Abstract
Recipients of anti-CD19 targeted therapies such as chimeric antigen receptor (CAR)-T cell are considered at high risk for complicated Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2) infection due to prolonged B cell aplasia and immunosuppression. These patients represent a unique cohort and so far, immune responses to SARS-CoV-2 have not been well characterized in this setting. We report a pediatric patient with B-cell acute lymphoblastic leukemia (B-ALL) who had asymptomatic SARS-CoV-2 infection while receiving blinatumomab, followed by lymphodepletion (LD) and tisagenlecleucel, a CD19 targeting CAR-T therapy. The patient had a complete response to tisagenlecleucel, did not develop cytokine release syndrome, or worsening of SARS-CoV-2 during therapy. The patient had evidence of ongoing persistence of IgG antibody responses to spike and nucleocapsid after LD followed by tisagenlecleucel despite the B-cell aplasia. Further we were able to detect SARS-CoV-2 specific T-cells recognizing multiple viral structural proteins for several months following CAR-T. The T-cell response was polyfunctional and predominantly CD4 restricted. This data has important implications for the understanding of SARS-CoV-2 immunity in patients with impaired immune systems and the potential application of SARS-CoV-2-specific T-cell therapeutics to treat patients with blood cancers who receive B cell depleting therapy.
Introduction
Children diagnosed with Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2), even those with underlying malignancies, seem to have less severe symptoms than adults with similar conditions.1,2 SARS-CoV-2 can result in significant lymphopenia especially in severe disease leading to suppression of interferon and cytokine expression, inhibition of effector cell activation and cellular mimicry.3,4
Immunocompromised patients, including those receiving lymphodepleting chemotherapy (LD) and hematopoietic stem cell transplant have depressed T cell function and humoral immunity.5,6 Evidence suggests that in such individuals, prolonged viral shedding can occur potentially increasing the risk of within host evolution and emergence of novel mutant strains.7
Recipients of CD19 targeting chimeric antigen receptor (CAR)-T therapy experience prolonged B cell aplasia or hypogammaglobinemia and hence are at high risk of complications from SARs-CoV-2 including hospitalization, ICU admission and death due to COVID-19.8–10 They also frequently have comorbidities such as cardiovascular and renal dysfunction that can lead to worse outcomes. In addition to the immunosuppression these patients experience, CAR-T recipients often experience complications such as cytokine release syndrome (CRS) and immune cell associated encephalopathy syndrome (ICANS) marked by intense systemic inflammation,11,12 all of which may place these patients at high risk for severe COVID-19 were they to contract the virus while receiving these targeted therapies.13 CAR-T recipients often receive intravenous immunoglobulin replacement to protect them against viral infections, but this may not confer complete protection.14–16 Although there have been recent reports describing the clinical course of COVID-19 in recipients of CAR-T therapy,13,17 few studies have examined how the immune system in such patients responds to SARS-CoV-2 infection or to the SARS-CoV-2 vaccine.18,19
We and others have shown that polyclonal and polyfunctional SARS-Cov-2 specific T cells can be recovered from healthy convalescent donors.19,20 The role of T-cell and antibody responses to SARS-CoV-2 for long-term immunity is largely unknown especially in patients who receive CD19-directed therapies. Here we evaluate the T-cell and antibody responses to structural SARS-CoV-2 antigens in a pediatric patient with B-cell acute lymphoblastic leukemia (B-ALL) who had SARS-CoV-2 while receiving blinatumomab, a bispecific T-cell engager antibody against CD19 followed by lymphodepleting chemotherapy and CD19 directed CAR-T cell (tisagenlecleucel) infusion.
Case description
Patient is a 17-year-old male referred to our institution for tisagenlecleucel, anti-CD19 CAR-T for treatment of relapsed B-ALL with involvement of bone marrow and testes. He was diagnosed with SARS-CoV-2, while receiving blinatumomab, by real time polymerase chain reaction (PCR) testing on nasopharyngeal swab performed in a clinical laboratory improvement amendments of 1998 (CLIA) facility using the BDMAX BioGX SARS-CoV-2 EUA test. Patient was asymptomatic but was tested due to exposure from a virus-positive family member. He did not develop any complications of COVID-19 while receiving blinatumomab and had persistent bone marrow disease after completion of the blinatumomab cycle. Given the uncertainty of the course of COVID-19 in this population at the onset of the pandemic, the patient’s leukapheresis was delayed by 2 weeks until he had a negative PCR test. He underwent leukapheresis for CAR-T manufacturing 37 days after the initial positive PCR test. Since the patient remained asymptomatic, he proceeded with the prescribed lymphodepletion (LD) with fludarabine and cyclophosphamide. Patient tested positive by PCR result at the time of LD (Cycle threshold 37 for target gene 1 and negative for target gene 2) and demonstrated SARS-CoV2 immunoglobulin G (IgG) antibodies and all subsequent PCR tests were negative. He received tisagenlecleucel without any further delay 75 days after his initial SARS-CoV-2 infection. Patient did not develop cytokine release syndrome or worsening of SARS-CoV-2 during therapy. Patient had minimal residual disease in the bone marrow at the time of CAR-T infusion and achieved complete remission (CR) of his B-ALL by morphology, minimal residual disease and next generation sequence testing at Day 30 post CAR-T. He has maintained a durable CR at 6 months with functional persistence of CAR-T cells as evident by persistent B cell aplasia in the peripheral blood.
Methods
Clinical data were obtained from the patient’s medical record. Peripheral blood (PB) for immune assays was collected at time of leukapheresis, Day 0 of CAR-T, Day 30, and Day 60 after obtaining informed consent on an IRB approved protocol in accordance with the Declaration of Helsinki.
Ex vivo expansion
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll and plasma cryopreserved for batch antibody testing. PBMCs were pulsed with a mix of overlapping peptide pools encompassing viral structural proteins in presence of cytokines and expanded in T cell medium as recently described.20 Cells were harvested on Day 10 and evaluated for immunophenotype, antigen specificity and functionality using IFN-γ enzyme-linked immunospot (ELISpot) assay (Millipore, Burlington, MA) and flow cytometry as recently described.20
Antibody serologies
Luciferase Immunoprecipitation Systems (LIPS) was used for measuring antibodies against the SARS-CoV-2 spike and nucleocapsid proteins as recently described.20
Results and Discussion
Given this patient’s SARS-CoV-2 infection in the context of B-cell depleting treatments, we evaluated the immune profile of the patient at various timepoints. Patient had diminished total absolute lymphocyte counts, and absolute T and B-cell counts at the time of SARS-CoV-2 infection as expected following LD and CAR-T. He had no B-cells detected in the peripheral blood consistent with effective CD19 targeting (Figure 1A).
Figure 1. Serologic and cellular immune responses to Sars-CoV-2 before and after CD19-CAR-T.
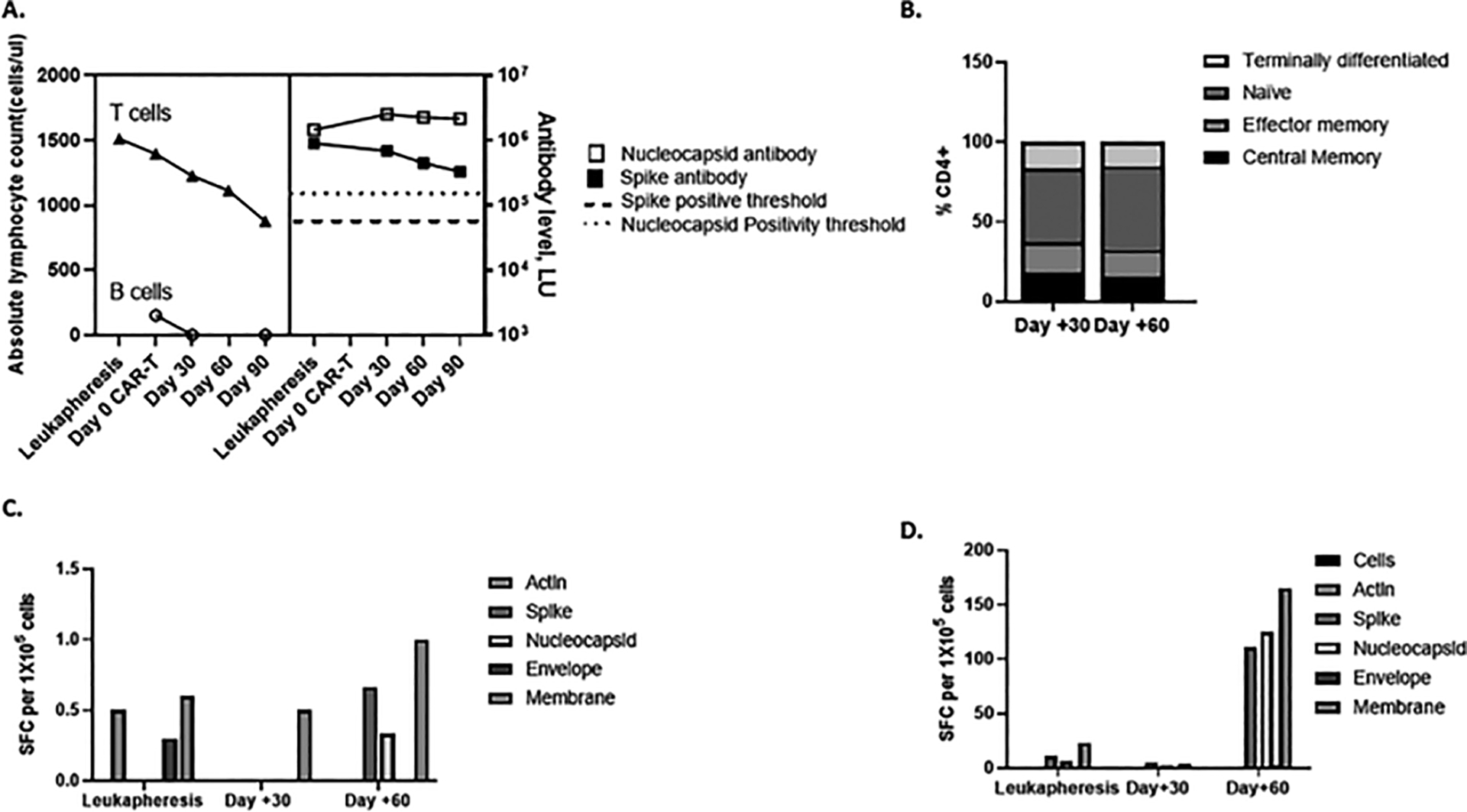
A T- and B-cell counts and antibody responses to spike and nucleocapsid as detected by LIPS assay over time. Antibody levels were tested using the Luciferase Immunoprecipitation assay (LIPS) and measured in luminescence units (LU). B Memory phenotype of SARS-CoV-2 specific T cells recovered at various timepoints. T-cell populations following expansion were determined by flow cytometry and denoted as central memory (CD45RO + CCR7+), effector memory (CD45RO − CCR7−), Naïve (CD45RO − CCR7+) and terminally differentiated (CD45RO − CCR7−). C. Detection of T-cell responses to SARS-CoV-2 from peripheral blood at various timepoints. Peripheral blood mononuclear cells (PBMC) from patient were tested for response to peptide libraries encompassing SARS-CoV-2 structural proteins by IFN-γ ELISpot pre-expansion and following ex vivo expansion for 10–12 days (D). Results are reported as spot forming cells (SFC) per 1 × 105 cells per well. Actin was used as negative control.
Despite the lymphopenia, antibody responses to spike and nucleocapsid proteins using the LIPS assay were detected in the plasma pre and post CAR-T at follow-up and remained elevated above the positivity threshold of 125, 000 LU and 45, 000 LU for nucleocapsid and spike antibodies respectively (Figure 1A) and are highly consistent with our observations in healthy adult control individuals in the 4–6 weeks following infection or a second vaccine. Furthermore, numerous studies have reported antibody responses to different vaccines, but the specific assays have varied and thus numerical values are not directly comparable.23 Next, we evaluated SARS-CoV-2 specific T-cell responses at the time of leukapheresis prior to LD and CAR-T infusion and days 30 and 60 post CAR-T. Post-expansion T-cells were predominantly CD4+ with central and effector memory subsets (Figure 1B). There was low level T-cell reactivity to SARS-CoV-2 antigens detected by the IFN-γ ELISpot assay following overnight stimulation of PBMCs with overlapping peptides of the structural proteins at all timepoints (denoted as pre-expansion in Figure 1C). To amplify this response, we expanded the PBMCS ex vivo for 10–14 days and repeated the ELISpot assay. T-cells recognizing spike, membrane and nucleocapsid proteins were detected from samples obtained pre and post CD19-CAR-T infusion (Figure 1C). The SARS-CoV-2 specific T-cells were polyfunctional with the highest TNF-α and IFN-γ responses observed to membrane protein (2.66% CD4+ T-cells) in CD4+ subsets (Figure 2A, C) at Day 60. No SARS-CoV2 CD8+ T-cell responses were detected (Figure 2B). This CD4+ specific T-cell response with a predominance to membrane was similar to our observations in healthy convalescent donors or following vaccination.21, 22 The absolute percentage of polyfunctional T cells detected in vitro that corresponds to a clinically significant in vivo response is unknown, but from our previous experience with many studies interrogating virus antigen specific and tumor associated antigen specific T cells, values consistently above negative controls and unstimulated cells in T cell products may correlate with clinical responses.24–26
Figure 2. Functional immune response of Sars-CoV-2 specific T cells.
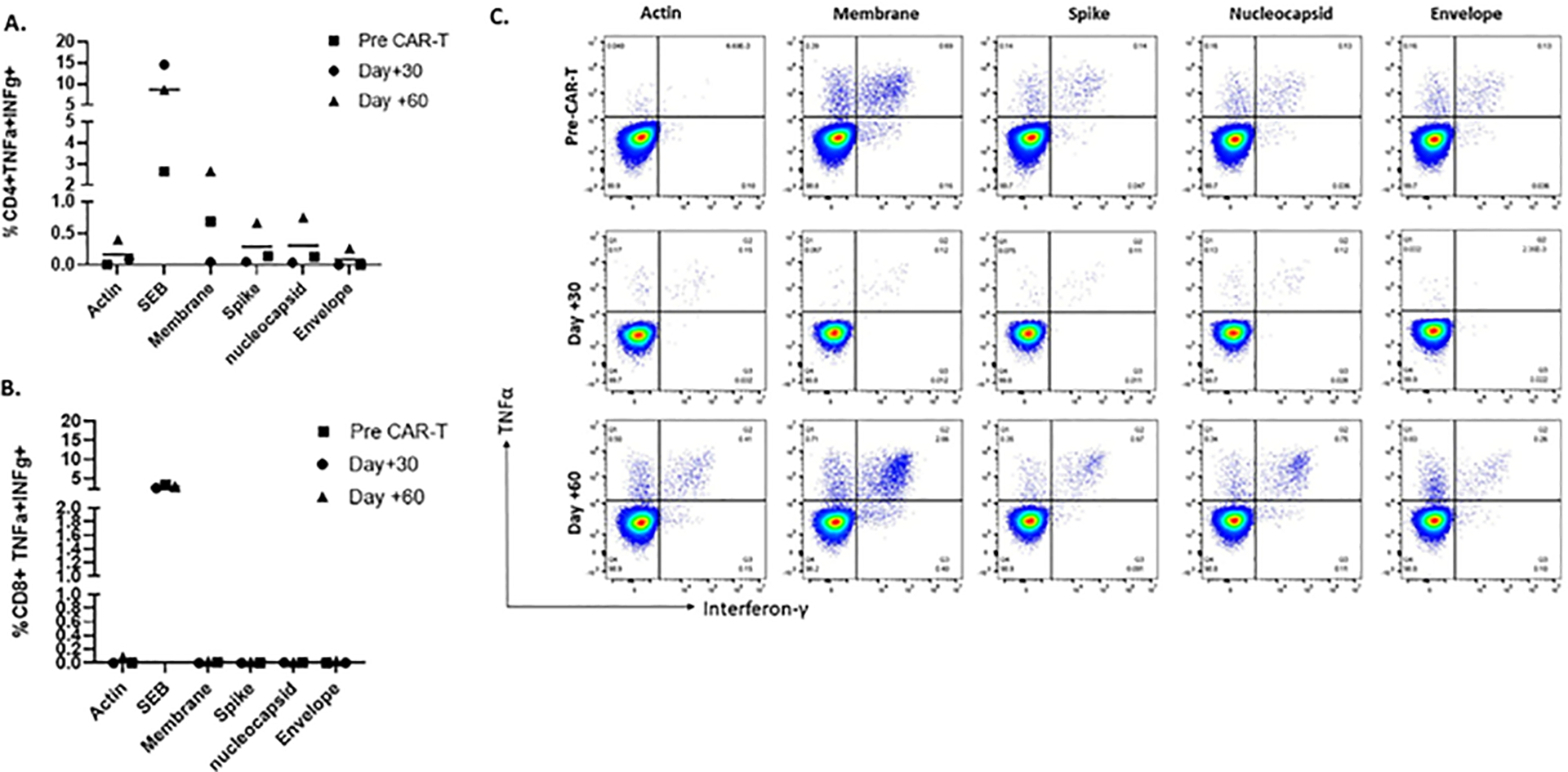
A, B. Polyfunctional T cell responses to SARS-CoV-2 antigens were tested via intracellular staining following 10–12 days of ex vivo expansion in the CD4+ (A) and CD8+ (B) subsets. C. Dot plots showing polyfunctional SARS-CoV-2 specific CD4+ T cells at various timepoints as detected by the secretion of proinflammatory cytokines TNFα and IFN-γ in response to restimulation to SARS-CoV-2 antigens.
Our patient had an asymptomatic infection prior to receiving CAR-T while receiving blinatumomab and had evidence of acquired immunity as evident by positive SARS-CoV-2 IgG antibodies. Equivocal PCR tests and chronic viral shedding has been reported in patients undergoing cancer therapy.27 The presence of IgG antibodies to spike and nucleocapsid proteins over time is suggestive of persistent humoral immune response after infection despite having no new B cells due to CD19-directed therapies. Recent evidence suggests that CD-19 negative plasma cells may contribute to long-term humoral immunity,28 as evidenced in one study which demonstrated that preexisting humoral immunity to vaccine-related antigens can persist in patients despite marked B-cell aplasia in patients receiving anti-CD19 CAR-T.29 Indeed evidence has shown that mature CD-19 negative plasma cells exist in substantial amounts within the bone marrow and seem to be somewhat protected from mobilization into the peripheral blood and may be important in maintaining humoral memory.30 The presence of IgG antibodies to various SARS-CoV-2 antigens several months after the primary infection and ongoing B-cell aplasia in our patient supports this observation and needs to be further explored in larger cohorts after primary infections or vaccination to understand the breadth of humoral immune deficiency induced by anti-CD19 CAR-T therapy. Of note, our patient started receiving IVIG replacement approximately 1 month after CAR-T therapy. At the time the patient was treated, IVIG produced in the US did not have high titers of neutralizing antibodies against SARS-CoV-2 but there are now reports of IVIG having substantial SARS-Co-V2 neutralizing antibodies that were not present in the pre-pandemic lots.31 Ongoing IVIG in our patient to current date could possibly provide additional level of protection against COVID-19.
Stimulation of patient T-cells with SARS-CoV-2 proteins ex vivo effectively expanded polyclonal and polyfunctional SARS-CoV-2 specific T-cells over time suggesting that this patient could mount an antigen-specific T-cell response when re-challenged.20 This data which has important implications regarding the ability for such individuals to elicit potentially protective immune responses, as well as for the application and timing of SARS-CoV-2 specific T-cell therapeutics to treat patients with blood cancers who receive B-cell depleting therapy. While the T-cells responding to SARS-CoV-2 in this patient were expanded ex vivo using a rapid expansion protocol stimulating the endogenous T cell receptors to different viral antigens; whether they contain the CD19 specific chimeric antigen receptor remains to be elucidated. Transgene levels of tisagenlecleucel have been detected up to 780 days in the peripheral blood with higher levels notable in patients in remission32 and we did not sort the peripheral blood mononuclear cells for lymphocytes prior to the ex vivo expansion of SARS-CoV-2 specific T cells. While the T-cells responding to SARS-CoV-2 in this patient are unlikely to contain the CD19 CAR, given their polyclonal and polyfunctional nature, it is possible that the T cells gain cross reactivity to non-CD19 antigens and future studies should explore the feasibility of dual antigen chimeric receptors targeting both CD19 and SARS-CoV-2. Indeed, previous studies have shown that virus specific T cells transduced with a retroviral vector expressing a CD19 CAR are effectively able to recognize CD19 and confer anti-tumor activity in vivo.33
Our study has limitations of it being a single case report and retrospective nature and larger longitudinal studies to analyze the cellular and humoral responses will help fully elucidate the clinical implications of preexisting T cell responses to SARS-CoV-2 antigens. While there is growing evidence about the efficacy of the SARS-CoV-2 vaccine in patients with hematologic malignancies,34 the vaccine is still limited to patients older than 5 years and emergence of variants remains a concern in this vulnerable population. The current guidelines from The American Society of Transplant and Cellular Therapy and American Society of Hematology support vaccinating all eligible patients, if feasible, prior to the CAR-T infusion without delaying this potentially curative therapy.35 Further studies are needed to explore the cellular and humoral immune responses to the SARS-CoV-2 vaccines to determine their efficacy and longevity in this high-risk population.
In summary, we provide unique longitudinal data in a pediatric patient with relapsed B-ALL who developed SARS-CoV-2 infection while receiving CD19-directed therapies. Further, this is the first report demonstrating robust adaptive immune responses to SARS-CoV-2 after CD19 CAR-T therapy.
Funding
This work was supported by grants The Safeway Foundation (H.D) in part by the intramural research programs of the National Institute of Dental and Craniofacial Research (PB) and National Institute of Allergy and Infectious Diseases (J.C.).
Footnotes
Disclosure statement
Conflict of interest disclosures: Catherine M. Bollard; Stock and Other Ownership Interests: Mana Therapeutics, NexImmune, Torque, Cabaletta Bio Consulting or Advisory Role: Repertoire Immune Medicines, NexImmune, Cellectis and Cabaletta Bio. Patents, Royalties, Other Intellectual Property: TAA-specific T cells and HIV-specific T cells. No other authors with conflicts of interest.
References
- 1.Bisogno G, Provenzi M, Zama D, et al. Clinical characteristics and outcome of Severe acute respiratory syndrome coronavirus 2 infection in Italian pediatric oncology patients: a study from the Infectious Diseases Working Group of the Associazione Italiana di Oncologia e Ematologia Pediatrica. Journal of the Pediatric Infectious Diseases Society. 2020;9(5):530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brar G, Pinheiro LC, Shusterman M, et al. COVID-19 severity and outcomes in patients with cancer: a matched cohort study. Journal of Clinical Oncology. 2020;38(33):3914–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astuti I. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14(4):407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultheiß C, Paschold L, Simnica D, et al. Next-generation sequencing of T and B cell receptor repertoires from COVID-19 patients showed signatures associated with severity of disease. Immunity. 2020;53(2):442–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19(5):318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storek J, Viganego F, Dawson MA, et al. Factors affecting antibody levels after allogeneic hematopoietic cell transplantation. Blood. 2003;101(8):3319–3324. [DOI] [PubMed] [Google Scholar]
- 7.Kemp SA C D, Datir RP, Ferreira IATM, Gayed S, Jahun A, Hosmillo M, Rees-Spear C, Mlcochova P, Lumb IU, Roberts DJ, Chandra A, Temperton N, Collaboration C-NBC-, COVID-19 Genomics UK (COG-UK) Consortium SK, Blan. SARS-CoV-2 evolution during treatment of chronic infection. Nature. 2021;592(7853):277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachanova V B M, Dahi P, Dholaria B, Grupp SA, Hayes-Lattin B, Janakiram M, Maziarz RT, McGuirk JP, Nastoupil LJ, Oluwole OO, Perales MA, Porter DL, Riedell PA, Consortium. CT-c. Chimeric Antigen Receptor T Cell Therapy During the COVID-19 Pandemic Biol Blood Marrow Transplant 2020;26(7):1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinana JL, Martino R, Garcia-Garcia I, Infectious Complications Subcommittee of the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH), et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9(1): 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vicent MG, Martinez AP, Trabazo Del Castillo M, et al. COVID-19 in pediatric hematopoietic stem cell transplantation: the experience of Spanish Group of Transplant (GETMON/GETH). Pediatr Blood Cancer. 2020;67(9):e28514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. [DOI] [PubMed] [Google Scholar]
- 13.Hensley MK, Bain WG, Jacobs J, et al. Intractable coronavirus disease 2019 (COVID-19) and prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication in a chimeric antigen receptor-modified T-cell therapy recipient: a case study. Clin Infect Dis. 2021;73(3):e815–e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gea-Banacloche J, Komanduri KV, Carpenter P, et al. National Institutes of Health hematopoietic cell transplantation late effects initiative: the immune dysregulation and Pathobiology Working Group Report. Biol Blood Marrow Transplant. 2017;23(6):870–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan KM, Kopecky KJ, Jocom J, et al. Immunomodulatory and antimicrobial efficacy of intravenous immunoglobulin in bone marrow transplantation. N Engl J Med. 1990;323(11):705–712. [DOI] [PubMed] [Google Scholar]
- 16.Valk SJ, Piechotta V, Chai KL, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;5:CD013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pensato U M L, Cani I, Janigro D, Zinzani PL, Guarino M, Cortelli P, Bisulli F. Brain dysfunction in COVID-19 and CAR-T therapy: cytokine storm-associated encephalopathy. Ann Clin Transl Neurol 2021;4:968–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Bert N, Tan AT, Kunasegaran K et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. [DOI] [PubMed] [Google Scholar]
- 19.Monin L, Laing AG, Munoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller MD, Harris KM, Jensen-Wachspress MA, Kankate VV, Lang H, Lazarski CA. SARS-CoV-2–specific T cells are rapidly expanded for therapeutic use and target conserved regions of the membrane protein. Blood, 2020:2905–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanojevic M, Geiger A, Ostermeier B, et al. Spike-directed vaccination elicits robust spike-specific T-cell response, including to mutant strains. Cytotherapy. 2021;24(1):10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burbelo PD, Riedo FX, Morishima C, et al. Sensitivity in detection of antibodies to nucleocapsid and spike proteins of severe acute respiratory syndrome coronavirus 2 in patients with coronavirus disease 2019. J Infect Dis. 2020;222(2):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021: 21: 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dave H, Luo M, Blaney JW, et al. Toward a rapid production of multivirus-specific T cells targeting BKV, adenovirus, CMV, and EBV from umbilical cord blood. Mol Ther Methods Clin Dev. 2017;5:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hont AB, Cruz CR, Ulrey R, et al. Immunotherapy of relapsed and refractory solid tumors with ex vivo expanded multi-tumor associated antigen specific cytotoxic T lymphocytes: a phase I study. J Clin Oncol. 2019;37(26):2349–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubinstein JD, Zhu X, Leemhuis T, et al. Virus-specific T cells for adenovirus infection after stem cell transplantation are highly effective and class II HLA restricted. Blood Adv. 2021;5(17):3309–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aydillo T G-R A, Aslam S, van de Guchte A, Khan Z, Obla A, Dutta J, van Bakel H, Aberg J, García-Sastre A, Shah G, Hohl T, Papanicolaou G, Perales MA, Sepkowitz K, Babady NE, Kamboj M. Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer. N Engl J Med. 2020;383(26):2586–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mei HE, Wirries I, Frolich D, et al. A unique population of IgG-expressing plasma cells lacking CD19 is enriched in human bone marrow. Blood. 2015;125(11):1739–1748. [DOI] [PubMed] [Google Scholar]
- 29.Bhoj VG, Arhontoulis D, Wertheim G, et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood. 2016;128(3):360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halliley JL, Tipton CM, Liesveld J, et al. Long-lived plasma cells are contained within the CD19 (−)CD38(hi) CD138(+) subset in human bone marrow. Immunity. 2015;43(1):132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volk A, Covini-Souris C, Kuehnel D, et al. SARS-CoV-2 neutralization in commercial lots of plasma-derived immunoglobulin. BioDrugs. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller KT, Maude SL, Porter DL, et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood. 2017;130(21):2317–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruz CR, Micklethwaite KP, Savoldo B, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122(17):2965–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khawaja KC, Dadwal S. ASH-ASTCT COVID-19 vaccination for hct and CAR T Cell Recipients: Frequently asked questions. 2021. [Google Scholar]


