Abstract
Objectives
To investigate roles of brain carbon monoxide (CO), an endogenous gasotransmitter, in regulation of the rat micturition reflex.
Methods
In urethane‐anesthetized (0.8 g/kg, ip) male rats, evaluation of urodynamic parameters was started 1 h before intracerebroventricular administration of CORM‐3 (CO donor) or ZnPP (non‐selective inhibitor of heme oxygenase, a CO producing enzyme) and continued for 2 h after the administration. We also investigated effects of centrally pretreated SR95531 (GABAA receptor antagonist) or SCH50911 (GABAB receptor antagonist) on the CORM‐3‐induced response.
Results
CORM‐3 significantly prolonged intercontraction intervals (ICIs) without changing maximal voiding pressure (MVP), while ZnPP significantly shortened ICI and reduced single‐voided volume and bladder capacity without affecting MVP, post‐voided residual volume, or voiding efficiency. The ZnPP‐induced ICI shortening was reversed by CORM‐3. The CORM‐3‐induced ICI prolongation was significantly attenuated by centrally pretreated SR95531 or SCH50911, respectively.
Conclusions
Brain CO can suppress the rat micturition reflex through brain γ‐aminobutyric acid (GABA) receptors.
Keywords: brain, carbon monoxide, GABAA receptor, GABAB receptor, micturition reflex
Abbreviations
- ANOVA
analysis of variance
- BC
bladder capacity
- CO
carbon monoxide
- DMF
N, N‐dimethylformamide
- GABA
γ‐aminobutyric acid
- HO
heme oxygenase
- ICI
intercontraction interval
- ICV
intracerebroventricularly
- LUTS
lower urinary tract symptoms
- MCAO
middle cerebral artery occlusion
- MVP
maximal voiding pressure
- PMC
pontine micturition center
- RV
post‐voided residual volume
- SON
supraoptic nucleus
- VE
voiding efficiency
- VV
single‐voided volume
INTRODUCTION
A toxic gas carbon monoxide (CO) is endogenously produced gasotransmitter 1 via heme oxygenase (HO)‐mediated degradation of heme to biliverdin. 2 CO has a variety of physical functions including vasomodulation, neuromodulation, and cytoprotection. 3 , 4 , 5 Alteration in endogenous CO synthesis is associated with several diseases such as atherosclerosis, 6 hypertension, 7 and neurodegenerative diseases. 5 Therefore, the HO‐mediated CO production can play an important role as a biodefense. Furthermore, low‐level CO has been explored as a potential therapeutic factor in many different pathogenesis models including cardiovascular and neurovegetative diseases. 5 In the brain, endogenous low‐level CO production can play several biological roles, including neuroprotection. 8
HO has three isozymes. 2 HO‐1 is an inducible type and is localized in the liver, spleen, brain, skin, and heart. 9 HO‐2 is a constitutive type, and its localization is widely distributed, with particularly higher concentrations in the brain and testis. 10 HO‐3 is only expressed in the rat brain, but its activity is extremely low 11 and is considered as a retrotransposon of HO‐2. 12 Under physiological conditions, HO‐2 is a major brain isoform, expressing in the olfactory bulb, cortex, hippocampus, hippocampal granule cell, and cerebellum. 11 HO‐2‐mediated CO production is increased through various physiological stimulations to maintain neural function, 13 whereas basal brain HO‐1 expression level is low, restricted in the dentate gyrus, ventromedial hypothalamus, and the brain stem. 11 However, under stressful stimulation such as hypothermia, cerebral ischemia, hemorrhage or trauma, brain HO‐1 expression is increased. 11
The coordination between the peripheral and central nervous systems is important for the control of urinary function. 14 The pontine micturition center (PMC) regulates micturition in an inhibitory manner with signals from the cerebrum. Regarding the role of CO in urinary function, there are a few reports showing the CO role in regulation of the micturition reflex as a relaxation factor in the urethral smooth muscle. 15 , 16 However, the regulatory role of brain CO remains unclear. Thus, we investigated the effects of centrally administered CORM‐3 (CO donor) and ZnPP (non‐selective HO inhibitor) on the rat micturition reflex to elucidate the role of brain CO in its regulation.
METHODS
Ethical approval and animals
The animal experimental protocol was approved by the Institutional Animal Care and Use Committee of Kochi University (protocol numbers: N‐84/O‐57/P‐67). The animal experiments are performed according to the ARRIVE guidelines. 17 A total of 112 male Wister rats (Japan SLC Inc., Hamamatsu, Japan) weighing 350–450 g were used. These rats housed two per cage and were maintained in an air‐conditioned room at 22–24°C under the 14/10 h light/dark cycle with lights on at 05:00 a.m. They were given food (laboratory chow, CE‐2; Clea Japan, Hamamatsu, Japan) and water ad libitum and were randomly divided into 16 groups and used for experiments.
Experimental surgery
Under urethane anesthesia (0.8 g/kg, IP), cystometry was performed in rats placed in a stereotaxic apparatus for the brain (SR‐6R; Narishige, Tokyo, Japan) according to previous procedures. 18 The stereotaxic coordinates of the tip of an injection stainless‐steel cannula (outer diameter, 0.3 mm) were as follows (in mm): AP −0.8, L 1.5, V 4.5 (AP, anterior from the bregma; L, lateral from the midline; V, below the surface of the brain), according to the rat brain atlas. 19 The cannula was injected into the left lateral ventricle 3 h after the surgery, and each drug was administered slowly. When drugs were intravenously administered, a PE50‐catheter filled with heparinized saline (100 U/mL) was cannulated into the femoral vein and the administration was performed through the catheter without placing in the stereotaxic apparatus.
Drug administration
CORM‐3, SR95531 (GABAA receptor antagonist), and SCH50911 (GABAB receptor antagonist) were dissolved in saline. ZnPP was dissolved in 1% N, N‐dimethylformamide (DMF)/saline. CORM‐3 (1 or 10 nmol in 10 μL/rat), ZnPP (10 or 30 nmol in 5 μL/rat), SR95531 (0.1 nmol in 10 μL/rat), and SCH50911 (0.1 nmol in 10 μL/rat) were injected slowly into the left lateral ventricle at the rate of 10 μL/min using a cannula connected to a 10‐μL Hamilton syringe (Hamilton, Reno, NV, USA). When CORM‐3 (10 nmol/rat), SR95531 (0.1 nmol/rat), or SCH50911 (0.1 nmol/rat) was pretreated, the cannula was retained in the ventricle for 15 min to circumvent each drug leakage and then removed from the ventricle. Thirty minutes after each pretreatment, ZnPP (30 nmol/rat) or CORM‐3 (10 nmol/rat) was intracerebroventricularly (ICV) administered. When CORM‐3 (10 nmol/rat) or ZnPP (30 nmol/rat) was intravenously administered, each drug solution was injected via a venous catheter (200 μL/rat).
Two hours after the last ICV injection, cresyl violet was administered into the same ventricular part; then, each rat was euthanized with an overdose of pentobarbital (80 mg/kg, IP). Subsequently, each rat was decapitated, and the brain was removed to check whether the cannula was injected into the exact brain location. Five rats were ruled out due to cannula misplacement; then, the data were obtained from 107 rats in this study.
Cystometry
Continuous and single cystometry were performed according to previous procedures to evaluate intercontraction interval (ICI), maximal voiding pressure (MVP), single‐voided volume (VV), post‐voided residual volume (RV), bladder capacity (BC), and voiding efficiency (VE). 18 When performing continuous cystometry, 2 h after the surgery, intravesical instillation of saline was started (12 mL/h), and each drug was injected 1 h after the start. Subsequently, the instillation was continued for 2 h after administration of CORM‐3 or ZnPP. When performing single cystometry, saline was intravesically instilled at a rate of 12 mL/h and the cystometry was performed for 30 min before and for 30 min after the ICV administration of ZnPP.
Data and statistical analyses
All data are presented as mean ± standard error of the mean. Relative values of ICI and MVP were calculated as the ratio of averaged ICI and MVP measured for each 30 min after CORM‐3 or ZnPP administration to those measured each 30 min before administration. Relative values of VV, RV, BC, and VE were calculated as the ratio of averaged each parameter measured for 30 min after ZnPP administration to those measured for 30 min before administration. These data were analyzed by an investigator (N.S.) blinded to experimental conditions. Based on the expected difference in a desired endpoint measurement between the test and control groups, the sample size in each experimental group was determined. Statistical differences were determined using one‐way analysis of variance (ANOVA) followed by a post hoc analysis with the Bonferroni method. When two means were compared, an unpaired Student's t‐test was used, and in single cystometry, a paired t‐test was used in the study before and after ICV‐administered ZnPP. p Values <0.05 were taken to indicate statistical significance.
Drugs and chemicals
We purchased urethane (Sigma Aldrich, St Louis, MO, USA), DMF (Nacalai Tesque, Kyoto, Japan), CORM‐3 (Tricarbonchloro(glycinato)ruthenium) (Tocris Bioscience, Bristol, UK), ZnPP (Zinc (II) Protoporphyrin IX) (MedChemExpress, NJ, USA), SR95531 (2‐(3‐carboxypropyl)‐6‐(4‐methoxyphenyl)‐2,3‐dihydropyridazin‐3‐iminium bromide) (Tocris Bioscience), and SCH50911 ((+)‐(2S)‐5,5‐dimethyl‐2‐morpholineacetic acid hydrochloride) (Tocris Bioscience). All other chemicals were commercially available.
RESULTS
Effect of ICV‐administered CORM‐3 on the micturition reflex
There was no significant difference in baseline values of ICI or MVP prior to vehicle or CORM‐3 administration among the three groups (Table 1).
TABLE 1.
Baseline values of intercontraction interval (ICI) and maximal voiding pressure (MVP) during the −30 to 0 min period in rats of Figures 2 and 4.
| ICI (s) | MVP (cmH2O) | |
|---|---|---|
| Vehicle (saline, 10 μL/rat, ICV) (n = 8) | 254.9 ± 37.5 | 42.2 ± 3.5 |
| CORM‐3 (1 nmol/rat, ICV) (n = 7) | 277.3 ± 46.1 | 50.3 ± 4.9 |
| CORM‐3 (10 nmol/rat, ICV) (n = 7) | 206.5 ± 26.8 | 38.1 ± 7.5 |
| Vehicle (1% DMF/saline, 5 μL/rat, ICV) (n = 7) | 288.2 ± 10.7 | 45.8 ± 2.5 |
| ZnPP (10 nmol/rat, ICV) (n = 8) | 285.4 ± 40.8 | 43.7 ± 2.5 |
| ZnPP (30 nmol/rat, ICV) (n = 9) | 213.2 ± 28.6 | 47.6 ± 3.8 |
Note: Values are means ± standard error of the mean. The number of animals per group is indicated in parentheses.
Abbreviations: DMF, N, N‐dimethylformamide; ICV, intracerebroventricularly.
ICV‐administered CORM‐3 did not affect ICI or MVP at a lower dose (1 nmol/rat), while at a higher dose (10 nmol/rat), CORM‐3 significantly prolonged ICI without affecting MVP during the 30–60 min period compared with the vehicle‐treated group (Figures 1 and 2).
FIGURE 1.
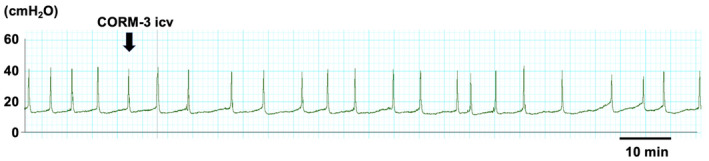
A representative in vivo continuous cystometry trace. CORM‐3 (10 nmol/rat) was administered via intracerebroventricularly injection. The arrow indicates the timing of CORM‐3 injection.
FIGURE 2.
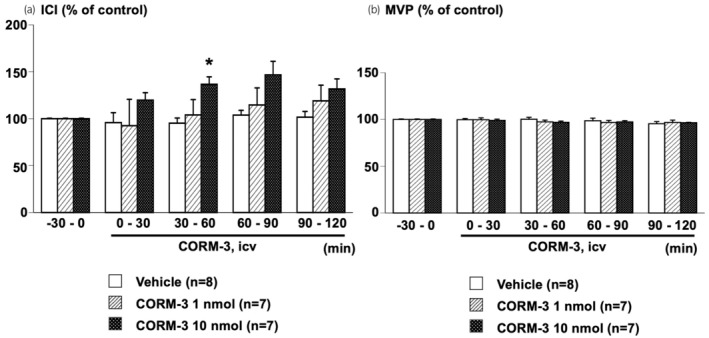
Effects of centrally administered CORM‐3 on urodynamics parameters, intercontraction interval (ICI) (a) and maximal voiding pressure (MVP) (b), of continuous cystometry. Data were calculated as a ratio of baseline values during the −30 to 0 min period before central administration of CORM‐3 (1 or 10 nmol/rat, intracerebroventricularly [ICV]) or vehicle (saline, 10 μL/rat, ICV). Values are means ± standard error of the mean. *p < 0.05, when compared to ANOVA, followed by post hoc analysis of the Vehicle group using the Bonferroni method. The number of animals per group is shown in parentheses.
Effect of intravenously administered CORM‐3 on the micturition reflex
There was no significant difference in baseline values of ICI prior to vehicle or CORM‐3 administration between the two groups (Table 2).
TABLE 2.
Baseline values of intercontraction interval (ICI) during the −30 to 0 min period in rats of Table 3.
| ICI (s) | |
|---|---|
| Vehicle (saline, 200 μL/rat, IV) (n = 5) | 207.3 ± 36.8 |
| CORM‐3 (10 nmol/rat, IV) (n = 5) | 247.2 ± 38.3 |
| Vehicle (1% N, N‐dimethylformamide/saline, 200 μL/rat, IV) (n = 5) | 227.5 ± 40.3 |
| ZnPP (30 nmol/rat, IV) (n = 5) | 265.2 ± 18.8 |
Note: Values are means ± standard error of the mean. The number of animals per group is indicated in parentheses.
Intravenously administered CORM‐3 showed no significant effect on ICI (Table 3).
TABLE 3.
Effect of intravenously administered CORM‐3 or ZnPP on intercontraction intervals.
| Before IV (%) | After IV (%) | ||||
|---|---|---|---|---|---|
| 0–30 min | 30–60 min | 60–90 min | 90–120 min | ||
| Vehicle (saline) (n = 5) | 100.0 ± 0.0 | 102.1 ± 3.9 | 105.5 ± 2.1 | 120.3 ± 11.9 | 111.3 ± 4.6 |
| CORM‐3 (10 nmol) (n = 5) | 100.0 ± 0.0 | 109.0 ± 5.4 | 97.4 ± 7.2 | 92.8 ± 1.9 | 101.3 ± 8.7 |
| Vehicle (1% DMF in saline) (n = 5) | 100.0 ± 0.0 | 105.5 ± 5.7 | 101.0 ± 5.5 | 106.2 ± 5.9 | 121.0 ± 10.5 |
| ZnPP (30 nmol) (n = 5) | 100.0 ± 0.0 | 101.3 ± 5.3 | 95.0 ± 3.1 | 96.3 ± 5.1 | 97.3 ± 6.7 |
Note: Data are calculated as a ratio of baseline values during the −30 to 0 min period. IV: intravenous administration of CORM‐3 (10 nmol/rat), vehicle (saline, 200 μL/rat), ZnPP (30 nmol/rat), or vehicle (1% N, N‐dimethylformamide in saline, 200 μL/rat). Values are means ± standard error of the mean. The number of animals per group is indicated in parentheses.
Abbreviation: DMF, N, N‐dimethylformamide.
Effect of ICV‐administered ZnPP on the micturition reflex
There was no significant difference in baseline values of ICI or MVP prior to vehicle or ZnPP administration among the three groups (Table 1).
Compared with the vehicle‐treated group, ICV‐administered ZnPP (30 nmol/rat) significantly shortened ICI without affecting MVP during the 0–30 min period. The ZnPP‐induced shortening gradually abolished 30 min after the ZnPP treatment (Figures 3 and 4).
FIGURE 3.
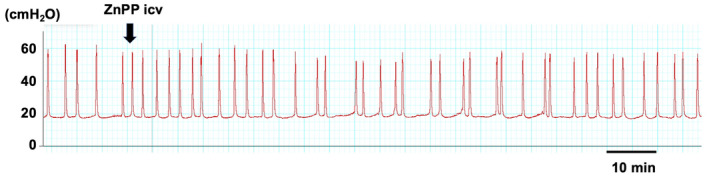
A representative in vivo continuous cystometry trace. ZnPP (30 nmol/rat) was administered through intracerebroventricularly injection. The arrow indicates the timing of ZnPP injection.
FIGURE 4.
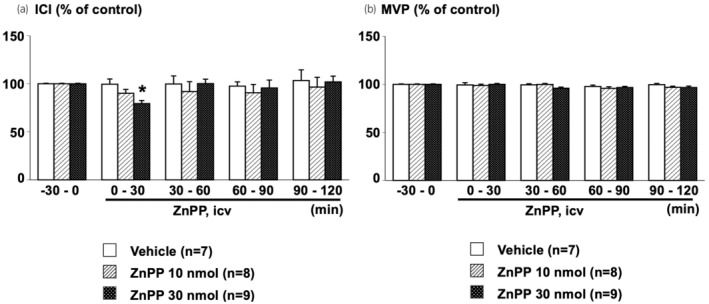
Effects of centrally administered ZnPP on urodynamic parameters, intercontraction interval (ICI) (a) and maximal voiding pressure (MVP) (b), of continuous cystometry. Data were calculated as a ratio of baseline values during the −30 to 0 min period before central administration of ZnPP (10 or 30 nmol/rat, intracerebroventricularly [ICV]) or vehicle (1% DMF in saline, 5 μL/rat, ICV). Values are means ± standard error of the mean. *p < 0.05, when compared to ANOVA, followed by post hoc analysis of the Vehicle group using the Bonferroni method. The number of animals per group is shown in parentheses.
Effect of ICV‐administered ZnPP on urodynamic parameters in single cystometry
The baseline values of VV, RV, BC, and VE during the −30 to 0 min period are shown in Table 4. ICV‐administered ZnPP (30 nmol/rat) significantly decreased VV and BC without changing RV or VE during the 0–30 min period compared with the −30 to 0 min period (Table 5).
TABLE 4.
Baseline values of VV, RV, BC and VE during the −30 to 0 min period in rats of Table 5.
| VV (mL) | RV (mL) | BC (mL) | VE (%) | |
|---|---|---|---|---|
| ZnPP (30 nmol/rat, ICV) (n = 9) | 1.0 ± 0.1 | 0.6 ± 0.1 | 1.6 ± 0.2 | 61.8 ± 7.3 |
Note: Values are means ± standard error of the mean. The number of animals per group is indicated in parentheses.
Abbreviations: BC, bladder capacity; ICV, intracerebroventricularly; RV, post‐voided residual volume; VE, voiding efficiency; VV, single‐voided volume.
TABLE 5.
Effects of centrally administered ZnPP on urodynamic parameters of single cystometry.
| Before ICV (%) | After ICV 0–30 min (%) | |
|---|---|---|
| VV | 100.0 ± 0.0 | 84.0 ± 6.8* |
| RV | 100.0 ± 0.0 | 84.0 ± 9.8 |
| BC | 100.0 ± 0.0 | 85.3 ± 6.6* |
| VE | 100.0 ± 0.0 | 100.8 ± 4.0 |
Note: Data are calculated as a ratio of baseline values before central administration of ZnPP (30 nmol/rat, ICV) (n = 9). Values are means ± standard error of the mean.
Abbreviations: BC, bladder capacity; ICV, intracerebroventricularly; RV, post‐voided residual volume; VE, voiding efficiency; VV, single‐voided volume.
p < 0.05, when compared to “Before ICV” using paired t‐test.
Effect of intravenously administered ZnPP on the micturition reflex
There was no significant difference in baseline values of ICI prior to vehicle or ZnPP administration between the two groups (Table 2).
Intravenously administered ZnPP showed no significant effect on ICI (Table 3).
Effects of ICV‐pretreated CORM‐3 on ZnPP‐induced ICI shortening
There was no significant difference in the baseline values of ICI prior to vehicle or ZnPP administration between the two groups (Table 6).
TABLE 6.
Baseline values of ICI during the −30 to 0 min period in rats of Figure 5.
| ICI (s) | |
|---|---|
| Vehicle (saline, 10 μL/rat, ICV) + ZnPP (30 nmol/rat, ICV) (n = 7) | 292.1 ± 36.0 |
| CORM‐3 (10 nmol/rat, ICV) + ZnPP (30 nmol/rat, ICV) (n = 7) | 325.4 ± 41.4 |
Note: Values are means ± standard error of the mean. The number of animals per group is indicated in parentheses.
Abbreviations: ICI, intercontraction interval; ICV, intracerebroventricularly.
ICV‐administered ZnPP (30 nmol/rat) significantly shortened ICI in the vehicle‐pretreated group, while in the CORM‐3‐pretreated group, ZnPP showed longer ICI compared with the vehicle‐pretreated group (Figure 5).
FIGURE 5.
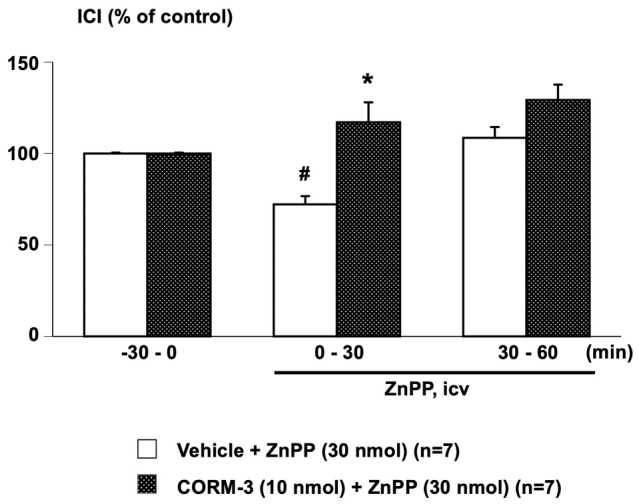
Effects of central pretreatment with CORM‐3 on ZnPP‐induced intercontraction interval (ICI) shortening. Data were calculated as a ratio to baseline values during the −30 to 0 min period before central administration of ZnPP. CORM‐3 (10 nmol/rat) was intracerebroventricularly (ICV) administrated 30 min before the central administration of ZnPP (30 nmol/rat, ICV). Values are means ± standard error of the mean. *p < 0.05, when compared to an unpaired Student's t‐test to the Vehicle + ZnPP group. The number of animals per group is shown in parentheses. # p < 0.05, when values were compared with ANOVA followed by post hoc analysis with the Bonferroni method to values prior to Vehicle + ZnPP administration (−30 to 0 min).
Effects of ICV‐pretreated SR95531 or SCH50911 on CORM‐3‐induced ICI prolongation
There was no significant difference in the baseline values of ICI prior to vehicle or CORM‐3 administration among the three groups (Table 7).
TABLE 7.
Baseline values of ICI during the −30 to 0 min period in rats of Figure 6.
| ICI (s) | |
|---|---|
| Vehicle (saline, 10 μL/rat, ICV) + CORM‐3 (10 nmol/rat, ICV) (n = 6) | 271.6 ± 15.8 |
| SR95531 (0.1 nmol/rat, ICV) + CORM‐3 (10 nmol/rat, ICV) (n = 6) | 338.0 ± 35.8 |
| SCH50911 (0.1 nmol/rat, ICV) + CORM‐3 (10 nmol/rat, ICV) (n = 6) | 310.5 ± 51.9 |
Note: Values are means ± standard error of the mean. The number of animals per group is indicated in parentheses.
Abbreviations: ICI, intercontraction interval; ICV, intracerebroventricularly.
ICV‐administered CORM‐3 significantly prolonged ICI during the 90–120 min period in the vehicle‐pretreated group, while ICV‐pretreated SR95531 or SCH50911 significantly suppressed the CORM‐3‐induced ICI prolongation (Figure 6).
FIGURE 6.
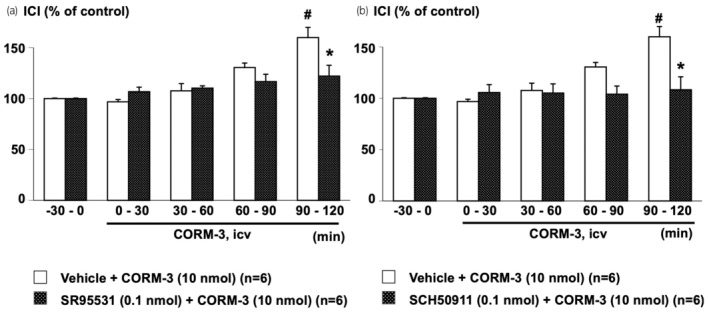
Effects of central pretreatment with SR95531 (a GABAA receptor antagonist, a) or SCH50911 (a GABAB receptor antagonist, b) on CORM‐3‐induced intercontraction interval (ICI) prolongation. Data were calculated as a ratio to baseline values during the −30 to 0 min period before central administration of CORM‐3. SR95531 (SR, 0.1 nmol/rat), SCH50911 (SCH, 0.1 nmol/rat), or vehicle (saline, 10 μL/rat) was intracerebroventricularly (ICV) pretreated 30 min before the central administration of CORM‐3 (10 nmol/rat, ICV). The Vehicle + CORM‐3 group is the same in (a) and (b). Values are means ± standard error of the mean. *p < 0.05, when compared to an unpaired Student's t‐test to the Vehicle + CORM‐3 group. # p < 0.05, when values were compared with ANOVA followed by post hoc analysis with the Bonferroni method to values prior to CORM‐3 administration (−30 to 0 min). The number of animals per group is shown in parentheses.
DISCUSSION
Our present data indicated that (1) ICV‐administered CORM‐3 prolonged ICI without affecting MVP, while intravenous administration of CORM‐3 had no effect on ICI; (2) although ICV administered ZnPP induced ICI shortening and reductions in VV and BC without affecting MVP or RV, intravenous administration of ZnPP had no effect on ICI; (3) the ZnPP‐induced ICI shortening was reversed by central pretreatment with CORM‐3; and (4) the CORM‐3‐induced ICI prolongation was suppressed by central pretreatment with SR95531 or SCH50911. These results suggest that brain CO can suppress the rat micturition reflex through brain γ‐aminobutyric acid (GABA) receptors.
First, we used CORM‐3, a CO donor delivering controlled amounts of CO to biological systems. 20 , 21 In general, responses induced by gasotransmitters are highly dependent on doses, the physiological response at lower doses and the non‐physiological effect at higher doses. In our preliminary experiments, ICV‐administered CORM‐3 at 1000 or 100 nmol per rat resulted in urinary retention or overflow incontinence, indicating that “non‐physiological” responses might be evoked by CORM‐3 at these higher doses. On the other hand, CORM‐3 at 10 nmol tended to prolong ICI. In this study, to evaluate effects of CORM‐3 on the micturition reflex under stable monitoring of the reflex, we investigated a dose‐dependency of CORM‐3 at 1 or 10 nmol per rat. ICV administered CORM‐3‐induced gradual prolongation of ICI without affecting MVP, suggesting that sustainable release of CO from CORM‐3 may induce the gradual prolongation. In addition, we examined whether intravenously administered CORM‐3 had effects on ICI to determine whether ICI prolongation evoked by ICV‐administered CORM‐3 was a central or peripheral effect due to the transfer of ICV‐administered CORM‐3 to the systemic circulation. Intravenously administered CORM‐3 at 10 nmol per rat, the same dose that induced ICI prolongation after ICV administration, had no effect on ICI. These results indicate that ICV‐administered CORM‐3 centrally suppressed the rat micturition reflex.
Subsequently, we used ZnPP (non‐selective HO inhibitor) to examine how inhibition of HO‐mediated CO production affected the micturition reflex. Endogenous CO is formed during degradation of heme to biliverdin by HO in the body. 2 In the rat brain, HO‐1 and HO‐2 are expressed predominantly. ZnPP primarily inhibits HO‐1 competitively but has also been reported to inhibit HO‐2. 22 In this study, ICV administered ZnPP induced ICI shortening without affecting MVP. In addition, we examined whether intravenously administered ZnPP had effects on ICI to determine whether ICI shortening evoked by ICV‐administered ZnPP was a central or peripheral effect due to the transfer of ICV‐administered ZnPP to the systemic circulation. Intravenously administered ZnPP at 30 nmol/rat, the same dose that induced ICI shortening after ICV administration, had no effect on ICI. These results indicate that ICV‐administered ZnPP centrally facilitated the rat micturition reflex.
ICI can be shortened via not only facilitation of the micturition reflex but also increment of residual urine volume. Therefore, we performed single cystometry to evaluate RV. ICV‐administered ZnPP reduced VV and BC without changing RV or VE. Based on these results, the ZnPP‐induced ICI shortening was due to promotion of the micturition reflex, but not due to increased RV. Since ICV‐administered ZnPP did not affect MVP, RV, or VE, parameters of bladder efferent activity, suppression of CO production by ZnPP may not excite the efferent pathway from the PMC. On the other hand, since ZnPP induced ICI shortening and reductions in VV and BC, ZnPP‐mediated suppression of CO production attenuated the inhibitory control from cerebral level to the PMC. From our results, it is presumed that the micturition reflex was promoted by ICV‐administered ZnPP. Taken together, ZnPP‐mediated inhibition of brain HO may promote the micturition reflex through reduction in brain CO production.
Since ZnPP is a non‐selective HO inhibitor, 22 we could not rule out the possibility that it acted on groups of enzymes other than the CO biosynthetic enzymes to promote the micturition reflex. Therefore, we examined the effect of CO supplementation in the brain on the ICV‐administered ZnPP‐induced ICI shortening. The ZnPP‐induced response was suppressed by ICV‐pretreated CORM‐3. These results indicate a possibility that supplementation of brain CO by central CORM‐3 pretreatment canceled the ICV‐administered ZnPP‐induced ICI shortening, suggesting that brain endogenous CO might play a suppressive role in regulation of the rat micturition reflex.
CO has been recognized as a neuromodulator that regulates vascular tone in response to glutamate 23 and effects on the hypothalamic supraoptic nucleus neuronal activity via GABA signaling. 24 The GABA is known as a central neurotransmitter involved in urinary suppression. 14 In fact, central administration of muscimol (GABAA receptor agonist) and baclofen (GABAB receptor agonist) suppressed the micturition reflex. 25 , 26 Therefore, we investigated the effect of ICV‐pretreated SR95531 (GABAA receptor antagonist) or SCH50911 (GABAB receptor antagonist) on the ICV‐administered CORM‐3‐induced ICI prolongation. We previously reported that SR95531 or SCH50911 at 0.1 nmol per rat showed no effect on ICI by itself, 27 while preliminary confirmed that each antagonist affected the micturition reflex by itself at a higher dose (0.3 nmol per rat). Therefore, pretreatment with each antagonist at 0.1 nmol per rat by itself showed no effect on the micturition reflex, indicating that the pretreatment could partially suppress the GABAergic nervous system at insufficient levels to change the micturition reflex. However, each pretreatment suppressed the CORM‐3‐induced ICI prolongation, indicating that brain CO inhibits the rat micturition reflex through both GABA receptors.
There are several limitations to this study. First, we performed experiments under anesthesia of urethane, which may influence urodynamics and neurotransmission. 28 , 29 Second, we did not measure the endogenous brain CO levels; therefore, we did not show that ICV‐administered ZnPP inhibited the production of endogenous brain CO. Since we injected ZnPP into the ventricle, we could not determine in which brain region the ZnPP influenced CO production and inhibited CO‐induced suppression of the micturition reflex. Therefore, it is difficult to determine in which brain region we should measure CO production. Further studies are necessary to investigate the specific brain regions that contribute to endogenous CO‐mediated regulation of the micturition reflex and to develop a method for control of the CO biosynthetic system in the brain. Furthermore, it is unknown by which neuronal circuit the produced CO act on the PMC via the GABAergic nervous system. Gaseous substances differ from liquid substances in that they easily pass through the cell membrane, interstitial spaces, and vascular wall by diffusion and move rapidly. Although this is a great advantage for signal transmitters, it is difficult to access their in vivo dynamics. Further research is needed in future regarding the methods used to regulate CO levels in the brain, which is by endogenously appropriated CO such as regulation of HO‐mediated heme degradation reaction, low‐concentration CO inhalation, or CORM‐3 administration.
Currently, there are a lot of elderly peoples with lower urinary tract symptoms (LUTS) including bladder overactivity. 30 For these patients, drugs targeting the lower urinary tract such as the bladder (muscarinic M3 receptors and β3 adrenoceptors) or the urethra (α1 adrenoceptors) are used, but there are some cases with insufficient therapeutic effects. 14 Especially, patients with central neurological diseases such as Parkinson's disease and stroke have LUTS including frequent urination due to disorders of the PMC, and in these cases, existing drugs targeting for the lower urinary tract may have poor efficacy. 31 , 32 In mouse model of transient middle cerebral artery occlusion (MCAO), inhalation of low doses of CO at the start of reperfusion reduced the infarction volume and improved neurological survival scores 33 and CORM‐3 administration by retro‐orbital injection at the start of reperfusion reduced neuroinflammation and alleviated blood–brain barrier disruption. 34 Therefore, exposure to CO has neuroprotective effects. Considering this CO property and our finding, brain CO can suppress the micturition reflex, low doses of CO might attenuate neurological damage and LUTS in patients with stroke.
In summary, brain CO can suppress the rat micturition reflex through brain GABA receptors. Thus, the brain CO might be a new therapeutic target for LUTS patients who cannot obtain sufficient therapeutic effects from conventional medications.
AUTHOR CONTRIBUTIONS
Masaki Yamamoto: Conceptualization; formal analysis; investigation; methodology; funding acquisition; writing—original draft; writing—review and editing. Takahiro Shimizu: Conceptualization; formal analysis; investigation; methodology; funding acquisition; writing—review and editing. Nobutaka Shimizu: Formal analysis; writing—review and editing. Mikiya Fujieda: Supervision; writing—review and editing. Motoaki Saito: Supervision; funding acquisition; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
Not applicable.
INFORMED CONSENT
Not applicable.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
Not applicable.
ANIMAL STUDIES
The animal experimental protocol was approved by the Institutional Animal Care and Use Committee of Kochi University (permit numbers: N‐84/O‐57/P‐67).
ACKNOWLEDGMENTS
This work was supported in part by a Grant‐in‐Aid for Scientific Research (C) (No. 20K07827 to T.S., No. 21K09428 to M.S.) from the Japan Society for the Promotion of Science, a grant from the Smoking Research Foundation in Japan (T.S.), and the Kochi Medical School Hospital President's Discretionary Grant (M.Y.).
REFERENCES
- 1. Hopper CP, Zambrana PN, Goebel U, Wollborn J. A brief history of carbon monoxide and its therapeutic origins. Nitric Oxide. 2021;111‐112:45–63. [DOI] [PubMed] [Google Scholar]
- 2. Ku BM, Joo Y, Mun J, Roh GS, Kang SS, Cho GJ, et al. Heme oxygenase protects hippocampal neurons from ethanol‐induced neurotoxicity. Neurosci Lett. 2006;405:168–171. [DOI] [PubMed] [Google Scholar]
- 3. Figueiredo‐Pereira C, Dias‐Pedroso D, Soares NL, Vieira HLA. CO‐mediated cytoprotection is dependent on cell metabolism modulation. Redox Biol. 2020;32:101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koçer G, Nasircilar Ülker S, Şentürk ÜK. The contribution of carbon monoxide to vascular tonus. Microcirculation. 2018;25:e12495. [DOI] [PubMed] [Google Scholar]
- 5. Queiroga CSF, Vercelli A, Vieira HLA. Carbon monoxide and the CNS: challenges and achievements. Br J Pharmacol. 2015;172:1533–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kishimoto Y, Kondo K, Momiyama Y. The protective role of heme oxygenase‐1 in atherosclerotic diseases. Int J Mol Sci. 2019;20:3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ndisang JF, Tabien HEN, Wang R. Carbon monoxide and hypertension. J Hypertens. 2004;22:1057–1074. [DOI] [PubMed] [Google Scholar]
- 8. Choi YK. Role of carbon monoxide in neurovascular repair processing. Biomol Ther. 2018;26:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang CY, Li XH, Zhang T, Fu J, Cui XD. Hydrogen sulfide upregulates heme oxygenase‐1 expression in rats with volume overload‐induced heart failure. Biomed Rep. 2013;1:454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mancuso C. The impact of heme oxygenase‐2 on pharmacological research: a bibliometric analysis and beyond. Front Pharmacol. 2023;14:1156333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ewing JF, Maines MD. Regulation and expression of heme oxygenase enzymes in aged‐rat brain: age related depression in HO‐1 and HO‐2 expression and altered sires‐response. J Neural Transm. 2006;113:439–454. [DOI] [PubMed] [Google Scholar]
- 12. Scapagnini G, D'Agata V, Calabrese V, Pascale A, Colombrita C, Alkon D, et al. Gene expression profiles of heme oxygenase isoforms in the rat brain. Brain Res. 2002;954:51–59. [DOI] [PubMed] [Google Scholar]
- 13. Parfenova H, Leffler CW. Cerebroproctive functions of HO‐2. Cuur Pharm Des. 2008;14:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Compr Physiol. 2015;5:327–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naseem KM, Mumtaz FH, Thompson CS, Sullivan ME, Khan MA, Morgan RJ, et al. Relaxation of rabbit lower urinary tract smooth muscle by nitric oxide and carbon monoxide: modulation by hydrogen peroxide. Eur J Pharmacol. 2000;387:329–335. [DOI] [PubMed] [Google Scholar]
- 16. Schroder A, Hedlund P, Andersson KE. Carbon monoxide relaxes the female pig urethra as effectively as nitric oxide in the presence of YC‐1. J Urol. 2002;167:1892–1896. [PubMed] [Google Scholar]
- 17. McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shimizu T, Shimizu S, Higashi Y, Nakamura K, Yoshimura N, Saito M. A stress‐related peptide bombesin centrally induces frequent urination through brain bombesin receptor type 1 and 2 in the rat. J Pharmacol Exp Ther. 2016;356:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Burlington: Elsevier Academic Press; 2005. [Google Scholar]
- 20. Adach W, Olas B. Carbon monoxide and its donors—their implications for medicine. Future Med Chem. 2019;11:61–73. [DOI] [PubMed] [Google Scholar]
- 21. Motterlini R, Mann BE, Foresti R. Therapeutic applications of carbon monoxide‐releasing molecules. Expert Opin Investig Drugs. 2005;14:1305–1318. [DOI] [PubMed] [Google Scholar]
- 22. Wong RJ, Vreman HJ, Schulz S, Kalish FS, Pierce NW, Stevenson DK. In vitro inhibition of heme oxygenase isoenzymes by metalloporphyrins. J Perinatol. 2011;31:S35–S41. [DOI] [PubMed] [Google Scholar]
- 23. Parfenova H, Neff RA 3rd, Alonso JS, Shlopov BV, Jamal CN, Sarkisova SA, et al. Cerebral vascular endothelial heme oxygenase: expression, localization, and activation by glutamate. Am J Physiol Cell Physiol. 2001;281:C1954–C1963. [DOI] [PubMed] [Google Scholar]
- 24. Reis WL, Biancardi VC, Son S, Antunes‐Rodrigues J, Stern JE. Carbon monoxide and nitric oxide interactions in magnocellular neurosecretory neurones during water deprivation. J Neuroendocrinol. 2015;27:111–122. [DOI] [PubMed] [Google Scholar]
- 25. Kanie S, Yokoyama O, Komatsu K, Kodama K, Yotsuyanagi S, Niikura S, et al. GABAergic contribution to rat bladder hyperactivity after middle cerebral artery occlusion. Am J Physiol Rerul Integr Comp Physiol. 2000;279:R1230–R1238. [DOI] [PubMed] [Google Scholar]
- 26. Shimizu S, Shimizu T, Nakamura K, Higashi Y, Saito M. Angiotensin II, a stress‐related neuropeptide in the CNS, facilitates micturition reflex in rats. Br J Pharmacol. 2018;175:3727–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamamoto M, Shimizu T, Zou S, Shimizu S, Higashi Y, Fujieda M, et al. Brain hydrogen sulfide suppresses the micturition reflex via brain GABA receptors in rats. Nitric Oxide. 2020;104‐105:44–50. [DOI] [PubMed] [Google Scholar]
- 28. Chang HY, Havton LA. Differential effects of urethane and isoflurane on external urethral sphincter electromyography and cystometry in rats. Am J Physiol Renal Physiol. 2008;295:F1248–F1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoshiyama M, Roppolo JR, Takeda M, de Groat WC. Effects of urethane on reflex activity of lower urinary tract in decerebrate unanesthetized rats. Am J Physiol Renal Physiol. 2013;304:F390–F396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gao Y, Wang M, Zhang H, Tan A, Yang X, Qin X, et al. Are metabolic syndrome and its components associated with lower urinary tract syndrome? Result from a Chinese male population survey. Urology. 2012;79:194–201. [DOI] [PubMed] [Google Scholar]
- 31. Sakakibara R, Panicker J, Finazzi‐Agro E, Lacovelli V, Bruschini H. A guideline for the management of bladder dysfunction in Parkinson's disease and other gait disorders. Neurourol Urodyn. 2016;35:551–563. [DOI] [PubMed] [Google Scholar]
- 32. Thor KB, Marson L, Katofiasc MA, Ricca DJ, Burgard EC. Recent developments in on‐demand voiding therapies. J Pharmacol Exp Ther. 2024. in press. 10.1124/jpet.123.002073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zeynalov E, Doré S. Low doses of carbon monoxide protect against experimental focal brain ischemia. Neurotox Res. 2009;15:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang J, Zhang D, Fu X, Yu L, Lu Z, Gao Y, et al. Carbon monoxide‐releasing molecule‐3 protects against ischemic stroke by suppressing neuroinflammation and alleviating blood‐brain barrier disruption. J Neuroinflammation. 2018;15:188. [DOI] [PMC free article] [PubMed] [Google Scholar]


