Abstract
Vepdegestrant (ARV-471) is an oral PROTAC ER degrader that binds an E3 ubiquitin ligase and ER to directly trigger ubiquitination of ER and its subsequent proteasomal degradation. In a first-in-human Phase I/II study, vepdegestrant monotherapy was well tolerated with clinical activity in pretreated patients with ER+/HER2- advanced breast cancer. The global, randomized Phase III VERITAC-2 study compares efficacy and safety of vepdegestrant versus fulvestrant in adults with ER+/HER2- advanced breast cancer after treatment with a CDK4/6 inhibitor plus endocrine therapy. Progression-free survival by blinded independent central review (primary end point) will be assessed in the intention-to-treat population and ESR1 mutation-positive subpopulation. Secondary end points include overall survival, tumor response, safety, pharmacokinetics, patient-reported outcomes, and circulating tumor DNA biomarkers.
Clinical trial registration: NCT05654623 (ClinicalTrials.gov)
Keywords: : ARV-471, breast cancer, ER+, fulvestrant, HER2-, PROTAC, targeted protein degradation, vepdegestrant
Plain Language Summary
VERITAC-2 is a clinical trial comparing vepdegestrant, a new drug that degrades estrogen receptors, to an existing treatment called fulvestrant in patients with ER+/HER2- advanced breast cancer: Estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2-) breast cancer grows in response to estrogen, a hormone in the body, and has low levels or no HER2 protein. People living with ER+/HER2- advanced breast cancer that has grown, spread to another part of the body, or cannot be removed by surgery are often treated with cyclin-dependent kinase (CDK) 4/6 inhibitors and endocrine therapies, but their cancer may get worse on these treatments and new treatments are needed. Fulvestrant, an endocrine therapy that attaches to estrogen receptors, lowers estrogen's effect on tumors and can slow or stop cancer growth. Vepdegestrant, a new medicine being tested for ER+ breast cancer, is a PROteolysis TArgeting Chimera (PROTAC) protein degrader that attaches to estrogen receptors and causes them to be tagged for removal by the cell's natural protein disposal system. By removing estrogen receptors, vepdegestrant may cause tumors to stop growing or shrink.
This paper describes the Phase III VERITAC-2 clinical study comparing vepdegestrant versus fulvestrant in people living with ER+/HER2- advanced breast cancer previously treated with a CDK4/6 inhibitor and endocrine therapy.
Patients will be randomly assigned to receive vepdegestrant (a pill taken once daily by mouth) or fulvestrant (a shot given into the muscle). The purpose of the study is to find out how long people live without their cancer getting worse with vepdegestrant or fulvestrant. VERITAC-2 will also look at how long people live during the study, side effects people may experience, and the overall well-being of people throughout the study.
Plain language summary
Article highlights.
VERITAC-2 study rationale
ER-mediated signaling is a key driver of breast cancer pathogenesis in ER+/HER2- breast cancer, which accounts for the majority of all breast cancers.
Despite the initial efficacy of first-line treatments that use endocrine therapies (ETs), the acquisition of mutations in ESR1 often leads to the development of ET resistance, leading to disease progression and poor outcomes; there is no clear standard of care in the second-line setting.
Vepdegestrant is a small-molecule PROteolysis TArgeting Chimera (PROTAC) ER degrader that has a unique mechanism of action that harnesses the ubiquitin-proteasome system to induce direct ubiquitination and subsequent degradation of ER, unlike selective ER degraders, which lead to conformational changes in ER that may indirectly result in ER degradation.
In preclinical studies, vepdegestrant demonstrated potent ER degradation and robust tumor growth inhibition and regression.
In a first-in-human Phase I/II dose escalation and cohort expansion clinical study, vepdegestrant was well tolerated and demonstrated antitumor activity; the 200-mg once-daily dose of vepdegestrant was chosen as the recommended Phase III monotherapy dose.
VERITAC-2 study design
VERITAC-2 is an open-label, randomized, global, multicenter, Phase III study comparing the efficacy and safety of vepdegestrant with fulvestrant in adults with ER+/HER2- advanced breast cancer.
Eligible patients have a confirmed diagnosis of ER+/HER2- locoregional recurrent or metastatic breast cancer not amenable to surgical resection or radiation.
Patients must also have had one line of prior treatment with CDK4/6 inhibitor therapy in combination with ET and no more than one additional line of ET wherein the most recent ET was given for ≥6 months before disease progression.
The primary end point is progression-free survival in both the intention-to-treat population and ESR1 mutation-positive subpopulation, and key secondary end points include overall survival, objective response rate, duration of response, clinical benefit rate, and safety and tolerability end points.
This study plans to enroll 560 patients; enrollment is ongoing at the time of publication.
Infographic
Infographic:
A PDF version of this infographic is available as supplemental material.
Video Abstract.
1. Background
1.1. Background & rationale
ER+/HER2- breast cancer is the most common subtype of all breast cancers [1]. ER-mediated signaling is a key driver of ER+ breast cancer pathogenesis. When estrogen binds its receptor, the ER dimerizes and translocates to the nucleus where it forms an active complex to induce expression of genes that promote tumor cell growth and proliferation [1,2]. ER+ breast cancer therapies have focused on disrupting the ER-mediated signaling pathway, including reducing estrogen production, modulating estrogen signaling through the ER, and/or antagonizing/degrading the ER itself [1].
The current preferred first-line therapy for ER+/HER2- advanced/metastatic breast cancer is endocrine therapy (ET) with an aromatase inhibitor in combination with a CDK4/6 inhibitor [1,3]. Unfortunately, despite initial efficacy in most patients, the acquisition of mutations in the estrogen receptor gene ESR1 and altered gene expression often lead to the development of ET resistance [1], and outcomes remain poor [4–6]. Approximately 40% of patients who received aromatase inhibitors as treatment for metastatic breast cancer have ESR1 mutations, primarily in the ligand-binding domain of ER [7]; the most common ESR1 mutations are D538G and Y537S [7]. There is no clear standard of care in the second-line setting, with treatment based upon a number of factors, including agents previously used and consideration of targeted agents based on tumor genomics [8–11]. However, sequential ET is generally recommended in patients with disease progression and presumed endocrine sensitivity [8–11]. Thus, targeting ER remains a cornerstone of disease management, and the development of novel ETs that can overcome resistance is an area of growing interest.
Fulvestrant is a first-in-class selective ER degrader (SERD) initially approved by the U.S. Food and Drug Administration (FDA) in 2002 for patients with ER+/HER2- advanced/metastatic breast cancer [12]. SERDs primarily immobilize ER in the cytoplasm limiting ER-directed gene transcription, and they have also been shown to indirectly lead to ER degradation secondary to conformational changes and/or immobilization of ER [13–15]. Fulvestrant is a standard therapy for patients with ER+/HER2- advanced/metastatic breast cancer; however, it must be administered intramuscularly [16], and at its labeled dose, ER protein degradation is 40–50% [17,18]. Recently, several targeted therapies for patients with advanced/metastatic breast cancer have been approved by the FDA, such as elacestrant for patients with ESR1-mutated ER+/HER2- disease [19], capivasertib in combination with fulvestrant for patients with one or more PIK3CA/AKT1/PTEN alterations [20], and alpelisib, a PI3K inhibitor approved in combination with fulvestrant for patients with PIK3CA mutations who progressed on ET [21]. Alpelisib also demonstrated efficacy among patients who progressed on CDK4/6 inhibitors [22], but those with ESR1 mutations show resistance to PI3K inhibitors [23]. Overall, there remains an unmet need for therapies with distinct mechanisms of action that can effectively target ER and overcome mechanisms of resistance, particularly in the second-line setting.
1.2. Vepdegestrant (ARV-471)
PROteolysis TArgeting Chimera (PROTAC) protein degraders are small molecules that harness the cell's natural protein disposal machinery, the ubiquitin-proteasome system, to induce degradation of a target protein of interest [24,25]. Vepdegestrant is an oral PROTAC ER degrader that induces degradation of wild-type ER and clinically relevant mutants [26]. Vepdegestrant has a half-maximal degradation concentration (DC50) of approximately 1 nM in ER+ breast cancer cell lines and induces a similar level of degradation of wild-type and mutant ER [26].
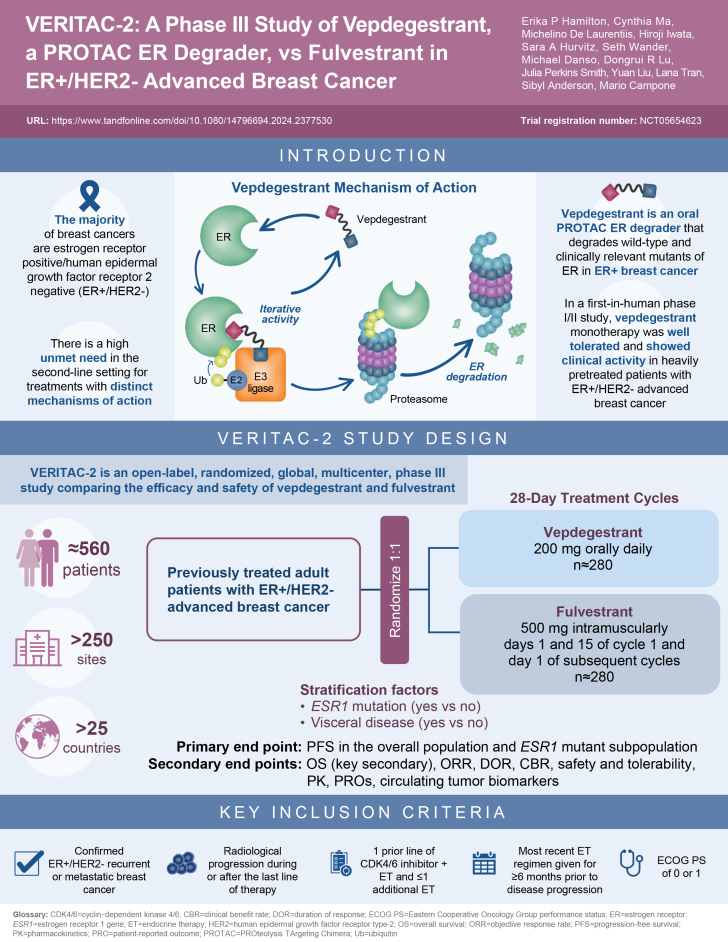
Unlike SERDs, which indirectly lead to ER degradation secondary to conformational changes and/or immobilization, vepdegestrant creates a trimer complex with the E3 ubiquitin ligase and ER to directly induce ubiquitination of ER and its subsequent proteasomal degradation (Figure 1) [25]. Vepdegestrant has iterative activity and can be recycled to bind additional ER target proteins, leading to their degradation.
Figure 1.
Mechanism of action of vepdegestrant.a
aGeneral PROTAC protein degrader, cereblon E3 ligase, and ER target protein are shown in the schematic. The chemical structure of vepdegestrant is shown in the inset.
PROTAC: PROteolysis TArgeting Chimera.
In preclinical studies of ER+ breast cancer models, vepdegestrant induced ≥90% degradation of wild-type ER and multiple forms of mutant ER in cell lines and inhibited ER-dependent breast cancer cell line proliferation [26]. In vivo, vepdegestrant at doses ranging from 3 to 30 mg/kg daily resulted in 85 to 120% tumor growth inhibition (TGI) in an MCF7 orthotopic xenograft model. In this same model, the 30-mg/kg vepdegestrant dose achieved complete TGI (105%) compared with 46% TGI for fulvestrant (200 mg/kg biweekly for 2 weeks and weekly for 2 weeks). In the hormone-independent ER Y537S patient-derived xenograft breast cancer model, vepdegestrant at doses of 10 and 30 mg/kg daily demonstrated tumor regression (99 and 107% TGI, respectively) and reduced ER levels by 79 and 88%, respectively [26]. Fulvestrant demonstrated 62% TGI and reduced ER levels by 63% in the same model in contrast [26].
1.3. First-in-human Phase I/II clinical study
Based on the promising preclinical data, a first-in-human Phase I/II study (NCT04072952) of vepdegestrant monotherapy and in combination with the CDK4/6 inhibitor palbociclib was initiated in patients with ER+/HER2- breast cancer; initial results have been presented at several scientific conferences [27–31]. In the Phase I dose escalation portion of this study, vepdegestrant demonstrated antitumor activity and was well tolerated at total daily doses ranging from 30 to 700 mg, with no dose-limiting toxicities [27]. Based on the results of the dose escalation study, vepdegestrant 200 mg once daily (QD) and 500 mg QD were further evaluated in VERITAC, the Phase II expansion cohort of the Phase I/II study. Preliminary results show encouraging clinical activity and a well-tolerated safety profile [28–30]. Vepdegestrant 200 mg QD was selected as the Phase III monotherapy dose based on comparable efficacy and favorable tolerability in heavily pretreated patients with ER+/HER2- advanced breast cancer versus 500 mg QD as well as robust ER degradation in the VERITAC study [28].
2. VERITAC-2
2.1. Study design
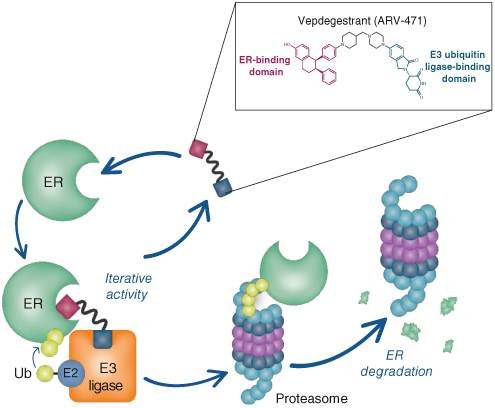
VERITAC-2 (NCT05654623) is an open-label, randomized, global, multicenter, Phase III study comparing the efficacy and safety of vepdegestrant with fulvestrant in adult patients with ER+/HER2- advanced breast cancer after prior combination CDK4/6 inhibitor therapy and ET (Figure 2) [32].
Figure 2.
VERITAC-2 study design.
2.2. Eligibility criteria
Eligible patients have a confirmed diagnosis of ER+/HER2- locoregional recurrent or metastatic breast cancer not amenable to surgical resection or radiation. Patients must have had one line of prior treatment with a CDK4/6 inhibitor therapy in combination with ET and no more than one additional line of ET wherein the most recent ET was given for ≥6 months before disease progression, and radiological disease progression was during or after the last line of therapy (Table 1).
Table 1.
VERITAC-2 key eligibility criteria.
| Key inclusion criteria | Key exclusion criteria |
|---|---|
| – Women or men aged ≥18 years ○ Women must be post-menopausal or if pre- or peri-menopausal,must agree to initiate or continue use of an LHRH agonist ○ Men must agree to initiate or continue use of an LHRH agonist ○ WOCBP and men must agree to use contraception – Confirmed ER+/HER2- locoregional recurrent or metastatic breast cancer – Prior therapies for locoregional recurrent or metastatic disease must fulfill all the following criteria: ○ 1 line of CDK4/6 inhibitor therapy in combination with ET (only 1 line of CDK4/6 inhibitor in any setting) ○ ≤1 ET (in addition to CDK4/6 inhibitor with ET) ○ Most recent ET given for ≥6 months prior to disease progressiona ○ Radiological progression during or after the last line of therapy – ECOG performance status of 0 or 1 – Measurable disease evaluable per RECIST v1.1 or nonmeasurable bone-only disease |
– Active brain metastases – Inflammatory breast cancer – Advanced, symptomatic visceral spread at risk of life-threatening complications in the short-term – Impaired cardiovascular function or clinically significant cardiovascular disease – Prior treatment with ○ Vepdegestrant ○ Fulvestrant ○ Elacestrant ○ mTOR, PI3K, or AKT pathway inhibitors ○ PARP inhibitors ○ Other investigational agents, including novel ET (SERDs, SERCAs, CERANs) ○ Chemotherapy for advanced/metastatic disease |
The most recent ET may be the ET component of the CDK4/6 inhibitor line of therapy.
CERAN: Complete estrogen receptor antagonist; ECOG: Eastern Cooperative Oncology Group; ET: Endocrine therapy; RECIST: Response Evaluation Criteria in Solid Tumors; SERCA: Selective estrogen receptor covalent agonist; SERD: Selective estrogen receptor degrader; WOCBP: Women of childbearing potential.
2.3. Objectives & outcome measures
The primary objective is to evaluate the efficacy of vepdegestrant compared with fulvestrant in both the intention-to-treat population and ESR1 mutation-positive subpopulation, which will be assessed by progression-free survival (PFS) based on blinded independent central review (BICR). Secondary objectives are to further evaluate the efficacy of vepdegestrant compared with fulvestrant, including overall survival (OS) and objective response rate (ORR) by BICR assessment, as well as to evaluate safety and tolerability, the effect of vepdegestrant on QTc, pharmacokinetics (PK) of vepdegestrant and its epimer, ARV-473, patient quality of life, and changes in tumor biomarkers. The primary and secondary end points of the VERITAC-2 study are summarized in Table 2.
Table 2.
VERITAC-2 study end points.
| End points | |
|---|---|
| Primary |
PFS in the ITT population and ESR1 mutation-positive subpopulation – Time interval from the date of randomization to the date of first documented tumor progression determined by BICR assessment as per RECIST v1.1 or death due to any cause, whichever comes first |
| Secondary |
OS – Time from date of randomization until the date of death due to any cause |
|
ORR – Proportion of patients with confirmed complete CR or PR by BICR |
|
|
DOR – Time from first documented evidence of CR or PR until progressive disease by BICR |
|
|
CBR – Proportion of patients with confirmed CR, PR, or SD ≥24 weeks by BICR |
|
| Incidence of AEs, SAEs, and ECG and laboratory abnormalities | |
| QT interval (QTc) | |
| Plasma concentrations of vepdegestrant and its epimer, ARV-473 | |
|
Patient disease-, health-, and treatment-related QOL, assessed by: – EQ-5D-5La – EORTC QLQ-C30b – EORTC QLQ-BR23c – BPI-SFd |
|
| Circulating tumor DNA changes |
The EQ-5D-5L assesses patients' current state of self-care, usual activities, mobility, pain/discomfort, and anxiety/depression. The visual analog scale score records the respondent's self-rated health status [33].
The EORTC QLQ-C30 contains 1 global QOL score and 5 multi-item functional subscales, including physical, role, emotional, cognitive, and social. Higher scores on QOL/functional domains are indicative of higher levels of functioning. Oncology-related symptoms are assessed using 9 symptom scales, where higher scores are reflective of a greater presence of symptoms [34].
The female and male versions of the EORTC QLQ-BR23 contain 4 functional scales to assess body image, sexual functioning, sexual enjoyment, and future perspective and 4 symptom scales to assess systemic therapy side effects, breast symptoms, arm symptoms, and being upset by hair loss. Higher scores represent greater severity [35].
The BPI-SF assesses pain levels and their effect on daily life [36].
AE: Adverse event; BICR: Blinded independent central review; BPI-SF: Brief Pain Inventory-short form; CBR: Clinical benefit rate; CR: Complete response; DOR: Duration of response; ECG: Electrocardiogram; EORTC QLQ-BR23: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Breast Cancer Module; EORTC QLQ-C30: EORTC Quality of Life Questionnaire Core; EQ-5D-5L: 5-level EQ-5D; ITT: Intention-to-treat; ORR: Objective response rate; OS: Overall survival; PFS: Progression-free survival; PR: Partial response; QTc: Corrected QT interval; QOL: Quality of life; RECIST: Response Evaluation Criteria in Solid Tumors; SAE: Serious AE; SD: Stable disease.
2.4. Sample size/recruitment
In total, VERITAC-2 will enroll approximately 560 patients who will be randomized 1:1 to receive vepdegestrant or fulvestrant. Patients will be stratified by the presence or absence of mutation(s) in the ESR1 gene (determined by blood sample), and the presence or absence of visceral disease, defined as lung, liver, brain, pleural, or peritoneal involvement.
2.5. Assignment to interventions
This is an unblinded, open-label study. Specific study intervention for each patient will be assigned using interactive response technology (IRT). Stratified randomization will be centrally allocated using the IRT system. Vepdegestrant will be administered as a 200-mg oral dose QD continuously in each 28-day cycle. Vepdegestrant must be taken with food at approximately the same time every morning. Fulvestrant will be administered as a 500-mg dose intramuscularly on days 1 and 15 of cycle 1, then on day 1 of each cycle starting from day 1 of cycle 2. Patients will continue to receive their assigned treatment until objective disease progression as determined by the investigator, global deterioration of health status requiring discontinuation, unacceptable toxicity, patient refusal of further treatment, pregnancy, significant protocol deviation, or death.
The study began March 3, 2023, and the estimated study completion date is May 15, 2028. Enrollment is ongoing globally; countries with currently enrolling and planned sites as of February 16, 2024, are shown in Figure 3.
Figure 3.
Countries with VERITAC-2 study sites.
aCountries with active study sites as of June 27, 2024.
2.6. Data collection & management
All data pertaining to an individual patient will be logged in a case report form. For all patients, baseline evaluations to assess efficacy will include computerized tomography (CT) or magnetic resonance imaging (MRI) scans of the chest, abdomen, and pelvis; patients will also receive a full-body bone scan. Scans will be repeated every 8 weeks for the first 48 weeks from the randomization date and then every 12 weeks thereafter (CT/MRI) and 24 weeks (bone scan) until radiographically and/or clinically documented disease progression as defined by Response Evaluation Criteria in Solid Tumors v1.1, study treatment discontinuation, discontinuation from overall participation by patient preference, loss to follow-up, or death. All imaging studies will be objectively verified by BICR, and all tumor-based efficacy analyses will be performed using BICR tumor assessments as the primary data source. All adverse events (AEs) and serious AEs (SAEs) will be recorded for each patient through at least 28 calendar days after the last administration of study intervention. AEs/SAEs will be coded using the Medical Dictionary for Regulatory Activities classification system. After treatment discontinuation, all patients will be followed for survival every 3 months until the date of death from any cause. Patient-reported outcome questionnaires will be administered at screening, on day 1 of cycles 1–6 and then on day 1 of subsequent even-numbered cycles, at end of treatment or withdrawal, and at the 28-day posttreatment follow-up.
2.7. Planned statistical analyses
All efficacy analyses will be performed using the full analysis set, which includes all enrolled patients who were randomized. All safety analyses will use the safety analysis set, which includes all randomized patients who receive at least one dose of study intervention. Patients will be analyzed according to the treatment they were randomized to receive.
Statistical hypothesis testing of the primary end point (PFS by BICR) and key secondary end point (OS) will be performed in two populations: 1) all randomized patients and 2) patients with ESR1 mutation. PFS and OS associated with each treatment will be estimated using the Kaplan-Meier method. The median PFS and OS (calculated in months) and associated 95% CIs will be presented by treatment arm. A stratified log-rank test will be used to compare PFS and OS between the two treatment arms.
All safety analyses will be summarized descriptively; summaries of AEs and other safety parameters will be provided as needed/appropriate. The number and percentage of patients who experience any AE, SAE, treatment-related AE, and treatment-related SAE will be summarized according to worst toxicity grades.
3. Conclusion
Vepdegestrant, a novel, oral PROTAC ER degrader, is a bifunctional small molecule that induces robust ER degradation by harnessing the ubiquitin-proteasome system [26]. In the first-in-human Phase I/II study, vepdegestrant monotherapy was well tolerated and showed evidence of clinical activity in heavily pretreated patients with ER+/HER2- advanced breast cancer [27–30].
The global, Phase III VERITAC-2 study is the first head-to-head study comparing the efficacy and safety of vepdegestrant with the intramuscular SERD fulvestrant in patients with ER+/HER2- advanced breast cancer after combination CDK4/6 inhibitor therapy and ET.
In addition, vepdegestrant is being investigated as a monotherapy and in combination approaches across multiple treatment settings for ER+ breast cancer, including a Phase III study comparing vepdegestrant in combination with palbociclib versus letrozole in combination with palbociclib (VERITAC-3; NCT05909397), a Phase II noncomparative study evaluating vepdegestrant or anastrozole as neoadjuvant monotherapy (TACTIVE-N; NCT05549505), a Phase Ib study evaluating vepdegestrant in combination with everolimus (TACTIVE-E; NCT05501769), and a Phase Ib/II study evaluating vepdegestrant in combination with the CDK4-selective inhibitor PF-07220060 (TACTIVE-K; NCT06206837). Vepdegestrant is also under evaluation in an umbrella study (TACTIVE-U) in combination with other anticancer treatments (ribociclib, NCT05573555; samuraciclib, NCT06125522; abemaciclib, NCT05548127) in patients with previously treated ER+ advanced/metastatic breast cancer.
Supplementary Material
Acknowledgments
We thank the patients who are participating in this study and their caregivers, as well as the investigators, researchers, and coordinators who are contributing to this study.
Funding Statement
This study is sponsored by Pfizer, Inc., in collaboration with Arvinas Estrogen Receptor, Inc. Protocol number: C4891001 (A Phase 3, Randomized, Open-Label, Multicenter Trial Of ARV-471 [PF-07850327] vs. Fulvestrant in Participants With Estrogen Receptor-Positive, HER2-Negative Advanced Breast Cancer Whose Disease Progressed After Prior Endocrine Based Treatment For Advanced Disease [VERITAC-2]).
Supplemental material
Supplemental data for this article can be accessed at https://doi.org/10.1080/14796694.2024.2377530
Author contributions
All authors contributed to the conception and drafting of the manuscript, participated in critical revisions that contributed to the intellectual content of the manuscript, and provided final approval of the draft to be published.
Financial disclosure
This study is sponsored by Pfizer, Inc., in collaboration with Arvinas Estrogen Receptor, Inc. Protocol number: C4891001 (A Phase 3, Randomized, Open-Label, Multicenter Trial Of ARV-471 [PF-07850327] vs. Fulvestrant in Participants With Estrogen Receptor-Positive, HER2-Negative Advanced Breast Cancer Whose Disease Progressed After Prior Endocrine Based Treatment For Advanced Disease [VERITAC-2]).
Competing interests disclosure
EP Hamilton has served in a consulting/advisory role for Arcus, AstraZeneca, Daiichi Sankyo, Ellipses Pharma, Genentech/Roche, Greenwich LifeSciences, iTeos, Janssen, Lilly, Loxo Oncology, Mersana, Novartis, Olema Pharmaceuticals, Orum Therapeutics, Pfizer, Inc., Relay Therapeutics, SeaGen, Stemline Therapeutics, Theratechnologies, Tubulis, and Verascity Science; received institutional research support from AbbVie, Accutar Biotechnology, Acerta Pharma, ADC Therapeutics, Akesobio Australia, Amgen, Aravive, Arqule, Artios, Arvinas, Inc., AstraZeneca, Atlas, BeiGene, Black Diamond, Bliss BioPharmaceuticals, Boehringer Ingelheim, Cascadian Therapeutics, Clovis, Compugen, Context Therapeutics, Cullinan-Florentine, Curis, CytomX, Daiichi Sankyo, Dana Farber Cancer Hospital, Dantari, Deciphera, Duality Biologics, eFFECTOR Therapeutics, Ellipses, Elucida Oncology, EMD Serono, FujiFilm, G1 Therapeutics, Genentech/Roche, H3 Biomedicine, Harpoon, Hutchinson MediPharma, Immunogen, Immunomedics, Incyte, Infinity Pharmaceuticals, InventisBio, Jacobio, K-Group Beta, Karyopharm, Kind Pharmaceuticals, Leap Therapeutics, Lilly, Loxo Oncology, Lycera, MabSpace Biosciences, Macrogenics, MedImmune, Mersana, Merus, Millenium, Molecular Templates, Novartis, Nucana, Olema, Oncomed, Onconova Therapeutics, Oncothyreon, ORIC Pharmaceuticals, Orinove, Orum Therapeutics, Pfizer, Inc., PharmaMar, Pieris Pharmaceuticals, Pionyr Immunotherapeutics, Plexxicon, Prelude Therapeutics, Profound Bio, Radius Health, Regeneron, Relay Therapeutics, Repertoire Immune Medicine, Rgenix, SeaGen, Sermonix Pharmaceuticals, Shattuck Labs, StemCentRx, Sutro, Syndax, Syros, Taiho, TapImmune, Tesaro, Tolmar, Torque Therapeutics, Treadwell Therapeutics, Verastem, Zenith Epigenetics, and Zymeworks. C Ma has served in a consulting role for AstraZeneca, Daiichi Sankyo, Genzyme, Gilead Sciences, Lilly, Novartis, Olaris, Pfizer, Inc., Regor, Stemline, Tempus, and TerSera; and has received research funding from Pfizer, Inc. and Puma Biotechnology. M De Laurentiis has received personal fees from AstraZeneca, Celgene, Daiichi Sankyo, Eisai, Eli Lilly, Exact Science, Gilead, Ipsen, Menarini-Stemline, MSD, Novartis, Pfizer, Inc., Pierre Fabre, Roche, Seagen, Takeda, and Veracyte, outside the submitted work. He has stock/ownership interests in Arvinas, Inc. H Iwata has served as invited speaker/advisory board member for AstraZeneca, Chugai, Daiichi Sankyo, Lilly, Pfizer, Sanofi, and Taiho; served as a steering committee member for Amgen, AstraZeneca, Chugai, Daiichi Sankyo, Kyowa Hakko Kirin, MSD, Novartis, Pfizer, Inc., and Sanofi; and served as a local principal investigator for Bayer, Boehringer, Lilly, and Nihon Kayaku. SA Hurvitz has served as an invited speaker for Axis Medical, Cancer Expert Now, Clinical Care Options, ICHE, MJH Associates, Peer Education, PER, PrecisCA, Primo, Projects in Knowledge, Prova Education, Research to Practice, Ultimate Medical Academy, Vaniam, and WebMD; has ownership interest in Ideal Implant and stocks and shares in ROM Tech; she receives royalties from Elsevier, McGraw, Sage, Springer, Wiley, and Wolters Kluwer; is a local principal investigator for Ambrx, Arvinas, Inc., AstraZeneca, Dantari, Dignitana, Eli Lilly, G1 Therapeutics, Genentech/Roche, Gilead, Greenwich Life Sciences, GSK, Immunomedics, Loxo Oncology, Macrogenics, Novartis, OBI Pharma, Orinove, Orum, Pfizer, Inc., Phoenix Molecular Designs, Pieris, Puma Biotechnology, Radius, Sanofi, Seattle Genetics, and Zymeworks; serves as coordinating principal investigator for Celcuity and Daiichi Sankyo; and has received research funding from Ambrx and Samumed. SA Hurvitz serves as a steering committee member for Greenwich Life Sciences and Orum; serves as principal investigator for Daiichi Sankyo, Genentech, and Seattle Genetics; and advisor for 4DPharma, Ambrx, Amgen, Artios, Arvinas, Inc., Daiichi Sankyo, Dantari, Immunomedics/Gilead, Lilly, Macrogenics, Novartis, Pieris, Pyxis, and Roche. SA Wander has served in a consulting/advisory board role for AstraZeneca, Biovica, Eli Lilly, Foundation Medicine, Genentech, Hologic, Novartis, Pfizer, Inc., Puma Biotechnology, and Veracyte; has done educational speaking for Eli Lilly, Guardant Health, and 2ndMD; and has received institutional research support from Eli Lilly, Genentech, Nuvation Bio, Pfizer, Inc., Regor Therapeutics Group, and Sermonix. M Danso has served in a consulting/advisory role for Immunomedics, Novartis, Pfizer, Inc., and Seattle Genetics; and has received honoraria from Amgen. DR Lu is employed by and has stock/ownership interests in Pfizer, Inc. J Perkins Smith is employed by and has stock/other ownership interests in Pfizer, Inc. Y Liu is employed by and has stock/ownership interests in Pfizer, Inc. L Tran is employed by and has stock/ownership interests in Pfizer, Inc. S Anderson was employed by Arvinas, Inc. at the time of manuscript development and had/has stock/ownership interests in Arvinas, Inc. M Campone has served in a consulting/advisory role for AstraZeneca, Daiichi Sankyo, Diaccurate, Gilead, Lilly, Menarini, Novartis, PET Therapy, Pfizer, Inc., Sanofi, and Seagen; has served as invited speaker for Lilly and Novartis; and received travel reimbursement fees from AstraZeneca, Lilly, Novartis, Pfizer, Inc., and Roche. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
Medical writing and editorial support was provided by Clare Gyorke, PhD, and Melissa Austin of Apollo Medical Communications, part of Helios Global Group, and Charlotte Pettigrew, PhD, and Lauren Hanlon, PhD, CMPP, of Red Nucleus, and was funded by Arvinas Operations, Inc.
Ethical conduct of research
All protocols/amendments have been approved by an Institutional Review Board at each study site. The study will be conducted in accordance with the ethical principles derived from international guidelines, including the Declaration of Helsinki and the Council for International Organizations of Medical Sciences International Ethical Guidelines, and are consistent with International Council of Harmonization/Good Clinical Practice and applicable regulatory requirements. Written consent will be obtained from each study patient. An external data monitoring committee, independent of the study team, will be responsible for ongoing monitoring of the safety of the participants in the trial.
Previous presentation
Presented at: European Society for Medical Oncology Breast Cancer Annual Conference. Berlin, Germany, 11 May-13 May 2023. Presentation 257TiP [32].
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Huppert LA, Gumusay O, Idossa D, et al. Systemic therapy for hormone receptor-positive/human epidermal growth factor receptor 2-negative early stage and metastatic breast cancer. CA Cancer J Clin. 2023;73:480–515. doi: 10.3322/caac.21777 [DOI] [PubMed] [Google Scholar]
- 2.Osborne CK, Wakeling A, Nicholson RI. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br J Cancer. 2004;90(Suppl. 1):S2–S6. doi: 10.1038/sj.bjc.6601629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Amico P, Cristofanilli M. Standard of care in hormone receptor-positive metastatic breast cancer: can we improve the current regimens or develop better selection tools? JCO Oncol Practice. 2021;18(5):331–334. doi: 10.1200/OP.21.00707 [DOI] [PubMed] [Google Scholar]
- 4.Lloyd MR, Wander SA, Hamilton E, et al. Next-generation selective estrogen receptor degraders and other novel endocrine therapies for management of metastatic hormone receptor-positive breast cancer: current and emerging role. Ther Adv Med Oncol. 2022;14:1–25. doi: 10.1177/17588359221113694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wander SA, Cohen O, Gong X, et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov. 2020;10(8):1174–1193. doi: 10.1158/2159-8290.CD-19-1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brett JO, Spring LM, Bardia A, Wander SA. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021;23(1):85. doi: 10.1186/s13058-021-01462-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burstein HJ, Somerfield MR, Barton DL, et al. Endocrine treatment and targeted therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO Guideline update. J Clin Oncol. 2021;39(35):3959–3977. doi: 10.1200/JCO.21.01392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gennari A, André F, Barrios CH, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–1495. doi: 10.1016/j.annonc.2021.09.019 [DOI] [PubMed] [Google Scholar]
- 10.ASCO Guidelines [Internet] . American Society of Clinical Oncology (ASCO). 2023. [cited 3 May 2024]. Algorithm for endocrine treatment and targeted therapy for HR-positive, HER2-negative metastatic breast cancer. Available from: https://old-prod.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2023-Algorithm-for-Endocrine-Treatment-Targeted-Therapy-HR%2BHER2-MBC%20%281%29.pdf [Google Scholar]
- 11.ASCO Breast Cancer [Internet] . American Society of Clinical Oncology (ASCO). 2024. [cited 2 May 2024]. Endocrine treatment and targeted therapy for HR-positive, HER2-negative metastatic breast cancer guideline and rapid update. Available from: https://old-prod.asco.org/practice-patients/guidelines/breast-cancer#/9326 [Google Scholar]
- 12.US FDA [Internet] . Faslodex (fulvestrant). Prescribing information. 2002. [cited 3 May 2023]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021344 [Google Scholar]
- 13.Guan J, Zhou W, Hafner M, et al. Therapeutic ligands antagonize estrogen receptor function by impairing its mobility. Cell. 2019;178(4):949–963. doi: 10.1016/j.cell.2019.06.026 [DOI] [PubMed] [Google Scholar]
- 14.Wardell SE, Marks JR, McDonnell DP. The turnover of estrogen receptor α by the selective estrogen receptor degrader (SERD) fulvestrant is a saturable process that is not required for antagonist efficacy. Biochem Pharmacol. 2011;82(2):122–130. doi: 10.1016/j.bcp.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bidard FC, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized Phase III EMERALD trial. J Clin Oncol. 2022;40(28):3246–3258. doi: 10.1200/JCO.22.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan MR, Schmid P. A review of fulvestrant in breast cancer. Oncol Ther. 2017;5(1):17–29. doi: 10.1007/s40487-017-0046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuter I, Gee JMW, Hegg R, et al. Dose-dependent change in biomarkers during neoadjuvant endocrine therapy with fulvestrant: results from NEWEST, a randomized Phase II study. Breast Cancer Res. Treat. 2012;133(1):237–246. doi: 10.1007/s10549-011-1947-7 [DOI] [PubMed] [Google Scholar]
- 18.Robertson JFR, Dixon JM, Sibbering DM, et al. A randomized trial to assess the biological activity of short-term (pre-surgical) fulvestrant 500 mg plus anastrozole versus fulvestrant 500 mg alone or anastrozole alone on primary breast cancer. Breast Cancer Res. 2013;15(2):R18. doi: 10.1186/bcr3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US FDA [Internet] . Orserdu (elacestrant). Prescribing information. 2023. [cited 3 May 2024]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=217639 [Google Scholar]
- 20.US FDA [Internet] . Truqap (capivasertib). Prescribing information. 2023. [cited 3 May 2024]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=218197 [Google Scholar]
- 21.US FDA [Internet] . Piqray (alpelisib). Prescribing information. 2024. [cited 11 June 2024]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/212526s009lbl.pdf [Google Scholar]
- 22.Rugo HS, Lerebours F, CIruelos E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive, advanced breast cancer (BYLieve): prior CDK4/6 inhibitor cohort in a Phase II, multicohort, multicentre, open-label, non-Comparative study. Lancet Oncol. 2021;22(4):489–498. doi: 10.1016/S1470-2045(21)00034-6 [DOI] [PubMed] [Google Scholar]
- 23.Razavi P, Dickler MN, Shah PD, et al. Alterations in PTEN and ESR1 promote clinical resistance to alpelisib plus aromatase inhibitors. Nat Cancer. 2020;1(4):382–393. doi: 10.1038/s43018-020-0047-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakamoto K, Kim KB, Kumagai A, et al. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci. 2001;98(15):8554–8559. doi: 10.1073/pnas.141230798 [DOI] [PMC free article] [PubMed] [Google Scholar]; • First report of PROTAC proof of concept.
- 25.Békés M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;12(3):181–200. doi: 10.1038/s41573-021-00371-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gough SM, Flanagan JJ, Teh J, et al. Oral estrogen receptor PROTAC® vepdegestrant (ARV-471) is highly efficacious as monotherapy and in combination with CDK4/6 or PI3K/mTOR pathway inhibitors in preclinical ER+ breast cancer models. Clin Cancer Res. 2024. doi: 10.1158/1078-0432.CCR-23-3465 [DOI] [PMC free article] [PubMed] [Google Scholar]; • First publication of in vitro and in vivo data about vepdegestrant.
- 27.Hamilton EP, Han HS, Schott AF, et al. Vepdegestrant, a PROteolysis TArgeting Chimera (PROTAC) estrogen receptor degrader, in estrogen receptor+/human epidermal growth factor receptor 2-advanced breast cancer: update of dose escalation results from a Phase I/II trial. Poster presented at: European Society for Medical Oncology Annual Meeting. Madrid, Spain; 2023 October 20–24; Poster 390P. [Google Scholar]; •• Results from the Phase I dose-escalation portion of the first-in-human Phase I/II of vepdegestrant.
- 28.Hurvitz SA, Schott AF, Ma C, et al. ARV-471, a PROTAC® estrogen receptor (ER) degrader in advanced ER-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer: Phase II expansion (VERITAC) of a Phase I/II study. Oral presentation at: San Antonio Breast Cancer Symposium. San Antonio, TX; 2022 December 6–10; Presentation GS3-03. [Google Scholar]; • Initial results from the VERITAC Phase II expansion cohort of the first-in-human Phase I/II of vepdegestrant.
- 29.Hurvitz SA, Schott AF, Ma C, et al. VERITAC update: Phase II study of ARV-471, a PROteolysis TArgeting Chimera (PROTAC) estrogen receptor (ER) degrader in ER+/human epidermal growth factor receptor 2 (HER2)- advanced breast cancer. Oral presentation at: European Society for Medical Oncology Breast Cancer Annual Conference. Berlin, Germany; 2023 May 11–13; Presentation 205P. doi: 10.1016/j.esmoop.2023.101394 [DOI] [Google Scholar]
- 30.Hurvitz SA, Schott AF, Ma C, et al. Updated results from VERITAC evaluating vepdegestrant, a PROteolysis TArgeting Chimera (PROTAC) estrogen receptor (ER) degrader, in ER-positive/human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer. Oral presentation at: San Antonio Breast Cancer Symposium. San Antonio, TX; 2023 December 5–9; Presentation PO3-05-08. [Google Scholar]; •• Updated results from the VERITAC Phase II expansion cohort of the first-in-human Phase I/II of vepdegestrant.
- 31.Hamilton EP, Jeselsohn R, Hurvitz SA, et al. Vepdegestrant, a PROteolysis TArgeting Chimera (PROTAC) estrogen receptor degrader, plus palbociclib in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: Phase IB cohort. Oral presentation at: San Antonio Breast Cancer Symposium. San Antonio, TX; 2023 December 5–9; Presentation PS15-03. [Google Scholar]
- 32.Hamilton EP, Ma C, De Laurentiis M, et al. VERITAC-2: a global, randomized Phase III study of ARV-471, a PROteolysis TArgeting Chimera (PROTAC) estrogen receptor (ER) degrader, vs. fulvestrant in ER+/human epidermal growth factor receptor 2 (HER2)- advanced breast cancer. Oral presentation at: European Society for Medical Oncology Breast Cancer Annual Conference. Berlin, Germany; 2023 May 11–13; Presentation 257TiP. [Google Scholar]; •• First poster presentation of VERITAC-2 TiP.
- 33.EUROQOL [Internet] . EuroQol Research Foundation. EQ-5D-5L. 2024. [cited 3 May 2024]. Available from: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ [Google Scholar]
- 34.EORTC Quality of Life [Internet] . European Organization for Research and Treatment of Cancer. Questionnaires. [cited 3 May 2024]. Available from: https://qol.eortc.org/questionnaires/ [Google Scholar]
- 35.EORTC Quality of Life [Internet] . European Organization for Research and Treatment of Cancer. Breast Cancer. [cited 3 May 2024]. Available from: https://qol.eortc.org/questionnaire/update-qlq-br23/ [Google Scholar]
- 36.MD Anderson Cancer Center [Internet] . The University of Texas MD Anderson Cancer Center. The Brief Pain Inventory. 2024. [cited 3 May 2024]. Available from: https://www.mdanderson.org/research/departments-labs-institutes/departments-divisions/symptom-research/symptom-assessment-tools/brief-pain-inventory.html [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.