Abstract
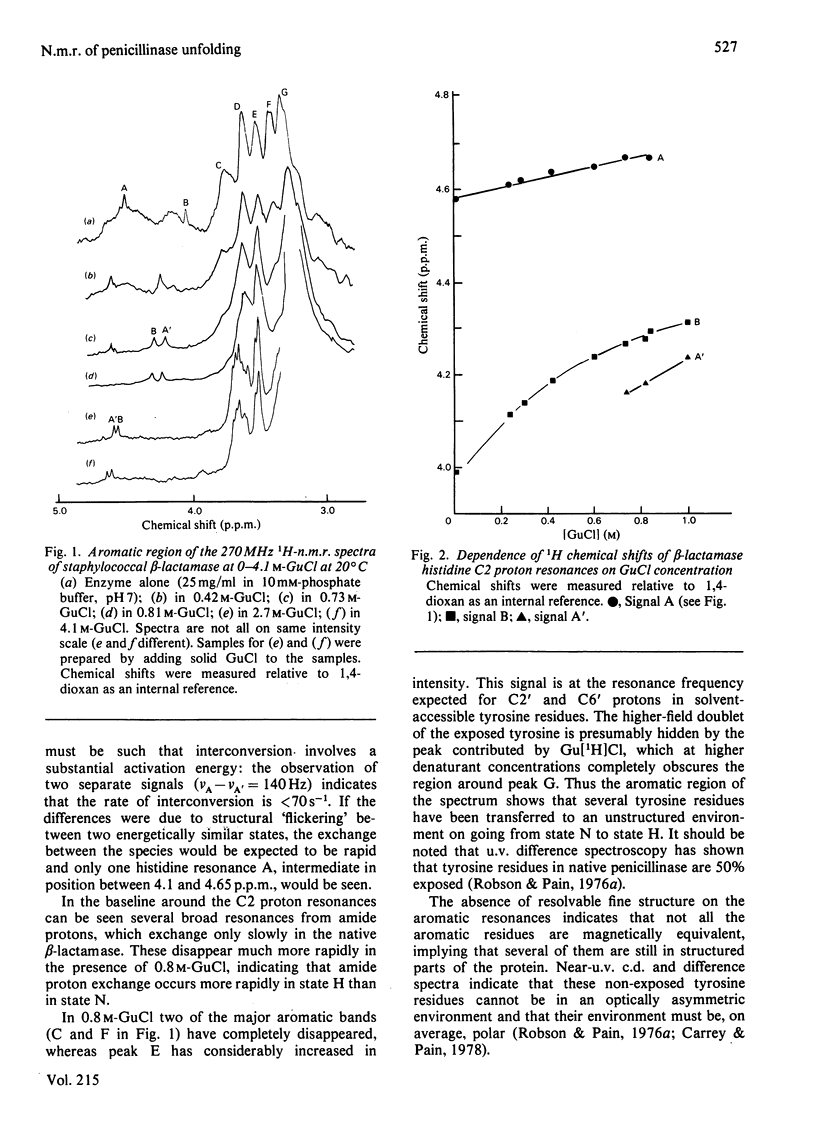
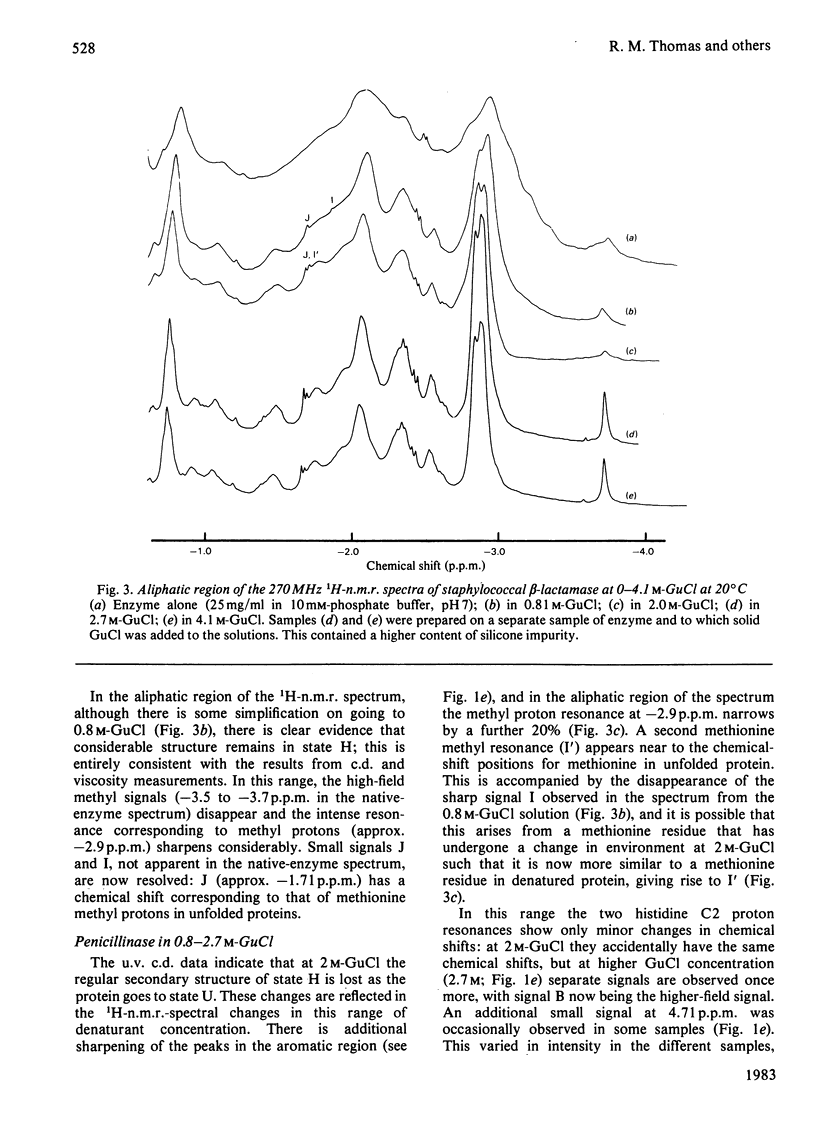
The unfolding of beta-lactamase (penicillinase) from Staphylococcus aureus by guanidinium chloride was followed by using n.m.r. spectroscopy. On the basis of the observation of resonances corresponding to histidine, tyrosine and other amino acid side chains, the existence of a stable partially folded species was demonstrated. These experiments provide detailed characterization of the intermediate that confirms and extends previous characterization by absorption and c.d. spectroscopy and by flow properties. In addition, they show that residues in the N-terminal third of the molecule are affected by the native-to-intermediate transition. Persistent non-equivalence of the two imidazole C2 proton resonances at high guanidinium chloride concentrations is discussed in terms of local sequence effects on the chemical shift.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The amino acid sequence of Staphylococcus aureus penicillinase. Biochem J. 1975 Nov;151(2):197–218. doi: 10.1042/bj1510197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrey E. A., Pain R. H. A reversibly refolding non-covalent complex of three peptides from Staphylococcus aureus penicillinase [proceedings]. Biochem Soc Trans. 1977;5(3):689–692. doi: 10.1042/bst0050689. [DOI] [PubMed] [Google Scholar]
- Carrey E. A., Pain R. H. Conformation of a stable intermediate on the folding pathway of Staphylococcus aureus penicillinase. Biochim Biophys Acta. 1978 Mar 28;533(1):12–22. doi: 10.1016/0005-2795(78)90542-1. [DOI] [PubMed] [Google Scholar]
- Creighton T. E., Pain R. H. Unfolding and refolding of Staphylococcus aureus penicillinase by urea-gradient electrophoresis. J Mol Biol. 1980 Mar 15;137(4):431–436. doi: 10.1016/0022-2836(80)90167-9. [DOI] [PubMed] [Google Scholar]
- Robson B., Pain R. H. The mechanism of folding of globular proteins. Equilibria and kinetics of conformational transitions of penicillinase from Staphylococcus aureus involving a state of intermediate conformation. Biochem J. 1976 May 1;155(2):331–344. doi: 10.1042/bj1550331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B., Pain R. H. The mechanism of folding of globular proteins. Suitability of a penicillinase from Staphylococcus Aureus as a model for refolding studies. Biochem J. 1976 May 1;155(2):325–330. doi: 10.1042/bj1550325. [DOI] [PMC free article] [PubMed] [Google Scholar]


