Abstract
Background:
An estimated one fourth of the world’s population is infected with Mycobacterium tuberculosis, and 5–10% of those infected develop tuberculosis in their lifetime. Preventing tuberculosis is one of the most underutilized but essential components of curtailing the tuberculosis epidemic. Moreover, current evidence illustrates that tuberculosis manifestations occur along a dynamic spectrum from infection to disease rather than a binary state as historically conceptualized. Elucidating determinants of transition between these states is crucial to decreasing the tuberculosis burden and reaching the END-TB Strategy goals as defined by the WHO. Vaccination, detection of infection, and provision of preventive treatment are key elements of tuberculosis prevention.
Objectives:
This review provides a comprehensive summary of recent evidence and state-of-the-art updates on advancements to prevent tuberculosis in various settings and high-risk populations.
Sources:
We identified relevant studies in the literature and synthesized the findings to provide an overview of the current state of tuberculosis prevention strategies and latest research developments.
Content:
We present the current knowledge and recommendations regarding tuberculosis prevention, with a focus on M. bovis Bacille-Calmette-Guérin vaccination and novel vaccine candidates, tests for latent infection with M. tuberculosis, regimens available for tuberculosis preventive treatment and recommendations in low- and high-burden settings.
Implications:
Effective tuberculosis prevention worldwide requires a multipronged approach that addresses social determinants, and improves access to tuberculosis detection and to new short tuberculosis preventive treatment regimens. Robust collaboration and innovative research are needed to reduce the global burden of tuberculosis and develop new detection tools, vaccines, and preventive treatments that serve all populations and ages.
Keywords: BCG, IGRA, LTBI, Novel vaccine candidates, Preventive treatment, TB spectrum, TPT, TST, Tuberculosis control
Background
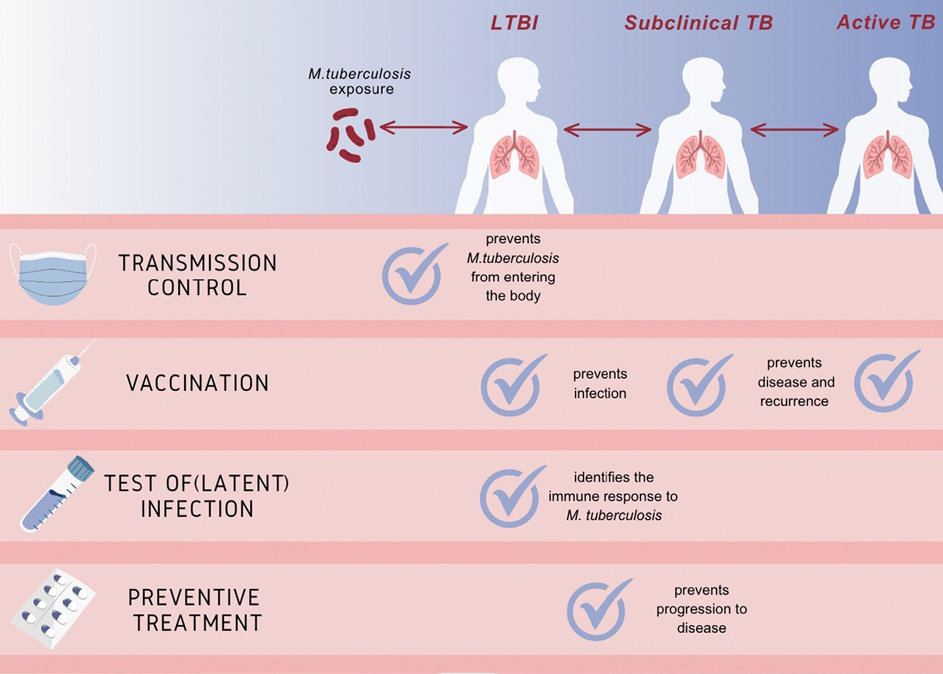
The WHO reported an alarming 10.6 million cases of tuberculosis in 2021 [1]. Indirect evidence suggests that one fourth of the world’s population is estimated to be infected with Mycobacterium tuberculosis [2], and 5–10% of those infected develop tuberculosis in their lifetime [3]. There is a paradigm shift in our understanding of tuberculosis natural history—instead of perpetuating a binary classification of latent M. tuberculosis infection (LTBI) and active tuberculosis, a dynamic spectrum of physiological states including incipient, subclinical, and active disease is now recognized [4,5]. Once infected with M. tuberculosis, the host immune response may eliminate the infection, contain the infection through immune response, or progress to subclinical, and thereafter active disease [4–7] (Fig. 1). Given that current diagnostic tests are unable to distinguish between these stages and produce both false positive and false negative results, the development of specific detection methods and targeted interventions to prevent disease progression and transmission are of paramount importance.
Fig. 1.
Spectrum of M. tuberculosis infection states and prevention strategies (model proposed by the authors, more evidence needed).
This review provides a comprehensive summary of recent evidence and state-of-the-art updates on advancements to prevent tuberculosis in various settings and high-risk populations.
Determinants of M. tuberculosis transmission
The risk of M. tuberculosis transmission is driven by a combination of host, pathogen, and environmental determinants. The main host-related factor is high bacillary load, as evidenced by a positive GeneXpert MTB/RIF, or cavitary disease [8,9], and exposure occurring in close proximity and for extended periods [10,11]. Regarding pathogen-related factors, genomic sequencing has revealed that distinct lineages possess varying degrees of virulence, thereby influencing their potential for transmission [12–14]. Next-generation sequencing now supports population level surveillance of tuberculosis by comparing the DNA sequences from patient isolates [15] and from environmental sources such as wastewater samples [16] to provide insight into transmission dynamics [17]. The main environmental determinant that increases the risk of M. tuberculosis transmission is overcrowding, as experienced in healthcare facilities [18], orphanages [19], prisons [20], and informal settlements [21].
The main prevention strategies currently available are vaccines (Bacillus Calmette-Guérin [BCG] or novel vaccine candidates still in the pipeline), identification of M. tuberculosis infection, and preventive treatment.
BCG vaccination
BCG vaccine remains the only licenced and widely used vaccination for tuberculosis prevention in humans [22,23]. BCG is effective in young children and mainly against severe forms of tuberculosis.
An individual-participant meta-analysis synthesized data from 26 studies including 68 552 participants. The studies were restricted to those with BCG vaccination at birth with the primary aim to investigate the age-specific impact of BCG vaccination on all forms of tuberculosis [24]. The overall effectiveness of BCG vaccination was of 18% (95% CI, 9–26); however, effectiveness was only seen in young children <5 years, suggesting novel vaccines are needed to prevent tuberculosis in older populations. BCG was protective against tuberculosis in those with a positive test of infection.
Whether BCG revaccination provides protection against tuberculosis has been debated for decades [25,26]. The Chingleput BCG vaccination trial (conducted in 1968) had shown no overall protection against active tuberculosis at 15 years in adults and limited protection in children [27]. A re-analysis of this study shows that among 2890 and 1546 participants of all ages in the BCG revaccination and placebo arms, the incidence of tuberculosis at 15 years post-vaccination was lower in the BCG revaccination arm (190 versus 296 cases per 100 000 population; hazard ratio, 0.64; 95% CI, 0.46–0.89) [28].
Better tuberculosis vaccines and innovative strategies are urgently needed to overcome the current tuberculosis crisis. Important evidence is expected from an on-going phase III BCG pre-travel study and a phase IIb booster BCG revaccination study; unfortunately, both studies include only adults.
Novel tuberculosis vaccines
After a long standstill, several tuberculosis vaccines are in development and at least a dozen candidates are currently moving through the clinical trial pipeline (see Table 1) [22,29–31].
Table 1.
Major TB vaccine candidates in clinical development
| Name | Composition | Most advanced clinical stage | Representative clinical trial number |
|---|---|---|---|
|
| |||
| TB protein: adjuvant formulations | |||
| H56:IC31 | Fusion protein of two antigens: IC31 as adjuvanta | Phase IIb ongoing | NCT03512249 |
| ID93: GLA-SE | Fusion protein of four antigens: GLA-SE as adjuvantb | Phase IIb ongoing | NCT03806686 |
| M72:AS01E | Fusion protein of two antigens: AS01E as adjuvantc | Phase IIb completed | NCT01755598 |
| AEC:BC02 | Combination of three protein antigens: BC02 as adjuvantd | Phase IIa ongoing | NCT05284812 |
| GamTBvac | Combination of three protein antigens: CpG as adjuvante | Phase III ongoing | NCT04975737 |
| Mtb-antigen encoding mRNA vaccines | |||
| BNT164a1/BNT164b1 | mRNA expressing multiple Mtb antigens in lipid nanoparticles | Phase I ongoing |
NCT05547464
NCT05537038 |
| TB antigen expressing viral vectors | |||
| ChadOx1.85A/MVA85A | ChadOx1 as carrier for prime, MVA as carrier for boost, both expressing same antigenf | Phase IIa ongoing | NCT00480558 |
| TB/FLU-04L | Non-replicating influenza virus expressing 2 antigensg | Phase I completed | NCT02501421 |
| Inactivated whole cell vaccines | |||
| Immuvac | Killed M. indicus pranii | Phase III ongoing | CTRI/2019/01/017026 |
| RUTI | Killed detoxified M. tuberculosis | Phase IIb ongoing | NCT04919239 |
| DAR-901 | Killed M. obuense | Phase IIb completed | NCT02712424 |
| Viable attenuated whole cell vaccines | |||
| MTBVAC | Genetically attenuated M. tuberculosish | Phase III for children and Phase IIa for adolescents and adults ongoing | NCT04975178 |
| VPM1002 | Genetically enhanced BCGi | Three phase III trials ongoing for (a) HIV-exposed/unexposed neonates, (b) adolescent and adult household contacts; (c) cured TB patients undergoing recurrence |
NCT04351685 |
BCG, Bacille-Calmette-Guérin; GLA-SE, glucopyranosyl lipid adjuvant - stable emulsion; TB, tuberculosis; TLR, toll-like receptor.
IC31 adjuvant: cationic peptide with a TLR-9 agonist.
GLA-SE: oil-in-water emulsion with TLR-4 agonist.
AS01E: liposome with TLR-4 agonist.
BC02: CpG adjuvant in aluminium hydroxide.
CpG adjuvant.
Chimpanzee adenovirus (ChadOxl) as prime and modified vaccinia Ankara (MVA) as boost both expressing same antigen.
Non-replicating influenza virus expressing 2 antigens.
2 independent gene deletions (phoP and fadD26) in Mtb.
Exchange of urease C by listeriolysin gene in BCG.
T lymphocytes which activate effector cells of innate immunity are critical for protection against tuberculosis induced by natural infection. Although antibodies participating in protective immunity activate secondary effector mechanisms [32], this activation itself is apparently insufficient. Neutralizing antibodies specific for protective antigens which could prevent infection with M. tuberculosis have not been identified. As a corollary, vaccine research and development is hampered by the lack of correlates of protection [33]. General agreement exists that Th1 type CD4 T-cells play a critical role in the protective immune response to M. tuberculosis. They are likely supported by IL-17 (interleukin-17) producing CD4 T-cells, unconventional T-cells, and CD8 T-cells which secrete cytokines and express cytolytic activity [34]. Pre-exposure vaccination involves administering a vaccine to individuals without exposure to M. tuberculosis, or without a detectable immune response to specific antigens. Post-exposure vaccination involves vaccinating individuals who have been exposed to M. tuberculosis or have risk of developing tuberculosis because of recent exposure and aims to prevent the development of tuberculosis or reduce its severity.
Vaccine candidates in clinical trials comprise subunit vaccines and whole cell vaccines. Subunit vaccines serve as boosters for individuals vaccinated at birth and are composed of few antigens considered relevant for protection. Attenuated live whole cell vaccines serve as boosters or replacements for BCG. Whole cell vaccines comprise a plethora of M. tuberculosis antigens. Inactivated vaccines are often composed of atypical mycobacteria which share numerous antigens with M. tuberculosis. Booster vaccines are administered either pre-exposure, in the absence of LTBI, or post-exposure, if evidence of LTBI exists. Replacement vaccines target neonates before exposure with M. tuberculosis.
Several vaccine trials are ongoing. Notably, the M72:AS01E vaccine candidate has shown a total efficacy of 54% (95% CI, 2.1–74.2) to prevent pulmonary tuberculosis in participants who received at least one dose [35]. The DAR-901 vaccine, an inactivated whole cell vaccine, was studied for the prevention of LTBI in 667 healthy Tanzanian adolescents; the primary efficacy outcome was time to interferon-γ release assays (IGRA) conversion. The vaccine candidate did not show a significant effect on new IGRA positivity or persistent LTBI—efficacy rates of 3% (95% CI, 13.9–17.7) and 4% (95% CI, 12.1–18.5), respectively [36]. However, lack of a direct and accurate measure of LTBI limits our ability to assess the effectiveness of vaccines designed to prevent infection [37]. Two live vaccines, MTBVAC and VPM1002, are based on M. tuberculosis and BCG, respectively. MTBVAC is an attenuated deletion mutant of M. tuberculosis lacking two independent virulence gene loci: phoP (transcription factor for several virulence factors) and fadD26 (involved in lipid synthesis). These deletions affect expression of hundreds of gene products including virulence factors. VPM1002 is a BCG vaccine in which the urease C gene has been replaced by the listeriolysin gene to strengthen attenuation and immunogenicity. The latest innovations in the vaccine area are mRNA vaccines which have entered phase I clinical trials (Table 1). These vaccines target the (a) healthy BCG-vaccinated and tuberculosis-infected people and (b) non–BCG vaccinated and non–tuberculosis infected people. There is insufficient knowledge to predict their potential efficacy.
Detecting latent M. tuberculosis infection
Test platforms routinely used in clinical care include the tuberculin skin test (TST) or blood-based interferon-γ (IFN-γ) IGRA [37]. The TST relies on the induction of a skin-test reaction after in vivo stimulation with tuberculin purified protein derivative. Commercial IGRA tests are based on IFN-γ production after specific stimulation of whole blood or peripheral blood mononuclear cells with two M. tuberculosis antigens, ESAT-6 and CFP-10. These antigens are encoded in the region of difference 1 present in the M. tuberculosis and M. bovis genome and absent in BCG and most environmental mycobacteria. Stimulation-induced IFN-γ is detected by either an ELISA, an ELISPOT assay, or flow cytometry. IGRAs are cross-reactive with only a few non-tuberculous mycobacteria but not with M. bovis, and are therefore more specific compared with TST [37]. IGRAs include negative and positive controls to assess validity and general immune function; thus, IGRAs are preferred in patients with immunodeficiency [38]. It is important to note that CD4 depletion in people living with HIV (PLHIV) may diminish test reliability [39] and the ability of ELISPOT-based assays to correct for the number of lymphocytes in the sample may preserve test reliability [40]. On the basis of established cut-off values, a positive result in exposed patients and patients at high risk of developing active tuberculosis represents an indication for preventive treatment. Neither IGRAs nor the TST differentiate LTBI from disease [41]. The use of IGRAs in low resource settings is prohibitive by its high price. TST requires cold chain and the patient to come to the health facility 48–72 hours after test placement for induration reading. Although quantitative information is not used routinely, tuberculosis incidence is higher among individuals with larger TST indurations or IGRA-levels [42]. The predictive value of IGRAs was shown to be higher than TST in low-incidence countries [43,44], whereas predictive utility of both tests is rather low in high-burden countries [45]. The performance of TST tends to be better in younger populations; nevertheless, for IGRAs no clear trend was identified in a meta-analysis [45] mainly because of one study where IGRAs performed poorer in children [46]. Therefore, newer generation tests are needed to better identify individuals who would benefit from tuberculosis preventive treatment (TPT). Novel skin-tests using M tuberculosis-specific antigens (region of difference 1 antigens) have been explored as alternatives and showed similar performance as IGRAs or TST [47] namely similar specificity to IGRA, and higher sensitivity than TST in children and in PLHIV [48–50].
Promising experimental approaches to differentiate between LTBI and tuberculosis exist based on altered cytokine expression profiles [51–53] or enrichment of specific T-cells from blood into pleural fluid or bronchoalveolar lavage [54–56]. Whole-blood signatures comprising different numbers of transcripts have been explored. Although whole-blood transcriptional signatures have demonstrated the potential to identify individuals at risk of developing tuberculosis, these individuals are mostly those who will develop incipient and subclinical disease [52,53,57]. The CORTIS trial analysed the diagnostic performance of a signature comprising 11 transcripts (RISK11) [58]. The signature identified active tuberculosis and confirmed its potential to predict progression to incident tuberculosis. However, when study participants were randomized to receive preventive chemotherapy based on the RISK11 signature, this did not reduce progression to tuberculosis. Although further studies are needed to define clinical applications, these novel test principles will have potential to better identify the states along the tuberculosis spectrum.
TPT
TPT recommended after exposure to tuberculosis is considered secondary prophylaxis. When recommended in PLHIV as part of a comprehensive package of HIV care regardless of tuberculosis exposure in high-burden settings. TPT is considered primary prophylaxis. Shorter recommended regimens can reduce the risk of tuberculosis development by 60–90% (Table 2) [59–61] and are preferred because of their association with higher completion rates. The shortest recommended regimen is 1 month of rifapentine and isoniazid daily [62]. Studies demonstrate that rifamycin-containing regimens have similar efficacy to 6 or 9 months of isoniazid monotherapy [63–65] and 3-month daily rifampicin is associated with a lower risk of hepatotoxicity [64,66]. Although low TPT coverage may be associated with acquired drug resistance, increased TPT coverage reduces acquired drug resistance [67].
Table 2.
TB preventive treatment regimens
| Regimen | Dosea | Comments |
|---|---|---|
|
| ||
| 4 mo of daily rifampicin | Age 10 y and older: 10 mg/kg/d Age <10 y: 15 mg/kg/d (range, 10–20 mg) | • Less hepatotoxicity than isoniazid monotherapy: 1.8% for 6H vs. 03% for 4R [64], • Less than 0.01 difference in confirmed tuberculosis when comparing rifampin and isoniazid after 28 mo of follow-up [66]. • Potent inducer of the cytochrome P450 enzyme system and can reduce concentrations of certain drugs (e.g. warfarin and protease inhibitors) significantly.b |
| 3 mo of daily rifampicin plus isoniazid | Isoniazid: Age 10 y and older: 5 mg/kg/d Age <10 y: 10 mg/kg/d (range, 7–15 mg) Rifampicin: Age 10 y and older: 10 mg/kg/d Age <10 y: 15 mg/kg/d (range, 10–20 mg) |
• Hepatotoxic risk not significantly different from 6H (OR 0.83, 95% Cl, 0.49–1.42) [59]. • Paediatric fixed dose formulations available; might be the preferred option in young children. • Potent inducer of the cytochrome P450 enzyme system and can reduce concentrations of certain drugs significantly (e.g. warfarin and protease inhibitors).b |
| 3 mo weekly rifapentine plus isoniazid (12 doses) | Age 2–14 y: Differ by weight band (see the WHO guidelines3) Age >14 y: Rifapentine 900 mg + Isoniazid 900 mg |
• Less hepatotoxicity than isoniazid monotherapy: 1.5% for 3HP vs. 5.5% for 6H (in HIV-positive)2 and 0.4% for 3HP vs. 2.7% for 9H (in HIV-negative) [65]. • There was a difference of 24% in TB occurrence in the 3HP group versus the isoniazid group [63]. • Systemic drug reactions appear to be more common than others: 3.5% for 3HP vs. 0.4% for 9H [60]. • Limited data in pregnant women and children <2 y. • Potent inducer of the cytochrome P450 enzyme system and can reduce concentrations of certain drugs significantly (e.g. warfarin and protease inhibitors).b |
| 1 mo of daily rifapentinec plus isoniazid (28 doses) | Age ≤13 y (regardless of weight band) Isoniazid, 300 mg/d Rifapentine, 600 mg/d |
• Hepatotoxicity less or similar to 9H: 2% for 1HP vs. 3% for 9H [62], • No hypersensitivity reactions in 1496 participants in one RCT [62], • There were 2% of TB cases in both isoniazid and 1HP group after 33 y [57], • Limited evidence in children <13 y. One prospective cohort study (n = 408) reported its' safety in 2–19 y [61]. • Potent inducer of the cytochrome P450 enzyme system and can reduce concentrations of certain drugs significantly (e.g. warfarin and protease inhibitors).b |
| 6 or 9 mo of daily isoniazid | Age 10 y and older: 5 mg/kg/d Age <10 y: 10 mg/kg/d (range, 7 –15 mg) |
• Less preferred to rifamycin-containing regimens. |
| 6 mo of daily levofloxacin | Age >14 y, by body weight: <46 kg, 750 mg/d; >45 kg, 1g/d Age <15 y (range, approx. 15 –20 mg/kg/d), by body weight: • 5–9 kg: 150 mg/d; • 10–15 kg: 200–300 mg/d; • 16–23 kg: 300–400 mg/d; • 24–34 kg: 500–750 mg/d. |
• Regimens should be developed for other types of drug resistance. |
1HP, 1 mo of daily rifapentine plus isoniazid; 3HP, 3 mo weekly rifapentine plus isoniazid; 4R, 4 mo of daily rifampicin; 6H, 6 mo of daily isoniazid; 9H, 9 mo of daily isoniazid.
Based on WHO consolidated guidelines on tuberculosis. Module 1: prevention—tuberculosis preventive treatment.
Detailed information is available elsewhere (e.g. https://reference.medscape.com/drug-interactionchecker).
Rifapentine is not currently available in many European countries [68].
The choice of regimen should be based upon the availability of medicines and formulations, the risk of adverse events, use of concomitant medications, and patients’ preferences. Notably, rifapentine is not available worldwide including countries within the European region of the WHO [68]. Furthermore, the cost of rifapentine can be a barrier, particularly in high-burden countries with resource constraints.
TPT in pregnant women and young children
Data on the use of rifapentine in pregnant women and children <2 years are limited [3]. One randomized controlled trial reported a higher risk of adverse pregnancy outcomes in women who were given isoniazid monotherapy during pregnancy compared with those who started therapy postpartum, whereas the same signal was not observed in observational studies [69,70]. Rifampicin is generally considered safe and might be the preferred option for pregnant women. The timing of TPT should be discussed with pregnant women, considering their two-fold increased risk of tuberculosis late in pregnancy and the post-partum period. Furthermore, maternal tuberculosis is associated with a six-fold increased risk of poor outcome in the neonate.
TPT in MDR/RR-tuberculosis
Among individuals in close contact with people with multidrug/rifampicin-resistant tuberculosis (MDR/RR-tuberculosis), the WHO recommends daily fluoroquinolone (levofloxacin or moxifloxacin) for 6 months alone or in combination with other agents such as ethionamide or ethambutol [3], although the recommendation was based on limited evidence. Two randomized controlled trials (RCTs) of daily levofloxacin alone for 6 months in contacts of MDR/RR-tuberculosis are expected to report results in 2023 [71–73]. For other types of drug resistance, the evidence on TPT is lacking.
Recommendations in low-burden settings
In accordance with the END-TB Strategy, low tuberculosis incidence countries (incidence rate <10/100 000 population) strive towards elimination (<1/100 000) by 2035 [74,75]. In addition to ensuring effective treatment for people with tuberculosis, active case finding and LTBI screening among risk-groups are key to achieving this ambitious goal [75]. Screening may focus on close contacts of index cases with pulmonary tuberculosis, though contacts of extra-pulmonary cases are also at risk [76].
Tuberculosis disproportionately affects specific populations in low-incidence settings, particularly recent migrants from high-burden countries, people undergoing immunosuppressive therapy, and PLHIV [77–79]. These groups, along with individuals recently exposed to tuberculosis, should be prioritized for active case finding and LTBI screening.
New-entrant screening may identify people at the point of entry in the destination country, or at pre-immigration screening, and is complemented by retrospective identification of people within 2 to 5 years of arrival [80]. For migrants, the risk of tuberculosis is known to decline with time since exposure or migration [81], highlighting that active tuberculosis/LTBI screening should be performed rapidly after arrival to maximize benefits. In the absence of tuberculosis, a discussion of the individual benefits and risks of preventative therapy can be supported using the personalized risk predictor tool PER1SKOPE-TB, which incorporates IGRA/TST results and individual risk factors (such as age, HIV status, history of contact, country of birth, date of migration, solid organ recipient) [81]. In parallel to efforts implemented within low-incidence countries, it is critical and cost-effective to support tuberculosis control efforts in high-burden settings, thereby contributing towards global progress and further reducing the risk of disease among recent entrants [82].
Recommendations in high-burden settings
Tuberculosis high incidence settings (incidence rate >100/100 000 population) carry a disproportionate burden of tuberculosis and are often within low- and middle-income countries. The tuberculosis epidemic in these settings is fuelled by socioeconomic determinants; poverty, malnutrition, and hunger increase susceptibility to LTBI, active tuberculosis, and severity of clinical outcomes [83–85]. A recent cluster randomized controlled trial estimated that provision of nutritional supplementation to 30 households would prevent one incident tuberculosis case [86]. Tuberculosis control can only be achieved by coordinated interventions related to social structural determinants, as well as timely diagnosis and support throughout the treatment. Several social and financial support strategies have been proposed to improve tuberculosis treatment adherence, including conditional cash transfers, which improved treatment completion rates [87]. Nevertheless, a meta-analysis of interventions using cash incentives has shown little to no effect on the number of people that are cured or complete tuberculosis treatment.
Evidence from high-burden settings has shown a three-fold increase in LTBI measured using TST/IGRAs in tuberculosis-affected households compared with tuberculosis-free households. Notably, RCTs demonstrate benefits of TPT regardless of TST/IGRA results in PLHIV [88]. Furthermore, the results of an individual-participant meta-analysis in PLHIV have demonstrated the utility of C-reactive protein alone and chest radiograph combined with symptom screening to effectively screen for tuberculosis [89]. The WHO symptom screen in household contacts had a pooled sensitivity of 89% (52–98%) and a specificity of 69% (51–83%) [90]. In young children, symptom screening and treating, without testing for LTBI, represents the most cost-effective strategy [91]. Therefore, the WHO recommends prioritizing TPT initiation in children under five who are household contacts of a person with bacteriologically confirmed tuberculosis, or in PLHIV [3]. TPT can be initiated in this population without the need for LTBI testing or a chest radiograph and through symptom-based exclusion of tuberculosis [92]. However, improved technologies such as portable radiographs and computer-aided detection could play a crucial role in improving tuberculosis detection [93]; unfortunately, computer-aided detection technologies have not been effectively evaluated in children.
Conclusion
Effective prevention of tuberculosis requires a multi-prong approach including novel vaccines, improved tests offering accuracy for each stage on the tuberculosis continuum, and shorter and accessible preventive treatment regimens. Although low-burden countries strive for elimination and narrowly combat tuberculosis primarily in migrants, high-burden settings still face numerous challenges and transmission ubiquitously plagues many high-risk groups. Robust collaboration and innovative research are needed to reduce the global burden of tuberculosis and develop new detection tools, vaccines, and treatment regimens that serve all populations.
Footnotes
Transparency declaration
SHEK is co-applicant of a patent for TB biomarkers, and coinventor and coholder of a patent for the TB vaccine, VPM1002, which has been licensed to Vakzine Projekt Management GmbH, Hannover/Germany and Serum Institute for India Ltd., Pune/India. CL reports personal fees from Insmed, Gilead, GSK, and Janssen for lecturing at sponsored symposia outside of the presented work. MS has received grants for investigator-initiated studies by Astellas and Biotest, and travel support and honoraria from Biotest, Moderna, MSD, Qiagen, and Takeda outside the presented work. CL is supported by the German Center for Infection Research. CL received support from the German Excellence Strategy – EXC 2167 Precision Medicine in Inflammation. No external funding has been received for this manuscript.
References
- [1].World Health Organization. Global tuberculosis report. Geneva: World Health Organization; 2022. [Google Scholar]
- [2].Houben RMGJ Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLOS Med 2016;13:e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].World Health Organization. WHO operational handbook on tuberculosis. Module 1: prevention - Tuberculosis preventive treatment. Geneva: World Health Organization; 2020. [Google Scholar]
- [4].Drain PK, Bajema KL, Dowdy D, Dheda K, Naidoo K, Schumacher SG, et al. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev 2018;31:e000211.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barry CE, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 2009;7:845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Achkar JM, Jenny-Avital ER. Incipient and subclinical tuberculosis: defining early disease states in the context of host immune response. J Infect Dis 2011; 204:S1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Migliori GB, Ong CWM, Petrone L, D’Ambrosio L, Centis R, Goletti D. The definition of tuberculosis infection based on the spectrum of tuberculosis disease. Breathe (Sheff). 2021:17:210079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Martinez L, Shen Y, Mupere E, Kizza A, Hill PC, Whalen CC. Transmission of Mycobacterium tuberculosis in households and the community: a systematic review and meta-analysis. Am J Epidemiol 2017;185:1327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kontturi A, Kekomäki S, Ruotsalainen E, Salo E. Tuberculosis contact investigation results among paediatric contacts in low-incidence settings in Finland. Eur J Pediatr 2021;180:2185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Reichler MR, Khan A, Yuan Y, Chen B, McAuley J, Mangura B, et al. Duration of exposure among close contacts of patients with infectious tuberculosis and risk of latent tuberculosis infection. Clin Infect Dis 2020;71:1627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Adetifa IMO, Kendall L, Donkor S, Lugos MD, Hammond AS, Owiafe PK, et al. Mycobacterium tuberculosis infection in close childhood contacts of adults with pulmonary tuberculosis is increased by secondhand exposure to tobacco. Am J Trop Med Hyg 2017;97:429–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chae H, Shin SJ. Importance of differential identification of Mycobacterium tuberculosis strains for understanding differences in their prevalence, treatment efficacy, and vaccine development. J Microbiol 2018;56:300–11. [DOI] [PubMed] [Google Scholar]
- [13].Saelens JW, Sweeney MI, Viswanathan G, Xet-Mull AM, Jurcic Smith KL, Sisk DM, et al. An ancestral mycobacterial effector promotes dissemination of infection. Cell 2022;185:4507–4525.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ashton PM, Cha J, Anscombe C, Thuong NTT, Thwaites GE, Walker TM. Distribution and origins of Mycobacterium tuberculosis L4 in southeast asia. bioRxiv; 2022. https://www.biorxiv.Org/content/10.1101/2022.08.01.502309v1. [Accessed 13 August 2023]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Diel R, Kohl TA, Maurer FP, Merker M, Meywald Walter K, Hannemann J, et al. Accuracy of whole-genome sequencing to determine recent tuberculosis transmission: an 11-year population-based study in Hamburg, Germany. Eur Respir J 2019:54:1901154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mtetwa HN, Amoah ID, Kumari S, Bux F, Reddy P. The source and fate of Mycobacterium tuberculosis complex in wastewater and possible routes of transmission. BMC Public Health 2022;22:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nikolayevskyy V, Niemann S, Anthony R, van Soolingen D, Tagliani E, Ködmön C, et al. Role and value of whole genome sequencing in studying tuberculosis transmission. Clin Microbiol Infect 2019;25:1377–82. [DOI] [PubMed] [Google Scholar]
- [18].Karat AS, Gregg M, Barton HE, Calderon M, Ellis J, Falconer J, et al. Evidence for the use of triage, respiratory isolation, and effective treatment to reduce the transmission of Mycobacterium tuberculosis in healthcare settings: a systematic review. Clin Infect Dis 2021;72:155–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mandalakas AM, Kirchner HL, Iverson S, Chesney M, Spencer MJ, Sidler A, et al. Predictors of Mycobacterium tuberculosis infection in international adoptees. Pediatrics 2007;120:e610–6. [DOI] [PubMed] [Google Scholar]
- [20].Sequera VG, Aguirre S, Estigarribia G, Cellamare M, Croda J, Andrews JR, et al. Increased incarceration rates drive growing tuberculosis burden in prisons and jeopardize overall tuberculosis control in Paraguay. Sci Rep 2020;10:21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wood R, Liang H, Wu H, Middelkoop K, Oni T, Rangaka MX, et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis 2010;14:406–12. [PMC free article] [PubMed] [Google Scholar]
- [22].Lange C, Aaby P, Behr MA, Donald PR, Kaufmann SHE, Netea MG, et al. 100 years of Mycobacterium bovis bacille Calmette-Guérin. Lancet Infect Dis 2022;22. e2–12. [DOI] [PubMed] [Google Scholar]
- [23].Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PEM, et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 2014;58:470–80. [DOI] [PubMed] [Google Scholar]
- [24].Martinez L, Cords O, Iiu Q, Acuna-Villaorduna C, Bonnet M, Fox GJ, et al. Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: a systematic review and individual participant data meta-analysis. Lancet Glob Health 2022;10:e1307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rodrigues LC, Pereira SM, Cunha SS, Genser B, Ichihara MY, de Brito SC, et al. Effect of BCG revaccination on incidence of tuberculosis in school-aged children in Brazil: the BCG-REVAC duster-randomised trial Lancet 2005;366:1290–5. [DOI] [PubMed] [Google Scholar]
- [26].Nemes E, Geldenhuys H, Rozot V, Rutkowski KT, Ratangee F, Bilek N, et al. Prevention of M. tuberculosis Infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med 2018;379:138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fifteen year follow up of trial of BCG vaccines in south India for tuberculosis prevention. Tuberculosis Research Centre (ICMR), Chennai. Indian J Med Res 1999;110:56–69. [PubMed] [Google Scholar]
- [28].Velayutham B, Thiruvengadam K, Kumaran PP, Watson B, Rajendran K, Padmapriyadarsini C. Revisiting the chingleput BCG vaccination trial for the impact of BCG revaccination on the incidence of tuberculosis disease. Indian J Med Res 2023;157:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kaufmann SHE. The TB vaccine development pipeline: present and future priorities and challenges for research and innovation. In: Essential tuberculosis. Heidelberg: Springer Nature Switzerland; 2021. p. 395–405. [Google Scholar]
- [30].Kaufmann SHE. Vaccine development against tuberculosis over the last 140 years: failure as part of success. Front Microbiol 2021:12:750124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Working Group on New TB Vaccines. TB vaccine pipeline, https://newtbvaccines.org/tb-vaccine-pipeline/. [Google Scholar]
- [32].Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, et al. A functional role for antibodies in tuberculosis. Cell 2016;167:433–443. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].World Health Organization. Correlates of vaccine-induced protection: methods and implications. Geneva: World Health Organization; 2013. [Google Scholar]
- [34].Flynn JL, Chan J. Immune cell interactions in tuberculosis. Cell 2022;185:4682–702. [DOI] [PubMed] [Google Scholar]
- [35].Tait DR, Hatherill M, Van Der Meeren O, Ginsberg AM, Van Brakel E, Salaun B, et al. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med 2019;381:2429–39. [DOI] [PubMed] [Google Scholar]
- [36].Munseri P, Said J, Amour M, Magohe A, Matee M, Rees CA, et al. DAR-901 vaccine for the prevention of infection with Mycobacterium tuberculosis among BCG-immunized adolescents in Tanzania: a randomized controlled, double-blind phase 2b trial. Vaccine 2020;38:7239–45. [DOI] [PubMed] [Google Scholar]
- [37].Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S, Goletti D, et al. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J 2009;33:956–73. [DOI] [PubMed] [Google Scholar]
- [38].Sester M, van Leth F, Bruchfeld J, Bumbacea D, Cirillo DM, Dilektasli AG, et al. Risk assessment of tuberculosis in immunocompromised patients. A TBNET study. Am J Respir Crit Care Med 2014;190:1168–76. [DOI] [PubMed] [Google Scholar]
- [39].Leidl L, Mayanja-Kizza H, Sotgiu G, Baseke J, Ernst M, Hirsch C, et al. Relationship of immunodiagnostic assays for tuberculosis and numbers of circulating CD4+ T-cells in HIV infection. Eur Respir J 2010;35:619–26. [DOI] [PubMed] [Google Scholar]
- [40].Mandalakas AM, Hesseling AC, Chegou NN, Kirchner HL, Zhu X, Marais BJ, et al. High level of discordant IGRA results in HIV-infected adults and children. Int J Tuberc Lung Dis 2008;12:417–23. [PubMed] [Google Scholar]
- [41].Sester M, Sotgiu G, Lange C, Giehl C, Girardi E, Migliori GB, et al. Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J 2011;37:100–11. [DOI] [PubMed] [Google Scholar]
- [42].Gupta RK, Lipman M, Jackson C, Sitch AJ, Southern J, Drobniewski F, et al. Quantitative IFN-γ release assay and tuberculin skin test results to predict incident tuberculosis. A prospective cohort study. Am J Respir Crit Care Med 2020;201:984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhou G, Luo Q Luo S, Teng Z, Ji Z, Yang J, et al. Interferon-γ release assays or tuberculin skin test for detection and management of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis 2020;20:1457–69. [DOI] [PubMed] [Google Scholar]
- [44].Abubakar I, Lalvani A Southern J, Sitch A, Jackson C, Onyimadu O, et al. Two interferon gamma release assays for predicting active tuberculosis: the UK PREDICT TB prognostic test study. Health Technol Assess 2018;22:1–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hamada Y, Gupta RK, Quartagno M, Izzard A, Acuna-Villaorduna C, Altet N, et al. Predictive performance of interferon-gamma release assays and the tuberculin skin test for incident tuberculosis: an individual participant data meta-analysis. EClinicalMedicine 2023:56:101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sharma SK, Vashishtha R Chauhan LS, Sreenivas V, Seth D. Comparison of TST and IGRA in diagnosis of latent tuberculosis infection in a high TB-burden setting. PLOS ONE 2017;12:e0169539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Krutikov M, Faust L, Nikolayevskyy V, Hamada Y, Gupta RK, Cirillo D, et al. The diagnostic performance of novel skin-based in-vivo tests for tuberculosis infection compared with purified protein derivative tuberculin skin tests and blood-based in vitro interferon-γ release assays: a systematic review and meta-analysis. Lancet Infect Dis 2022;22:250–64. [DOI] [PubMed] [Google Scholar]
- [48].World Health Organization. Rapid communication: TB antigen-based skin tests for the diagnosis of TB infection. Geneva: World Health Organization; 2022. [Google Scholar]
- [49].Badaro R Machado BAS, Duthie MS, Araujo-Neto CA, Pedral-Sampaio D, Nakatani M, et al. The single recombinant M. tuberculosis protein DPPD provides enhanced performance of skin testing among HIV-infected tuberculosis patients. AMB Express 2020;10:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lozovskaya ME, Belushkov W, Gurina OP, Vasilyeva YB, Klochkova LV. Comparative evaluation of innovative diagnostic tests for latent and active TB infection in children. Pediatrician (St Petersburg) 2014;5:46–50. [Google Scholar]
- [51].Sester U, Fousse M, Dirks J, Mack U, Prasse A, Singh M, et al. Whole-blood flow-cytometric analysis of antigen-specific CD4 T-cell cytokine profiles distinguishes active tuberculosis from non-active states. PLOS ONE 2011;6:e17813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gupta RK, Turner CT, Venturini C, Esmail H, Rangaka MX, Copas A et al. Concise whole blood transcriptional signatures for incipient tuberculosis: a systematic review and patient-level pooled meta-analysis. Lancet Respir Med 2020;8:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zak DE, Penn-Nicholson A Scriba TJ, Thompson E, Suliman S, Amon LM, et al. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet 2016;387:2312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Jafari C, Emst M, Kalsdorf B, Greinert U, Diel R Kirsten D, et al. Rapid diagnosis of smear-negative tuberculosis by bronchoalveolar lavage enzyme-linked immunospot. Am J Respir Crit Care Med 2006;174:1048–54. [DOI] [PubMed] [Google Scholar]
- [55].Jafari C, Ernst M, Strassburg A Greinert U, Kalsdorf B, Kirsten D, et al. Local immunodiagnosis of pulmonary tuberculosis by enzyme-linked immunospot. Eur Respir J 2008;31:261–5. [DOI] [PubMed] [Google Scholar]
- [56].Jafari C, Olara ID, Daduna F, Ernst M, Heyckendorf J, Lange C, et al. Rapid diagnosis of pulmonary tuberculosis by combined molecular and immunological methods. Eur Respir J 2018:51:1702189. [DOI] [PubMed] [Google Scholar]
- [57].Roe J, Venturini C, Gupta RK, Gurry C, Chain BM, Sun Y, et al. Blood transcriptomic stratification of short-term risk in contacts of tuberculosis. Clin Infect Dis 2020;70:731–7. [DOI] [PubMed] [Google Scholar]
- [58].Scriba TJ, Fiore-Gartland A, Penn-Nicholson A, Mulenga H, Mbandi SK, Borate B, et al. Biomarker-guided tuberculosis preventive therapy (CORTIS): a randomised controlled trial. Lancet Infect Dis 2021;21:354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zenner D, Beer N, Harris RJ, Lipman MC, Stagg HR, van der Werf MJ. Treatment of latent tuberculosis infection: an updated network meta-analysis. Ann Intern Med 2017;167:248–55. [DOI] [PubMed] [Google Scholar]
- [60].Sterling TR Mora RN, Borisov AS, Phillips E, Shepherd G, Adkinson NF, et al. Flu-like and Other systemic drug reactions among persons receiving weekly rifapentine plus isoniazid or daily isoniazid for treatment of latent tuberculosis infection in the PREVENT tuberculosis study. Clin Infect Dis 2015;61:527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Malik AA Farooq S, Jaswal M, Khan H, Nasir K, Fareed U, et al. Safety and feasibility of 1 month of daily rifapentine plus isoniazid to prevent tuberculosis in children and adolescents: a prospective cohort study. Lancet Child Adolesc Health 2021;5:350–6. [DOI] [PubMed] [Google Scholar]
- [62].Swindells S, Ramchandani R, Gupta A, Benson CA, Leon-Cruz J, Mwelase N, et al. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med 2019;380:1001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Martinson NA Barnes GL, Moulton LH, Msandiwa R, Hausier H, Ram M, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med 2011;365:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Menzies D, Adjobimey M, Ruslami R, Trajman A, Sow O, Kim H, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med 2018;379:440–53. [DOI] [PubMed] [Google Scholar]
- [65].Sterling TR Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 2011;365:2155–66. [DOI] [PubMed] [Google Scholar]
- [66].Hamada Y, Ford N, Schenkel K, Getahun H. Three-month weekly rifapentine plus isoniazid for tuberculosis preventive treatment: a systematic review. Int J Tuberc Lung Dis 2018;22:1422–8. [DOI] [PubMed] [Google Scholar]
- [67].Kunkel A Colijn C, Lipsitch M, Cohen T. How could preventive therapy affect the prevalence of drug resistance? Causes and consequences. Philos Trans R Soc Lond B Biol Sci 2015:370:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Guglielmetti L, Günther G, Leu C, Cirillo D, Duarte R, Garcia-Basteiro AL, et al. Rifapentine access in Europe: growing concerns over key tuberculosis treatment component. Eur Respir J 2022:59:2200388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gupta A Montepiedra G, Aaron L, Theron G, McCarthy K, Bradford S, et al. Isoniazid preventive therapy in HIV-infected pregnant and postpartum women. N Engl J Med 2019;381:1333–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hamada Y, Figueroa C, Martín-Sánchez M, Falzon D, Kanchar A The safety of isoniazid tuberculosis preventive treatment in pregnant and postpartum women: systematic review and meta-analysis. Eur Respir J 2020:55:1901967. [DOI] [PubMed] [Google Scholar]
- [71].Fox GJ, Nguyen CB, Nguyen TA Tran PT, Marais BJ, Graham SM, et al. Levofloxacin versus placebo for the treatment of latent tuberculosis among contacts of patients with multidrug-resistant tuberculosis (the VQUIN MDR trial): a protocol for a randomised controlled trial. BMJ Open 2020;10:e033945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Seddon JA Garcia-Prats AJ, Purchase SE, Osman M, Demers AM, Hoddinott G, et al. Levofloxacin versus placebo for the prevention of tuberculosis disease in child contacts of multidrug-resistant tuberculosis: study protocol for a phase III cluster randomised controlled trial (TB-CHAMP). Trials 2018;19:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kherabi Y, Tunesi S, Kay A, Guglielmetti L. Preventive therapy for contacts of drug-resistant tuberculosis. Pathogens 2022:11:1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].WHO. The end TB strategy. 2015. http://www.who.int/tb/strategy/End_TB_Strategy.pdf?ua=1. [Google Scholar]
- [75].World Health Organization. Towards tuberculosis elimination: an action framework for low-incidence countries. Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- [76].Wingfield T, Macpherson P, Cleary P, Ormerod LP. High prevalence of TB disease in contacts of adults with extrapulmonary TB. Thorax 2018;73:210202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cole B, Nilsen DM, Will L, Etkind SC, Burgos M, Chorba T. Essential components of a public health tuberculosis prevention, control, and elimination program: recommendations of the advisory council for the elimination of tuberculosis and the National Tuberculosis Controllers Association. MMWR Recomm Rep 2020;69:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].National Institute for health and care excellent guidance and guidelines. Tuberculosis; 2016. https://www.nice.org.uk/guidance/ng33. [Google Scholar]
- [79].Programmatic management of latent tuberculosis infection in the European Union. 2018. https://www.ecdc.europa.eu/en/publications-data/programmatic-management-latent-tuberculosis-infection-european-union. [Google Scholar]
- [80].UKHSA TB action plan for England 2021 to 2026. 2021. https://www.gov.uk/govemment/publications/tuberculosis-tb-action-plan-for-england/tuberculosis-tb-action-plan-for-england-2021-to-2026. [Google Scholar]
- [81].Gupta RK, Calderwood CJ, Yavlinsky A, Krutikov M, Qpartagno M, Alchelburg MC, et al. Discovery and validation of a personalized risk predictor for incident tuberculosis in low transmission settings. Nat Med 2020;26:1941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Schwartzman K, Menzies D. Tuberculosis screening of immigrants to low-prevalence countries. A cost-effectiveness analysis. Am J Respir Crit Care Med 2000;161:780–9. [DOI] [PubMed] [Google Scholar]
- [83].Hargreaves JR, Boccia D, Evans CA, Adato M, Petticrew M, Porter JDH. The social determinants of tuberculosis: from evidence to action. Am J Public Health 2011;101:654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Carwile ME, Hochberg NS, Sinha P. Undernutrition is feeding the tuberculosis pandemic: a perspective.J Clin Tuberc Mycobact Dis 2022:27:100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Boccia D, Hargreaves J, Ayles H, Fielding K, Simwinga M, Godfrey-Faussett P. Tuberculosis infection in Zambia: the association with relative wealth. Am J Trap Med Hyg 2009;80:1004–11. [PMC free article] [PubMed] [Google Scholar]
- [86].Bhargava A, Bhaigava M, Meher A, Benedetti A, Velayutham B, Teja GS, et al. Nutritional supplementation to prevent tuberculosis incidence in household contacts of patients with pulmonary tuberculosis in India (RATIONS): a field-based, open-label, cluster-randomised, controlled trial. Lancet 2023;402:627–40. [DOI] [PubMed] [Google Scholar]
- [87].Dave JD, Rupani MP. Does direct benefit transfer improve outcomes among people with tuberculosis? – a mixed-methods study on the need for a review of the cash transfer policy in India. Int J Health Policy Manag 2022;11:2552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Badje A Moh R, Gabillard D, Guéhi C, Kabran M, Ntakpé JB, et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the Temprano ANRS 12136 trial. Lancet Glob Health 2017;5:e1080–9. [DOI] [PubMed] [Google Scholar]
- [89].Dhana A, Hamada Y, Kengne AP, Kerkhoff AD, Rangaka MX, Kredo T, et al. Tuberculosis screening among ambulatory people living with HIV: a systematic review and individual participant data meta-analysis. Lancet Infect Dis 2022;22:507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Vonasek B, Ness T, Takwoingi Y, Kay AW, Wyk SS van, Ouellette L, et al. Screening tests for active pulmonary tuberculosis in children. Cochrane Database Syst Rev 2021;6:CD013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mandalakas AM, Hesseling AC, Gie RP, Schaaf HS, Marais BJ, Sinanovic E. Modelling the cost-effectiveness of strategies to prevent tuberculosis in child contacts in a high-burden setting. Thorax 2013;68:247–55. [DOI] [PubMed] [Google Scholar]
- [92].Vasiliu A, Abelman RA, Casenghi M, Cohn J, Bonnet M. Symptom-based screening versus chest radiography for TB child contacts: a systematic review and meta-analysis. Pediatr Infect Dis J 2021;40:1064–9. [DOI] [PubMed] [Google Scholar]
- [93].Melendez J, Hogeweg L, Sánchez Cl, Philipsen RHHM, Aldridge RW, Hayward AC, et al. Accuracy of an automated system for tuberculosis detection on chest radiographs in high-risk screening. Int J Tuberc Lung Dis 2018;22:567–71. [DOI] [PMC free article] [PubMed] [Google Scholar]