ABSTRACT
Subarachnoid-pleural fistula (SPF), a rare complication following transthoracic spinal surgery, results in the accumulation of cerebrospinal fluid (CSF) in the pleural space. Hindered spontaneous closure, attributed to negative pleural pressure, gives rise to CSF hypotension and subdural blood collections. Despite numerous reported cases, achieving consensus on management remains elusive. Treatment options encompass conservative measures, surgical repair, epidural blood patch, and diverse approaches such as multilayer dural closure or meningocele resection. Presented herein is a distinctive case following lateral thoracic meningocele surgery, where SPF-induced CSF hypotension found successful resolution through the innovative use of titanium hemostatic clips to occlude the meningocele. This novel approach, emphasizing the utility of titanium clips, deviates from conventional strategies. Surgical SPF exclusion, particularly leveraging titanium clips, emerges as a potential solution, effectively alleviating symptoms of CSF hypotension. The article also aims to present a personal experience, contributing an effective and alternative approach for the etiological treatment of thoracic meningocele.
Keywords: Intracranial hypotension, subarachnoid-pleural fistula, thoracic meningocele
INTRODUCTION
Subarachnoid-pleural fistula (SPF) is a rare and feared complication of spinal surgery, consisting of a pathological shunt between the subarachnoid and pleural spaces.[1] It can lead to the accumulation of cerebrospinal fluid (CSF) in the pleural space. Spontaneous closure of this communication is hindered by the negative pleural pressure acting as a suction force in the cavity, determining continuous CSF loss. This may lead to CSF hypotension with secondary subdural blood collections. Nonetheless, there is no unanimous consensus regarding the management of this complication due to the paucity of cases reported so far.
Spinal meningocele is a rare congenital malformation characterized by the protrusion of the dura mater and CSF through a vertebral foramen or bony defect.[2] Thoracic meningoceles, though rare, are often associated with neurofibromatosis type I, Marfan syndrome, or other connective tissue disorders.[2] Their prevalence in the thoracic region is probably due to the relatively weaker paravertebral muscles and the pressure gradient between the subdural and pleural spaces, leading to neuroforamen dilation and bony remodeling, such as vertebral pedicle erosion.[2] Patients can be asymptomatic or exhibit neurological or pulmonary symptoms such as cough, dyspnea, and palpitations due to lung and mediastinal compression. In exceptional cases, it may cause spinal cord compression (e.g. paraparesis and pain) or symptoms of intracranial hypotension (IH).[3]
IH, characterized by reduced CSF volume and pressure, often results from dural defects along the neuraxis.[4,5] It clinically presents with bilateral subdural hygromas or subdural hematomas (SDHs). Symptoms may include postural headaches, nausea, vomiting, disorientation, memory impairment, diplopia, gait disturbances, and sudden hearing loss.[4] Sometimes, localization of the leak/dural defect site is not obtainable. Therefore, the first-line therapeutic approach is the epidural blood patch (EBP), especially for moderate-to-severe headaches unresponsive to conservative management.[6] However, in cases with an identifiable and accessible dural defect, direct repair becomes the preferred treatment option.[7]
Several cases of iatrogenic SPFs determining IH, primarily associated with laminectomy procedures or consequent to lung tumor resections, are reported to date.[8,9] Treatment management typically involves pleural and lumbar drainage.[9] Direct surgical correction remains the preferred approach when feasible. Successful repair of dorsal fistulas using dural grafts,[10] omental flaps obtained from cadavers,[11] and the application of noninvasive positive pressure ventilation (NPPV) have been described.[12] When the point of the leak is unknown or difficult to treat, an EBP may be performed.[8]
Herein, we present the case of a patient who developed an SPF after undergoing transthoracic surgery for a lateral thoracic meningocele. Following the surgery, the patient experienced secondary CSF hypotension, successfully managed by occluding the meningocele by means of hemostatic clips. Notably, while this complication is not rare, the treatment method employed in this specific case is distinctive, with no similar cases reported in the available literature to the best of our knowledge.
CASE REPORT
A 54-year-old woman patient came to our attention with a 6-month history of worsening cervical and right dorsal pain unresponsive to analgesics, bringing a computed tomography (CT) and magnetic resonance (MR) imaging of the cervicothoracic spine revealing an extradural right paramedian lesion with probable cystic content at the T9-11 level, extending into the thoracic cavity, apparently excluded from the subarachnoid space [Figure 1]. Then, the patient underwent transthoracic cyst marsupialization and decompression, performed through a right mini-thoracotomy in the T10-11 space. During the procedure, puncture and aspiration of the cyst were followed by refilling of the cyst wall, thus revealing communication with the subarachnoid space. Therefore, we performed cyst decompression and occlusion of the communication with a dural patch and fibrin glue. The postoperative course was uneventful, and the patient experienced transitory relief of the right hemithorax pain. A thoracic CT examination documented partial decompression of the cyst. Several weeks later, the patient developed positional headaches and cervicalgia. Subsequent thorax and brain CT examinations revealed right pleural effusion and subdural fluid collection, respectively, indicating CSF hypotension due to CSF leakage into the thoracic compartment [Figure 1]. Then, based on the clinical and radiological findings, we decided to perform a posterior approach with the aim of repairing the fistula by direct surgical exclusion. A T10 right hemilaminectomy, partial T9, and T10 right pediculectomy were performed. After exposing the dural sac, we identified two necks of the meningoceles occupying the two enlarged neuroforamina and, after isolating them, three 11 mm titanium aneurysm clips were strategically used to exclude the caudal “neck,” and another clip was used to exclude the cranial one [Figure 2]. A right chest drainage was subsequently inserted and maintained for 6 days, during which a chest radiographic examination demonstrated improved pleural effusion and lung reexpansion. Postoperatively, the patient experienced significant relief from preoperative headaches, even in an upright position. Postoperative CT and MR imaging showed the absence of communication between the thoracic meningocele and the dural sac, confirming the surgical procedure’s effectiveness [Figure 2]. Serial cranial CT examinations showed a progressive reduction of bilateral subdural collections until complete regression [Figure 3]. Furthermore, serial MR examination of the thoracic spine documented progressive regression of the cyst. At the 6-month follow-up, the patient displayed complete resolution of symptoms, indicating the success of the surgical strategy.
Figure 1.
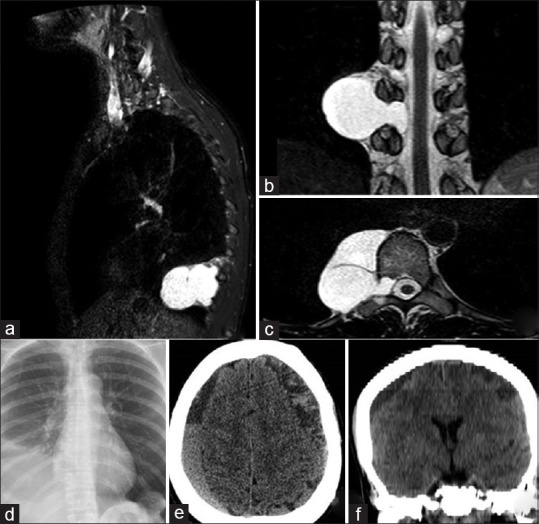
Sagittal (a), coronal (b), and axial (c) T2-weighted magnetic resonance images depicting the right thoracic meningocele located at the T9-T11 level. Postoperative (thoracotomy) chest radiography (d) shows the pleural effusion along with the cyst residual. Axial computed tomography (CT) image (e), and coronal CT multiplanar reconstruction (f) demonstrate bilateral subdural hematoma secondary to intracranial hypotension
Figure 2.
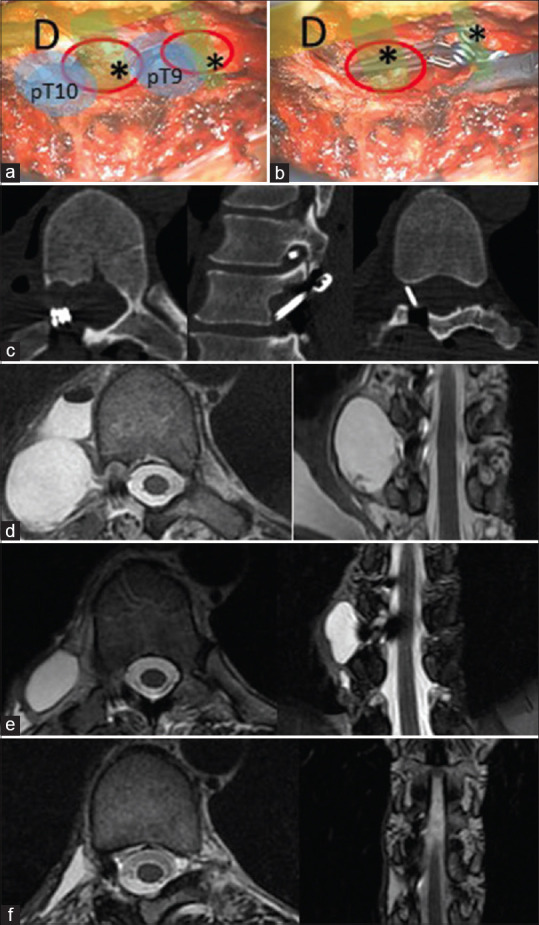
Intraoperative images after isolation (a) and clipping (b) of cyst’s neck. D: dural sac; pT9: T9 pedicle; pT10:T10 pedicle; *cyst’s caudal neck; *cyst’s cranial neck. Postoperative (laminectomy and cyst exclusion) images: bone window axial, and sagittal reconstruction computed tomography images (c) show cyst’s necks clipping; postoperative (d), 3-month (e) and 6-month (f) follow-up thoracic spine magnetic resonance images show progressive cyst regression
Figure 3.
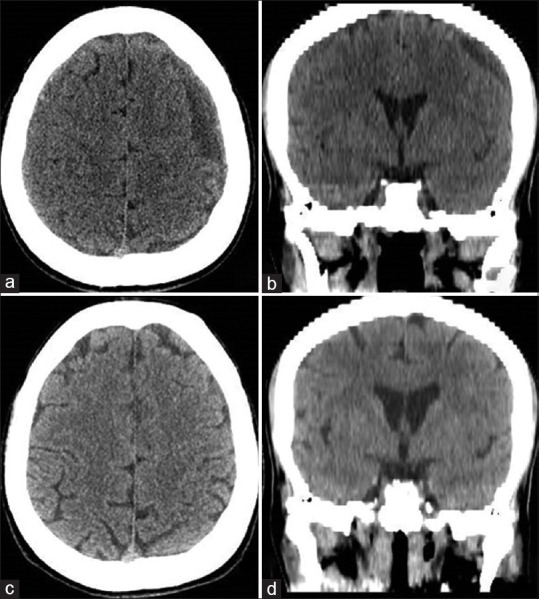
Three-month (a and b) and 6-month (c and d) follow-up cranial computed tomography images show reduction and disappearance of bilateral subdural hematoma
DISCUSSION
SPF is an uncommon complication of spinal surgery through a transthoracic approach. It can lead to the accumulation of CSF in the pleural space. Spontaneous closure of this communication is hindered by the negative pleural pressure acting as a suction force in the cavity, determining continuous loss of CSF. This may lead to CSF hypotension with secondary subdural blood collections. Nonetheless, there is no unanimous consensus regarding the management of this complication due to the paucity of cases reported so far.
Treatments for SPF include conservative measures (i.e. chest drainage, bed rest, intravenous fluid supplementation, NPPV, etc.) and surgical repair.[13] Some authors[2] have advocated for the use of a multilayer dural closure along with lumboperitoneal or cystoperitoneal shunts with a high failure rate. Previous studies[14] report resection or ligation of the meningocele posteriorly through a laminectomy, with or without instrumentation if the patient has kyphosis/kyphoscoliosis or laterally through thoracotomy.[3]
In addition, there is evidence suggesting the effectiveness of EBP in managing spontaneous IH. EBP is occasionally employed based on the premise that it induces a mass effect, compressing the subarachnoid space and thereby regulating CSF pressure within the cranial cavity. Simultaneously, the formation of an “epidural plug” is facilitated by clot formation, where the clot adheres to the thecal sac and may potentially develop into a permanent seal.[6]
The utilization of titanium clips for lateral meningocele surgical exclusion is uncommon. Nevertheless, clips can be very useful, thanks to the different existing configurations, adaptability, and maneuverability of the clip holder in narrow spaces, such as at the neuroforamen, providing atraumatic and complete wrapping of the neck.
Regarding SDHs secondary to IH, some authors[15] advocate surgical evacuation in case of clinical deterioration and consciousness impairment. Others[15] note that subdural fluid collections can be managed safely by directing treatment at the underlying CSF leak, without hematoma evacuation, and some emphasize that surgical evacuation might increase the risk of brain herniation. There are several reports[15] of transient complications, morbid outcomes, and even death.
In our case, after the etiologic treatment of IH, we opted for conservative management of the SDH with serial CT examinations and close clinical monitoring, although the patient was symptomatic in terms of positional headaches.
CONCLUSIONS
In cases of symptomatic CSF hypotension secondary to a SPF, the surgical treatment of excluding/closing the fistula may be adequate to effectively resolve even the secondary signs related to subsequent CSF hypotension, such as SDHs. In our case, a simple and etiologic approach, in line with a “to do more with less” philosophy, was employed, directly identifying and closing the communication between the subdural and pleural spaces.
Declaration
The material used for this case report is stored in the archives of Policlinico Gemelli. It is available for consultation by other researchers or professionals interested in the study.
I declare that all authors have significantly contributed to the writing of the text in their respective areas of expertise. Each author provided substantial contributions to the design, data collection, analysis, and interpretation of the results.
This study did not require ethics approval as it did not involve interventions on human subjects, sensitive data, or any procedures falling under the jurisdiction of an ethics committee. All aspects of the research were conducted in accordance with ethical principles, and patient privacy and confidentiality were strictly maintained throughout the study.
This declaration is made in accordance with ethical and scientific guidelines. Any updates or changes will be promptly communicated.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chaudhry MS. Intracranial hypotension caused by dural-pleural fistula. J Neuroimaging. 2012;22:208–9. doi: 10.1111/j.1552-6569.2010.00549.x. [DOI] [PubMed] [Google Scholar]
- 2.Close LN, Park B, Woodroffe RW, Hitchon PW. Thoracic meningocele and cervical syringomyelia treated with ventriculoperitoneal shunt. World Neurosurg. 2019;129:322–6. doi: 10.1016/j.wneu.2019.05.204. [DOI] [PubMed] [Google Scholar]
- 3.Das P, Goyal T, Hunt MA. Intrathoracic meningocele associated with neurofibromatosis type 1 and a novel technique for surgical repair: Case report. J Neurosurg Spine. 2017;27:291–4. doi: 10.3171/2017.2.SPINE16699. [DOI] [PubMed] [Google Scholar]
- 4.Signorelli F, Caccavella VM, Giordano M, Ioannoni E, Caricato A, Polli FM, et al. A systematic review and meta-analysis of factors affecting the outcome of the epidural blood patching in spontaneous intracranial hypotension. Neurosurg Rev. 2021;44:3079–85. doi: 10.1007/s10143-021-01505-5. [DOI] [PubMed] [Google Scholar]
- 5.Ferrante E, Trimboli M, Rubino F. Spontaneous intracranial hypotension: Review and expert opinion. Acta Neurol Belg. 2020;120:9–18. doi: 10.1007/s13760-019-01166-8. [DOI] [PubMed] [Google Scholar]
- 6.Huang H, Wei TT, Niu ZF, Yu L, He FF. Case report: Epidural blood patches are effective in treating intracranial hypotension due to a subarachnoid-pleural fistula. Front Surg. 2022;9:936949. doi: 10.3389/fsurg.2022.936949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He KD, Rymarczuk GN, Clark SW, Gillick JL, Vahedi P, Sharan AD. A novel technique for prevention of subarachnoid-pleural fistula after incidental durotomy during transthoracic spinal surgery. Oper Neurosurg (Hagerstown) 2019;16:451–4. doi: 10.1093/ons/opy170. [DOI] [PubMed] [Google Scholar]
- 8.Hawk MW, Kim KD. Review of spinal pseudomeningoceles and cerebrospinal fluid fistulas. Neurosurg Focus. 2000;9:e5. doi: 10.3171/foc.2000.9.1.5. [DOI] [PubMed] [Google Scholar]
- 9.Malca SA, Roche PH, Touta A, Pellet W. Pneumocephalus after thoracotomy. Surg Neurol. 1995;43:398–401. doi: 10.1016/0090-3019(95)80072-o. [DOI] [PubMed] [Google Scholar]
- 10.Raffa SJ, Benglis DM, Levi AD. Treatment of a persistent iatrogenic cerebrospinal fluid-pleural fistula with a cadaveric dural-pleural graft. Spine J. 2009;9:e25–9. doi: 10.1016/j.spinee.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Sahota S, Nassr A, Khan MH, Marsh RW, Moran SL, Arnold PM, et al. Treatment of a thoracic dural-pleural fistula with a vascularized omental flap: A case report. Spine (Phila Pa 1976) 2012;37:E683–5. doi: 10.1097/BRS.0b013e31824a3f73. [DOI] [PubMed] [Google Scholar]
- 12.Schlag HR, Muquit S, Hristov TB, Morassi G, Boszczyk BM, Shafafy M. Subarachnoidal pleural fistula after resection of intradural thoracic disc herniation and multimodal treatment with noninvasive positive pressure ventilation (NPPV) Eur Spine J. 2016;25:155–9. doi: 10.1007/s00586-015-4137-1. [DOI] [PubMed] [Google Scholar]
- 13.Federspiel CK, Kelsen J, Fugleholm K. Delayed iatrogenic intracranial hypotension after thoracotomy. Ann Thorac Surg. 2020;110:e35–7. doi: 10.1016/j.athoracsur.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Ebara S, Yuzawa Y, Kinoshita T, Takahashi J, Nakamura I, Hirabayashi H, et al. A neurofibromatosis type 1 patient with severe kyphoscoliosis and intrathoracic meningocele. J Clin Neurosci. 2003;10:268–72. doi: 10.1016/s0967-5868(03)00003-1. [DOI] [PubMed] [Google Scholar]
- 15.Ferrante E, Rubino F, Beretta F, Regna-Gladin C, Ferrante MM. Treatment and outcome of subdural hematoma in patients with spontaneous intracranial hypotension: A report of 35 cases. Acta Neurol Belg. 2018;118:61–70. doi: 10.1007/s13760-017-0845-0. [DOI] [PubMed] [Google Scholar]


