Abstract
Objective
Evaluation of the accuracy of the wrist-type fully automatic blood pressure (BP) monitor: DBP-2242 in adolescent and adult populations according to ISO 81060-2 : 2018+Amd.1 : 2020.
Methods
BP measurements were taken from the subjects using the same-arm sequential method, and BP data measured by a mercury sphygmomanometer was used as the standard.
Results
This study analyzed 262 sets of data from 89 subjects. According to criterion 1, the mean difference between the SBP of the test and reference devices was −0.57 ± 7.31 mmHg, and the DBP was −2.27 ± 7.17 mmHg, which is in accordance with the requirements. According to criterion 2, the average difference between the SBP was −0.57 ± 6.25 mmHg and the DBP was −2.27 ± 5.99 mmHg, which is in accordance with the requirements.
Conclusion
Wrist-type fully automatic BP monitor: DBP-2242 complies with ISO 81060-2 : 2018+Amd.1 : 2020 and can be used for BP measurement in adolescent and adult populations.
Keywords: accuracy, blood pressure, blood pressure monitor, validation, wrist-type
Introduction
Hypertension is a common chronic disease that, as it progresses, leading to heart attacks, heart failure, strokes, and renal failure [1]. Accurate blood pressure (BP) measurement facilitates early detection and allows for lifestyle and, where needed, pharmacological intervention to reduce and prevent the complications of high BP [2]. In accordance with the most recent approved validation standards for noninvasive BP measurement devices (ISO 81060-2 and ISO 81060-2 : 2018, AMD 1, 2020-01, consensus on the best validation procedures have been published [3–6].
In accordance with those standards, the study of DBP-2422 was undertaken to validate its accuracy in adolescent and adult populations.
Methods
This study was approved by the hospital ethics committee before it was conducted. The ethics number was IRB2023-001-02.
Study population
Subjects were recruited in the Suzhou Ninth Hospital affiliated to Soochow University in this study. Inclusion criteria for subjects: (1) age >12 years, regardless of sex; (2) voluntarily participated in BP measurement and signed an informed consent form; (3) ability to communicate well with the investigators and good compliance to follow the requirements of the clinical study. Exclusion criteria: (1) allergy to the materials of the cuffs of the sphygmomanometers; (2) severe arrhythmia; (3) persons with impaired consciousness, unable to give adequate information; (4) other conditions that the researcher considers inappropriate for participation in this study.
Instruments
The test device is DBP-2242 (manufactured by JOYTECH Healthcare Co., Ltd.), which measures SBP, DBP, and pulse rate through the wrist area of the human body on the principle of the oscillometric method. The device can measure BP in the range of 0–300 mmHg and pulse rate in the range of 30–180 beats per minute. The cuff is suitable for the wrist circumference range: 13.5 cm–21.5 cm. The reference device was a mercury sphygmomanometer: XJ11D (Shanghai Medical Instruments CO., LTD.).
Validation team
The survey team consisted of three trained members. Two observers took BP measurements using the mercury sphygmomanometers and recorded the measurements independently, while a third supervisor took BP measurements using the test device and recorded BP readings from the test device.
Procedures
The subject should be in a comfortable, relaxed state during BP measurements. During the test, we had the patient’s arm bent upward so that the wrist was level with the heart. According to ISO standards, a subject should rest for 5 min before taking the first blood pressure measurement. Measurements were performed using the same-arm sequential method. Measurements were taken at intervals of at least 60 s. Two surveyors used a double-headed stethoscope to simultaneously auscultate reference BP measurements. The last audible Korotkoff sound (fifth phase) was used as the reference DBP. If the fifth phase (K5) was not heard, the participant was excluded. Any difference between any two reference SBPs greater than 12 mmHg, or any difference between any two reference DBPs greater than 8 mmHg, all data for this subject shall be excluded. Any pair of readings with a SBP or DBP difference of 4 mmHg or greater are excluded, as per ISO testing standards.
Analysis
Means and standard deviations (SD) were used for descriptive statistics for quantitative information, and rates or composition ratios (%) were used for qualitative information. The accuracy of the test device was verified according to criterion 1 and 2 of ISO 81060-2 : 2018+Amd.1 : 2020. For criterion 1: the overall mean difference and SD of the SBP and DBP of the test and reference devices taken from each valid data set shall not be greater than 5 mmHg or 8 mmHg. Bland–Altman plots were used to describe the average of the differences between the test and reference device measurements. For criterion 2, the SD of the overall mean of the subject’s BP for each group of test and reference devices shall not be greater than the maximum allowable SD (Table 1).
Table 1.
Maximum allowable SD for criterion 2
| n | Maximum permissible SD (mmHg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.0 _ | 0.1 _ | 0.2 _ | 0.3 _ | 0.4 _ | 0.5 _ | 0.6 _ | 0.7 _ | 0.8 | 0.9 | |
| ±0 | 6.95 | 6.95 | 6.95 | 6.95 | 6.93 | 6.92 | 6.91 | 6.90 | 6.89 | 6.88 |
| ±1 | 6.87 | 6.86 | 6.84 | 6.82 | 6.80 | 6.78 | 6.76 | 6.73 | 6.71 | 6.68 |
| ±2 | 6.65 | 6.62 | 6.58 | 6.55 | 6.51 | 6.47 | 6.43 | 6.39 | 6.34 | 6.30 |
| ±3 | 6.25 | 6.20 | 6.14 | 6.06 | 6.03 | 5.97 | 5.89 | 5.83 | 5.77 | 5.70 |
| ±4 | 5.64 | 5.56 | 5.49 | 5.41 | 5.33 | 5.25 | 5.16 | 5.08 | 5.01 | 4.90 |
| ±5 | 4.79 | – | – | – | – | – | – | – | – | – |
For a mean value of ± 4.2 mmHg, the maximum permissible SD is 5.49 mm Hg.
Results
In total 262 data sets from 89 individuals were included in this analysis. There were 56 (62.9%) males and 33 (37.1%) females. The average wrist circumference was 17.31 ± 2.14 cm. Gender, wrist circumference, BP distribution, and special population distribution met the standard requirements (Table 2).
Table 2.
Characteristics of the study participants (n = 89)
| Variable | the ISO 81060-2 : 2018 standard (n, %) | Value (%) |
|---|---|---|
| Age, year (range) | 46.79 ± 16.96 years (13–78 years) | |
| >12 years and <18 years | / | 5 (5.6%) |
| ≥18 years old and <60 years old | / | 62 (69.7%) |
| ≥60 years | / | 22 (24.7%) |
| Gender, n (%) | Men (≥26, ≥30%); women (≥26, ≥30%) | 56 (62.9%): 33 (37.1%) |
| Wrist circumstance, cm (range) | 17.31 ± 2.14 cm (13.60–21.50 cm) | |
| >13.5 cm and ≤15.5 cm | ≥17, ≥20% | 22 (24.7%) |
| >15.5 cm and ≤17.5 cm | ≥17, ≥20% | 29 (32.6%) |
| >17.5 cm and ≤19.5 cm | ≥17, ≥20% | 18 (20.2%) |
| >19.5 cm and ≤21.5 cm | ≥17, ≥20% | 20 (22.5%) |
| ≥13.5 cm and ≤14.5 cm | ≥9, ≥10% | 10 (11.2%) |
| ≥20.5 cm and ≤21.5 cm | ≥9, ≥10% | 15 (16.9%) |
| SBP, mmHg (range) | ||
| ≤100 mmHg | ≥5% | 49 (14.0%) |
| ≥160 mmHg | ≥5% | 18 (5.1%) |
| ≥140 mmHg | ≥20% | 73 (20.8%) |
| DBP, mmHg (range) | ||
| ≤60 mmHg | ≥5% | 23 (6.6%) |
| ≥100 mmHg | ≥5% | 21 (6.0%) |
| ≥85 mmHg | ≥20% | 113 (32.2%) |
Data are expressed as the means ± SD or percentages or numbers.
/ means not applicable.
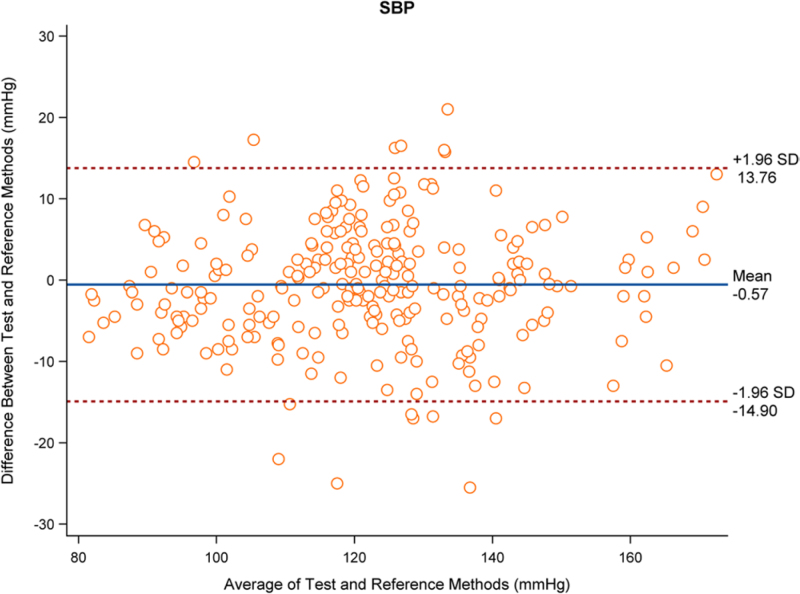
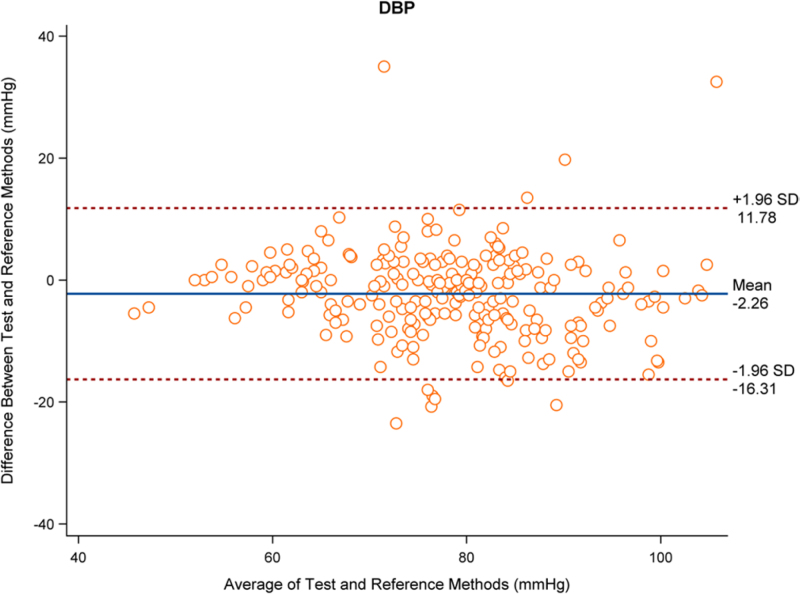
The analysis results based on 262 sets of data showed that the mean difference in SBP between the test and reference devices was −0.57 mmHg with an SD of 7.31 mmHg, and the mean difference in DBP was −2.27 mmHg with an SD of 7.17 mmHg. The mean difference in SBP and DBP were both less than 5 mmHg, and the SD was less than 8 mmHg, which complied with criterion 1. Figures 1 and 2 show the distribution of SBP and DBP differences measured by the test and reference devices, respectively.
Fig. 1.
Distribution diagram for difference value of SBP (mmHg) measured by the test device and the reference device (262 sets of data).
Fig. 2.
Distribution diagram for difference value of DBP (mmHg) measured by the test device and the reference device (262 sets of data).
The results of the analysis based on 89 individuals showed that the mean difference in SBP between the test and reference devices was −0.57 mmHg, with an SD of 6.25 mmHg, which was less than 6.91 mmHg. The mean difference in DBP was −2.27 mmHg, with an SD of 5.99 mmHg, which was less than 6.55 mmHg and thus meet criterion 2 requirements.
Discussion
Accurate BP measurement is essential and is an important tool in the prevention and treatment of hypertension. Wrist-type fully automatic BP monitors are inexpensive and easy to use, making them an ideal self-help BP monitoring device. The accuracy of BP measurement devices directly affects the diagnosis, treatment, and adjustment of BP medication, so it is important to evaluate the accuracy of BP measurement devices.
This study validated the accuracy of wrist-type fully automatic BP monitor DBP-2242 measurements in adolescent and adult populations based on ISO 81060-2 : 2018+Amd.1 : 2020. The results of the study showed that the test device met the requirements of criterion 1 and criterion 2 of the international standard for accuracy, indicating that they can accurately and reliably measure BP in both adolescent and adult populations.
In addition, the DBP-2242 was stable with no device malfunctions during the trial, and the investigators found the product to be very easy to use. The DBP-2242’s features such as irregular heartbeat reminder and BP data storage also operated well during the trial.
Conclusion
The DBP-2242 met the requirements of the AAMI/ESH/ISO common criteria ISO 81060-2 : 2018+Amd.1 : 2020 and can be recommended for the measurement of BP in adolescents and adults.
Acknowledgements
This study was funded by the JOYTECH Healthcare Co., Ltd., Hangzhou, China, through the Suzhou Ninth Hospital affiliated to Soochow University Account for Research.
Data analysis: Y.J. and X.B. Writing–original draft: Y.J., Yu.J., and X.B. Writing–review & editing: Y.J.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Yuehong Jin and Xiaoming Bei contributed equally to the writing of this article.
References
- 1.Bilo G, Sala O, Perego C, Faini A, Gao L, Głuszewska A, et al. Impact of cuff positioning on blood pressure measurement accuracy: may a specially designed cuff make a difference? Hypertens Res. 2017; 40:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Committee of Chinese Journal of Cardiology. Chinese guidelines for cardiovascular disease prevention (2017). Chinese Journal of Cardiology. 2018; 01:10–25. [Google Scholar]
- 3.Jointly by Technical Committee ISO/TC 121, Anaesthetic and respiratory equipment, Subcommittee SC 3, Respiratory devices and related equipment used for patient care, and Technical Committee IEC/TC 62, Electrical equipment in medical practice, Subcommittee SC D, Electromedical equipment; 2018. https://www.iso.org/standard/73339.html. [Accessed 15 November 2022] [Google Scholar]
- 4.Jointly by Technical Committee ISO/TC 121, Anaesthetic and respiratory equipment, Subcommittee SC 3, Respiratory devices and related equipment used for patient care, and Technical Committee IEC/TC 62, Electrical equipment in medical practice, Subcommittee SC D, Electromedical equipment; 2020. https://www.iso.org/standard/75432.html. [Accessed 2 July 2022] [Google Scholar]
- 5.Stergiou GS, Palatini P, Asmar R, Ioannidis JP, Kollias A, Lacy P, et al.; European Society of Hypertension Working Group on Blood Pressure Monitoring. Recommendations and practical guidance for performing and reporting validation studies according to the Universal Standard for the validation of blood pressure measuring devices by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO). J Hypertens. 2019; 37:459–466. [DOI] [PubMed] [Google Scholar]
- 6.Stergiou GS, Alpert B, Mieke S, Asmar R, Atkins N, Eckert S, et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) collaboration statement. Hypertension. 2018; 71:368–374. [DOI] [PubMed] [Google Scholar]