [DOI] [PubMed] [Google Scholar]
- 23. Popovics P, Skalitzky KO, Schroeder E, Jain A, Silver SV, Van Fritz F, et al. Steroid hormone imbalance drives macrophage infiltration and Spp1/osteopontin(+) foam cell differentiation in the prostate. J Pathol. 2023;260:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hammarsten J, Damber JE, Karlsson M, Knutson T, Ljunggren O, Ohlsson C, et al. Insulin and free oestradiol are independent risk factors for benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2009;12:160–165. [DOI] [PubMed] [Google Scholar]
- 25. Ellem SJ, Risbridger GP. The dual, opposing roles of estrogen in the prostate. Ann N Y Acad Sci. 2009;1155:174–186. [DOI] [PubMed] [Google Scholar]
- 26. Nicholson TM, Moses MA, Uchtmann KS, Keil KP, Bjorling DE, Vezina CM, et al. Estrogen receptor‐alpha is a key mediator and therapeutic target for bladder complications of benign prostatic hyperplasia. J Urol. 2015;193:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 2011;82:184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang Y, Sheng J, Hu S, Cui Y, Xiao J, Yu W, et al. Estrogen and G protein‐coupled estrogen receptor accelerate the progression of benign prostatic hyperplasia by inducing prostatic fibrosis. Cell Death Dis. 2022;13:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang DL, Xu JW, Zhu JG, Zhang YL, Xu JB, Sun Q, et al. Role of GPR30 in estrogen‐induced prostate epithelial apoptosis and benign prostatic hyperplasia. Biochem Biophys Res Commun. 2017;487:517–524. [DOI] [PubMed] [Google Scholar]
- 31. Wang Z, Hu L, Salari K, Bechis SK, Ge R, Wu S, et al. Androgenic to oestrogenic switch in the human adult prostate gland is regulated by epigenetic silencing of steroid 5alpha‐reductase 2. J Pathol. 2017;243:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rastrelli G, Vignozzi L, Corona G, Maggi M. Testosterone and benign prostatic hyperplasia. Sex Med Rev. 2019;7:259–271. [DOI] [PubMed] [Google Scholar]
- 33. Habenicht UF, Schwarz K, Neumann F, el Etreby MF. Induction of estrogen‐related hyperplastic changes in the prostate of the cynomolgus monkey (Macaca fascicularis) by androstenedione and its antagonization by the aromatase inhibitor 1‐methyl‐androsta‐1,4‐diene‐3,17‐dione. Prostate. 1987;11:313–326. [DOI] [PubMed] [Google Scholar]
- 34. Gingell JC, Knonagel H, Kurth KH, Tunn UW. Placebo controlled double‐blind study to test the efficacy of the aromatase inhibitor atamestane in patients with benign prostatic hyperplasia not requiring operation. The Schering 90.062 study group. J Urol. 1995;154:399–401. [DOI] [PubMed] [Google Scholar]
- 35. De Nunzio C, Presicce F, Tubaro A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat Rev Urol. 2016;13:613–626. [DOI] [PubMed] [Google Scholar]
- 36. Kohnen PW, Drach GW. Patterns of inflammation in prostatic hyperplasia: a histologic and bacteriologic study. J Urol. 1979;121:755–760. [DOI] [PubMed] [Google Scholar]
- 37. Di Silverio F, Gentile V, De Matteis A, Mariotti G, Giuseppe V, Luigi PA, et al. Distribution of inflammation, pre‐malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol. 2003;43:164–175. [DOI] [PubMed] [Google Scholar]
- 38. Delongchamps NB, de la Roza G, Chandan V, Jones R, Sunheimer R, Threatte G, et al. Evaluation of prostatitis in autopsied prostates—is chronic inflammation more associated with benign prostatic hyperplasia or cancer? J Urol. 2008;179:1736–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nickel JC, Roehrborn CG, O'Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol. 2008;54:1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Robert G, Descazeaud A, Nicolaiew N, Terry S, Sirab N, Vacherot F, et al. Inflammation in benign prostatic hyperplasia: a 282 patients' immunohistochemical analysis. Prostate. 2009;69:1774–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Inamura S, Ito H, Shinagawa T, Tsutsumiuchi M, Taga M, Kobayashi M, et al. Prostatic stromal inflammation is associated with bladder outlet obstruction in patients with benign prostatic hyperplasia. Prostate. 2018;78:743–752. [DOI] [PubMed] [Google Scholar]
- 42. Torkko KC, Wilson RS, Smith EE, Kusek JW, van Bokhoven A, Lucia MS. Prostate biopsy markers of inflammation are associated with risk of clinical progression of benign prostatic hyperplasia: findings from the MTOPS study. J Urol. 2015;194:454–461. [DOI] [PubMed] [Google Scholar]
- 43. Meng Y, Yu W, Liu Z, Zhang M, Chen Y, Li S, et al. The inflammation patterns of different inflammatory cells in histological structures of hyperplasic prostatic tissues. Transl Androl Urol. 2020;9:1639–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qian Q, He W, Liu D, Yin J, Ye L, Chen P, et al. M2a macrophage can rescue proliferation and gene expression of benign prostate hyperplasia epithelial and stroma cells from insulin‐like growth factor 1 knockdown. Prostate. 2021;81:530–542. [DOI] [PubMed] [Google Scholar]
- 45. Taoka R, Tsukuda F, Ishikawa M, Haba R, Kakehi Y. Association of prostatic inflammation with down‐regulation of macrophage inhibitory cytokine‐1 gene in symptomatic benign prostatic hyperplasia. J Urol. 2004;171:2330–2335. [DOI] [PubMed] [Google Scholar]
- 46. Kakehi Y, Segawa T, Wu XX, Kulkarni P, Dhir R, Getzenberg RH. Down‐regulation of macrophage inhibitory cytokine‐1/prostate derived factor in benign prostatic hyperplasia. Prostate. 2004;59:351–356. [DOI] [PubMed] [Google Scholar]
- 47. Song H, Shen Q, Hu S, Jin J. The role of macrophage migration inhibitory factor in promoting benign prostatic hyperplasia epithelial cell growth by modulating COX‐2 and P53 signaling. Biol Open. 2020;9:bio053447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lu T, Lin WJ, Izumi K, Wang X, Xu D, Fang LY, et al. Targeting androgen receptor to suppress macrophage‐induced EMT and benign prostatic hyperplasia (BPH) development. Mol Endocrinol. 2012;26:1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fujita K, Ewing CM, Getzenberg RH, Parsons JK, Isaacs WB, Pavlovich CP. Monocyte chemotactic protein‐1 (MCP‐1/CCL2) is associated with prostatic growth dysregulation and benign prostatic hyperplasia. Prostate. 2010;70:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schauer IG, Ressler SJ, Tuxhorn JA, Dang TD, Rowley DR. Elevated epithelial expression of interleukin‐8 correlates with myofibroblast reactive stroma in benign prostatic hyperplasia. Urology. 2008;72:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Penna G, Fibbi B, Amuchastegui S, Cossetti C, Aquilano F, Laverny G, et al. Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno‐mediated inflammation. J Immunol. 2009;182:4056–4064. [DOI] [PubMed] [Google Scholar]
- 52. Wu D, Tang HX, Wu Y, Qian SB, Xu D, Qi J. The possible association between serum interleukin 8 and acute urinary retention in Chinese patients with benign prostatic hyperplasia. Andrologia. 2020;52:e13763. [DOI] [PubMed] [Google Scholar]
- 53. Wu D, Shi ZE, Xu D, Wu Y, Qian SB, Qi J. Serum interleukin 6 and acute urinary retention in elderly men with benign prostatic hyperplasia in China: a cross‐sectional study. Transl Androl Urol. 2021;10:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith DK, Hasanali SL, Wang J, Kallifatidis G, Morera DS, Jordan AR, et al. Promotion of epithelial hyperplasia by interleukin‐8‐CXCR axis in human prostate. Prostate. 2020;80:938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arivazhagan J, Nandeesha H, Dorairajan LN, Sreenivasulu K. Association of elevated interleukin‐17 and angiopoietin‐2 with prostate size in benign prostatic hyperplasia. Aging Male. 2017;20:115–118. [DOI] [PubMed] [Google Scholar]
- 56. Hamakawa T, Sasaki S, Shibata Y, Imura M, Kubota Y, Kojima Y, et al. Interleukin‐18 may lead to benign prostatic hyperplasia via thrombospondin‐1 production in prostatic smooth muscle cells. Prostate. 2014;74:590–601. [DOI] [PubMed] [Google Scholar]
- 57. Hata H, Tonokura K. Impact of next‐generation vehicles on tropospheric ozone estimated by chemical transport model in the Kanto region of Japan. Sci Rep. 2019;9:3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cannarella R, Condorelli RA, Barbagallo F, La Vignera S, Calogero AE. Endocrinology of the aging prostate: current concepts. Front Endocrinol (Lausanne). 2021;12:554078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kumar S, Yadav RN, Gupta P, Gaspar BL, Kakkar N, Verma A, et al. Prostatic hyperplasia in acromegaly, a myth or reality: a case‐control study. Eur J Endocrinol. 2015;172:97–106. [DOI] [PubMed] [Google Scholar]
- 60. Du Y, Long Q, Shi Y, Liu X, Li X, Zeng J, et al. Insulin‐like growth factor binding protein‐3 mediates interleukin‐24‐induced apoptosis through inhibition of the mTOR pathway in prostate cancer. Oncol Rep. 2015;34:2273–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sreenivasulu K, Nandeesha H, Dorairajan LN, Rajappa M, Vinayagam V. Elevated insulin and reduced insulin like growth factor binding protein‐3/prostate specific antigen ratio with increase in prostate size in benign prostatic hyperplasia. Clin Chim Acta. 2017;469:37–41. [DOI] [PubMed] [Google Scholar]
- 62. Giacomini A, Grillo E, Rezzola S, Ribatti D, Rusnati M, Ronca R, et al. The FGF/FGFR system in the physiopathology of the prostate gland. Physiol Rev. 2021;101:569–610. [DOI] [PubMed] [Google Scholar]
- 63. Mori H, Maki M, Oishi K, Jaye M, Igarashi K, Yoshida O, et al. Increased expression of genes for basic fibroblast growth factor and transforming growth factor type beta 2 in human benign prostatic hyperplasia. Prostate. 1990;16:71–80. [DOI] [PubMed] [Google Scholar]
- 64. Ropiquet F, Giri D, Lamb DJ, Ittmann M. FGF7 and FGF2 are increased in benign prostatic hyperplasia and are associated with increased proliferation. J Urol. 1999;162:595–599. [PubMed] [Google Scholar]
- 65. Saez C, Gonzalez‐Baena AC, Japon MA, Giraldez J, Segura DI, Rodriguez‐Vallejo JM, et al. Expression of basic fibroblast growth factor and its receptors FGFR1 and FGFR2 in human benign prostatic hyperplasia treated with finasteride. Prostate. 1999;40:83–88. [DOI] [PubMed] [Google Scholar]
- 66. O'Brien TS, Smith K, Cranston D, Fuggle S, Bicknell R, Harris AL. Urinary basic fibroblast growth factor in patients with bladder cancer and benign prostatic hypertrophy. Br J Urol. 1995;76:311–314. [DOI] [PubMed] [Google Scholar]
- 67. Giri D, Ittmann M. Interleukin‐1alpha is a paracrine inducer of FGF7, a key epithelial growth factor in benign prostatic hyperplasia. Am J Pathol. 2000;157:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yan G, Fukabori Y, Nikolaropoulos S, Wang F, McKeehan WL. Heparin‐binding keratinocyte growth factor is a candidate stromal‐to‐epithelial‐cell andromedin. Mol Endocrinol. 1992;6:2123–2128. [DOI] [PubMed] [Google Scholar]
- 69. Sugimura Y, Foster BA, Hom YK, Lipschutz JH, Rubin JS, Finch PW, et al. Keratinocyte growth factor (KGF) can replace testosterone in the ductal branching morphogenesis of the rat ventral prostate. Int J Dev Biol. 1996;40:941–951. [PubMed] [Google Scholar]
- 70. Planz B, Wang Q, Kirley SD, Lin CW, McDougal WS. Androgen responsiveness of stromal cells of the human prostate: regulation of cell proliferation and keratinocyte growth factor by androgen. J Urol. 1998;160:1850–1855. [DOI] [PubMed] [Google Scholar]
- 71. Mehta PB, Robson CN, Neal DE, Leung HY. Serum keratinocyte growth factor measurement in patients with prostate cancer. J Urol. 2000;164:2151–2155. [PubMed] [Google Scholar]
- 72. Wang Q, Stamp GW, Powell S, Abel P, Laniado M, Mahony C, et al. Correlation between androgen receptor expression and FGF8 mRNA levels in patients with prostate cancer and benign prostatic hypertrophy. J Clin Pathol. 1999;52:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sciarra F, Monti S, Adamo MV, Palma E, Toscano V, d'Eramo G, et al. Regional distribution of epidermal growth factor, testosterone and dihydrotestosterone in benign prostatic hyperplasia tissue. Urol Res. 1995;23:387–390. [DOI] [PubMed] [Google Scholar]
- 74. Kim SK, Park HK, Choi HS, Yoo KH, Chung JH. Association study of polymorphisms of epidermal growth factor and epidermal growth factor receptor with benign prostatic hyperplasia in a Korean population. Int Neurourol J. 2016;20:363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Frydenberg M, Foo TM, Jones AS, Grace J, Hensley WJ, Rogers J, et al. Benign prostatic hyperplasia—video image analysis and its relationship to androgen and epidermal growth factor receptor expression. J Urol. 1991;146:872–876. [DOI] [PubMed] [Google Scholar]
- 76. Marquardt H, Hunkapiller MW, Hood LE, Todaro GJ. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science. 1984;223:1079–1082. [DOI] [PubMed] [Google Scholar]
- 77. De Miguel P, Royuela BR, Ruiz A, Fraile B, Paniagua R. Immunohistochemical comparative analysis of transforming growth factor alpha, epidermal growth factor, and epidermal growth factor receptor in normal, hyperplastic and neoplastic human prostates. Cytokine. 1999;11:722–727. [DOI] [PubMed] [Google Scholar]
- 78. Shah A, Shah AA, Nandakumar K, Lobo R. Mechanistic targets for BPH and prostate cancer‐a review. Rev Environ Health. 2021;36:261–270. [DOI] [PubMed] [Google Scholar]
- 79. Kassen A, Sutkowski DM, Ahn H, Sensibar JA, Kozlowski JM, Lee C. Stromal cells of the human prostate: initial isolation and characterization. Prostate. 1996;28:89–97. [DOI] [PubMed] [Google Scholar]
- 80. Peehl DM, Sellers RG. Induction of smooth muscle cell phenotype in cultured human prostatic stromal cells. Exp Cell Res. 1997;232:208–215. [DOI] [PubMed] [Google Scholar]
- 81. Sampson N, Zenzmaier C, Heitz M, Hermann M, Plas E, Schafer G, et al. Stromal insulin‐like growth factor binding protein 3 (IGFBP3) is elevated in the diseased human prostate and promotes ex vivo fibroblast‐to‐myofibroblast differentiation. Endocrinology. 2013;154:2586–2599. [DOI] [PubMed] [Google Scholar]
- 82. Deering RE, Bigler SA, Brown M, Brawer MK. Microvascularity in benign prostatic hyperplasia. Prostate. 1995;26:111–115. [DOI] [PubMed] [Google Scholar]
- 83. Wieszczeczynski M, Krakowski L, Opielak G, Krakowska I, Furmaga J, Brodzki P, et al. MicroRNA and vascular endothelial growth factor (VEGF) as new useful markers in the diagnosis of benign prostatic hyperplasia in dogs. Theriogenology. 2021;171:113–118. [DOI] [PubMed] [Google Scholar]
- 84. Hatano T, Ishii G, Endo K, Kishimoto K, Egawa S. Shrinkage of prostate volume in sunitinib‐treated patients with renal cell carcinoma. Jpn J Clin Oncol. 2013;43:1282–1285. [DOI] [PubMed] [Google Scholar]
- 85. Liu D, Liu J, Li Y, Liu H, Hassan HM, He W, et al. Upregulated bone morphogenetic protein 5 enhances proliferation and epithelial‐mesenchymal transition process in benign prostatic hyperplasia via BMP/Smad signaling pathway. Prostate. 2021;81:1435–1449. [DOI] [PubMed] [Google Scholar]
- 86. Gao Y, Liu P, He F, Yang X, Wu R, Chen W, et al. Fibroblast growth factor 2 promotes bladder hypertrophy caused by partial bladder outlet obstruction. Front Cell Dev Biol. 2021;9:630228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang LW, Li JL, Yu Y, Xiao RH, Huang HW, Kuang RR, et al. Association of increased urine brain derived neurotrophic factor with lower urinary tract symptoms in men with benign prostatic hyperplasia. J Huazhong Univ Sci Technolog Med Sci. 2017;37:531–535. [DOI] [PubMed] [Google Scholar]
- 88. Fowke JH, Murff HJ, Signorello LB, Lund L, Blot WJ. Race and socioeconomic status are independently associated with benign prostatic hyperplasia. J Urol. 2008;180:2091–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hellwege JN, Stallings S, Torstenson ES, Carroll R, Borthwick KM, Brilliant MH, et al. Heritability and genome‐wide association study of benign prostatic hyperplasia (BPH) in the eMERGE network. Sci Rep. 2019;9:6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cheng G, Dai M, Xin Q, Wang L, Kong F, Xu D. Patients with benign prostatic hyperplasia show shorter leukocyte telomere length but no association with telomerase gene polymorphisms in Han Chinese males. Int J Clin Exp Pathol. 2020;13:2123–2129. [PMC free article] [PubMed] [Google Scholar]
- 91. Bayat H, Narouie B, Ziaee SM, Mowla SJ. Two long non‐coding RNAs, Prcat17.3 and Prcat38, could efficiently discriminate benign prostate hyperplasia from prostate cancer. Prostate. 2018;78:812–818. [DOI] [PubMed] [Google Scholar]
- 92. Greco F, Inferrera A, La Rocca R, Navarra M, Casciaro M, Grosso G, et al. The potential role of MicroRNAs as biomarkers in benign prostatic hyperplasia: a systematic review and meta‐analysis. Eur Urol Focus. 2019;5:497–507. [DOI] [PubMed] [Google Scholar]
- 93. Ali A, Du Feu A, Oliveira P, Choudhury A, Bristow RG, Baena E. Prostate zones and cancer: lost in transition? Nat Rev Urol. 2022;19:101–115. [DOI] [PubMed] [Google Scholar]
- 94. Yoshizako T, Wada A, Hayashi T, Uchida K, Sumura M, Uchida N, et al. Usefulness of diffusion‐weighted imaging and dynamic contrast‐enhanced magnetic resonance imaging in the diagnosis of prostate transition‐zone cancer. Acta Radiol. 2008;49:1207–1213. [DOI] [PubMed] [Google Scholar]
- 95. Garcia JJ, Al‐Ahmadie HA, Gopalan A, Tickoo SK, Scardino PT, Reuter VE, et al. Do prostatic transition zone tumors have a distinct morphology? Am J Surg Pathol. 2008;32:1709–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. McNeal J. Pathology of benign prostatic hyperplasia. Insight into etiology. Urol Clin North Am. 1990;17:477–486. [PubMed] [Google Scholar]
- 97. Alonso‐Magdalena P, Brossner C, Reiner A, Cheng G, Sugiyama N, Warner M, et al. A role for epithelial‐mesenchymal transition in the etiology of benign prostatic hyperplasia. Proc Natl Acad Sci USA. 2009;106:2859–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fraga CH, True LD, Kirk D. Enhanced expression of the mesenchymal marker, vimentin, in hyperplastic versus normal human prostatic epithelium. J Urol. 1998;159:270–274. [DOI] [PubMed] [Google Scholar]
- 99. Sakurai A, Nakai A, DeGroot LJ. Expression of three forms of thyroid hormone receptor in human tissues. Mol Endocrinol. 1989;3:392–399. [DOI] [PubMed] [Google Scholar]
- 100. Eldhose A, Nandeesha H, Dorairajan LN, Sreenivasulu K, Arul Vijaya Vani S. Thyroid and parathyroid hormones in benign prostatic hyperplasia. Br J Biomed Sci. 2016;73:94–96. [DOI] [PubMed] [Google Scholar]
- 101. McKeehan WL, Adams PS, Rosser MP. Direct mitogenic effects of insulin, epidermal growth factor, glucocorticoid, cholera toxin, unknown pituitary factors and possibly prolactin, but not androgen, on normal rat prostate epithelial cells in serum‐free, primary cell culture. Cancer Res. 1984;44:1998–2010. [PubMed] [Google Scholar]
- 102. Hammarsten J, Hogstedt B. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur Urol. 2001;39:151–158. [DOI] [PubMed] [Google Scholar]
- 103. Xia BW, Zhao SC, Chen ZP, Chen C, Liu TS, Yang F, et al. The underlying mechanism of metabolic syndrome on benign prostatic hyperplasia and prostate volume. Prostate. 2020;80:481–490. [DOI] [PubMed] [Google Scholar]
- 104. Chen Z, Miao L, Gao X, Wang G, Xu Y. Effect of obesity and hyperglycemia on benign prostatic hyperplasia in elderly patients with newly diagnosed type 2 diabetes. Int J Clin Exp Med. 2015;8:11289–11294. [PMC free article] [PubMed] [Google Scholar]
- 105. Bonkhoff H. Neuroendocrine cells in benign and malignant prostate tissue: morphogenesis, proliferation, and androgen receptor status. Prostate Suppl. 1998;8:18–22. [PubMed] [Google Scholar]
- 106. Xue Y, Smedts F, Verhofstad A, Debruyne F, de la Rosette J, Schalken J. Cell kinetics of prostate exocrine and neuroendocrine epithelium and their differential interrelationship: new perspectives. Prostate Suppl. 1998;8:62–73. [PubMed] [Google Scholar]
- 107. Cockett AT, di Sant'Agnese PA, Gopinath P, Schoen SR, Abrahamsson PA. Relationship of neuroendocrine cells of prostate and serotonin to benign prostatic hyperplasia. Urology. 1993;42:512–519. [DOI] [PubMed] [Google Scholar]
- 108. Kyoda Y, Ichihara K, Hashimoto K, Kobayashi K, Fukuta F, Masumori N. Distribution of neuroendocrine cells in the transition zone of the prostate. Adv Urol. 2017;2017:8541697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kyoda Y, Ichihara K, Hashimoto K, Kobayashi K, Fukuta F, Masumori N. Sustained density of neuroendocrine cells with aging precedes development of prostatic hyperplasia in spontaneously hypertensive rats. BMC Urol. 2019;19:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ratajczak W, Lubkowski M, Lubkowska A. Heat shock proteins in benign prostatic hyperplasia and prostate cancer. Int J Mol Sci. 2022;23:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hata J, Machida T, Matsuoka K, Hoshi S, Akaihata H, Hiraki H, et al. Complement activation by autoantigen recognition in the growth process of benign prostatic hyperplasia. Sci Rep. 2019;9:20357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kim HT, Kim YJ, Park SR, Ryu SY, Jung JY. NAD(P)H‐quinone oxidoreductase 1 silencing aggravates hormone‐induced prostatic hyperplasia in mice. Andrologia. 2018;50:e12906. [DOI] [PubMed] [Google Scholar]
- 113. Smart JA, Oleksak JE, Hartsough EJ. Cell adhesion molecules in plasticity and metastasis. Mol Cancer Res. 2021;19:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Li F, Pascal LE, Stolz DB, Wang K, Zhou Y, Chen W, et al. E‐cadherin is downregulated in benign prostatic hyperplasia and required for tight junction formation and permeability barrier in the prostatic epithelial cell monolayer. Prostate. 2019;79:1226–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Roumeguere T, Sfeir J, El Rassy E, Albisinni S, Van Antwerpen P, Boudjeltia KZ, et al. Oxidative stress and prostatic diseases. Mol Clin Oncol. 2017;7:723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Vital P, Castro P, Ittmann M. Oxidative stress promotes benign prostatic hyperplasia. Prostate. 2016;76:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Aydin A, Arsova‐Sarafinovska Z, Sayal A, Eken A, Erdem O, Erten K, et al. Oxidative stress and antioxidant status in non‐metastatic prostate cancer and benign prostatic hyperplasia. Clin Biochem. 2006;39:176–179. [DOI] [PubMed] [Google Scholar]
- 118. Minutoli L, Rinaldi M, Marini H, Irrera N, Crea G, Lorenzini C, et al. Apoptotic pathways linked to endocrine system as potential therapeutic targets for benign prostatic hyperplasia. Int J Mol Sci. 2016;17:1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zenzmaier C, Untergasser G, Hermann M, Dirnhofer S, Sampson N, Berger P. Dysregulation of Dkk‐3 expression in benign and malignant prostatic tissue. Prostate. 2008;68:540–547. [DOI] [PubMed] [Google Scholar]
- 120. Zenzmaier C, Sampson N, Plas E, Berger P. Dickkopf‐related protein 3 promotes pathogenic stromal remodeling in benign prostatic hyperplasia and prostate cancer. Prostate. 2013;73:1441–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Minutoli L, Altavilla D, Marini H, Rinaldi M, Irrera N, Pizzino G, et al. Inhibitors of apoptosis proteins in experimental benign prostatic hyperplasia: effects of serenoa repens, selenium and lycopene. J Biomed Sci. 2014;21:19. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 122. Takezawa K, Fujita K, Matsushita M, Motooka D, Hatano K, Banno E, et al. The Firmicutes/Bacteroidetes ratio of the human gut microbiota is associated with prostate enlargement. Prostate. 2021;81:1287–1293. [DOI] [PubMed] [Google Scholar]
- 123. An J, Song Y, Kim S, Kong H, Kim K. Alteration of gut microbes in benign prostatic hyperplasia model and finasteride treatment model. Int J Mol Sci. 2023;24:5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Li LY, Han J, Wu L, Fang C, Li WG, Gu JM, et al. Alterations of gut microbiota diversity, composition and metabonomics in testosterone‐induced benign prostatic hyperplasia rats. Mil Med Res. 2022;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Dong W, Zheng J, Huang Y, Tan H, Yang S, Zhang Z, et al. Sodium butyrate treatment and fecal microbiota transplantation provide relief from ulcerative colitis‐induced prostate enlargement. Front Cell Infect Microbiol. 2022;12:1037279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Matsushita M, Fujita K, Motooka D, Hatano K, Hata J, Nishimoto M, et al. Firmicutes in gut microbiota correlate with blood testosterone levels in elderly men. World J Mens Health. 2022;40:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Shin JH, Park YH, Sim M, Kim SA, Joung H, Shin DM. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol. 2019;170:192–201. [DOI] [PubMed] [Google Scholar]
- 128. Lewis DA, Brown R, Williams J, White P, Jacobson SK, Marchesi JR, et al. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front Cell Infect Microbiol. 2013;3:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tsai KY, Wu DC, Wu WJ, Wang JW, Juan YS, Li CC, et al. Exploring the association between gut and urine microbiota and prostatic disease including benign prostatic hyperplasia and prostate cancer using 16S rRNA sequencing. Biomedicine. 2022;10:2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lee HY, Wang JW, Juan YS, Li CC, Liu CJ, Cho SY, et al. The impact of urine microbiota in patients with lower urinary tract symptoms. Ann Clin Microbiol Antimicrob. 2021;20:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Thomas S, Dunn CD, Campbell LJ, Strand DW, Vezina CM, Bjorling DE, et al. A multi‐omic investigation of male lower urinary tract symptoms: potential role for JC virus. PLoS One. 2021;16:e0246266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yu H, Meng H, Zhou F, Ni X, Shen S, Das UN. Urinary microbiota in patients with prostate cancer and benign prostatic hyperplasia. Arch Med Sci. 2015;11:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Okada K, Takezawa K, Tsujimura G, Imanaka T, Kuribayashi S, Ueda N, et al. Localization and potential role of prostate microbiota. Front Cell Infect Microbiol. 2022;12:1048319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jain S, Samal AG, Das B, Pradhan B, Sahu N, Mohapatra D, et al. Escherichia coli, a common constituent of benign prostate hyperplasia‐associated microbiota induces inflammation and DNA damage in prostate epithelial cells. Prostate. 2020;80:1341–1352. [DOI] [PubMed] [Google Scholar]
- 135. Breyer BN, Huang WY, Rabkin CS, Alderete JF, Pakpahan R, Beason TS, et al. Sexually transmitted infections, benign prostatic hyperplasia and lower urinary tract symptom‐related outcomes: results from the prostate, lung, colorectal and ovarian cancer screening trial. BJU Int. 2016;117:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Mitteregger D, Aberle SW, Makristathis A, Walochnik J, Brozek W, Marberger M, et al. High detection rate of trichomonas vaginalis in benign hyperplastic prostatic tissue. Med Microbiol Immunol. 2012;201:113–116. [DOI] [PubMed] [Google Scholar]
- 137. Kim JH, Kim SS, Han IH, Sim S, Ahn MH, Ryu JS. Proliferation of prostate stromal cell induced by benign prostatic hyperplasia epithelial cell stimulated with trichomonas vaginalis via crosstalk with mast cell. Prostate. 2016;76:1431–1444. [DOI] [PubMed] [Google Scholar]
- 138. Kim SS, Kim JH, Han IH, Ahn MH, Ryu JS. Inflammatory responses in a benign prostatic hyperplasia epithelial cell line (BPH‐1) infected with trichomonas vaginalis. Korean J Parasitol. 2016;54:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kim JH, Han IH, Shin SJ, Park SY, Chung HY, Ryu JS. Signaling role of adipocyte leptin in prostate cell proliferation induced by trichomonas vaginalis. Korean J Parasitol. 2021;59:235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kristal AR, Arnold KB, Schenk JM, Neuhouser ML, Goodman P, Penson DF, et al. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol. 2008;167:925–934. [DOI] [PubMed] [Google Scholar]
- 141. Lagiou P, Wuu J, Trichopoulou A, Hsieh CC, Adami HO, Trichopoulos D. Diet and benign prostatic hyperplasia: a study in Greece. Urology. 1999;54:284–290. [DOI] [PubMed] [Google Scholar]
- 142. Vignozzi L, Morelli A, Sarchielli E, Comeglio P, Filippi S, Cellai I, et al. Testosterone protects from metabolic syndrome‐associated prostate inflammation: an experimental study in rabbit. J Endocrinol. 2012;212:71–84. [DOI] [PubMed] [Google Scholar]
- 143. Zhang ZH, Luo B, Xu S, Fu L, Chen YH, Zhang C, et al. Vitamin D deficiency promotes prostatic hyperplasia in middle‐age mice through exacerbating local inflammation. J Steroid Biochem Mol Biol. 2018;182:14–20. [DOI] [PubMed] [Google Scholar]
- 144. Li YZ, Shi BK, Li JY, Zhu XW, Liu J, Liu YL. Role of p‐ERK1/2 in benign prostatic hyperplasia during hyperinsulinemia. Urol J. 2020;18:225–229. [DOI] [PubMed] [Google Scholar]
- 145. Shibamori K, Kyoda Y, Shindo T, Hashimoto K, Kobayashi K, Tanaka T, et al. Maternal diet during gestation affect prostatic tissue component in SHR/Izm offspring. Prostate. 2024;84:303–314. [DOI] [PubMed] [Google Scholar]


