Abstract
Objectives:
The preferred endovascular therapy (EVT) for large-vessel occlusion in intracranial atherosclerosis (ICAS) is unknown. We compared the efficacy of preferred stent thrombectomy and preferred angioplasty in patients with acute large-vessel occlusion in ICAS.
Methods:
Data from consecutive EVT patients (May 2020 to September 2023) with acute middle cerebral artery occlusion in ICAS were retrospectively analyzed. Preferred angioplasty was performed if there was a preoperative “microcatheter first-pass effect;” otherwise, preferred stent thrombectomy was performed. Analyses were grouped according to the two EVT treatments. Clinical data of all patients, including the time from puncture to recanalization, rate of successful reperfusion, early neurological improvement, intracranial hemorrhage, and modified Rankin Scale score at 90 days, were recorded and analyzed.
Results:
Six-two patients were enrolled in this study (mean age was 60.66±13.21 y, 22.6% female). The preferred angioplasty group had a higher first-pass recanalization rate than the preferred stent thrombectomy group (61.3% vs. 21.9%, P<0.001) and a higher proportion of patients who were functionally independent (defined as a modified Rankin Scale score of 0 to 3) at 90 days [odds ratio,3.681; 95% confidence interval (CI):1.009 to 13.428; P=0.048]. There was no significant difference between the groups in the time from puncture to recanalization, the frequency of successful reperfusion, and early neurological improvement, or intracranial hemorrhage (P>0.05).
Conclusions:
This study suggests that for acute middle cerebral artery occlusion in ICAS, preferred angioplasty may be a safe and effective procedure.
Key Words: acute ischaemic stroke, middle cerebral artery occlusion, intracranial atherosclerosis, preferred angioplasty, preferred stent thrombectomy
Acute ischemic stroke caused by large-vessel occlusion (LVO) frequently results in death or disability. The main causes of acute occlusion of large vessels are cardioembolism and atherosclerosis.1 In contrast to European and American populations, intracranial atherosclerosis-related occlusion(ICAS-LVO) is higher in the Chinese population. It reaches 30% to 50%.2 Mechanical thrombectomy has a low rate of successful reperfusion in patients with ICAS-LVO,3,4 these patients often require a rescue angioplasty. In contrast, repeated endovascular therapy (EVT) may exacerbate intimal and arterial injury and are prone to secondary thrombosis, leading to a high rate of reocclusion.5 Currently, there are no clear guideline recommendations for emergency EVT treatments for ICAS-LVO.6 The strategy for mechanical thrombectomy in ICAS-LVO is to start with stent thrombectomy. Rescue angioplasty is performed if residual stenosis is severe and blood flow cannot be maintained. Angioplasty included balloon angioplasty and stenting.7 Fewer studies have reported favoring preferred angioplasty for ICAS-related acute large-vessel occlusion. Therefore, in this study, we retrospectively analyzed patients with acute middle cerebral artery occlusion due to ICAS, assessed them preoperatively or intraoperatively, and compared the efficacy and prognosis of using preferred angioplasty or preferred stent thrombectomy.
METHODS
Study Participants
Consecutive EVT patients (from May 2020 to September 2023) with acute middle cerebral artery occlusion in ICAS admitted to Zhangjiagang Hospital, affiliated with Soochow University, were enrolled and retrospectively analyzed. A total of 62 patients were enrolled in the study according to the inclusion and exclusion criteria. We conducted a retrospective cohort study based on the type of EVT treatment.
Inclusion criteria were (1) age ≥18 years, (2) digital subtraction angiography(DSA) confirmed occlusion of the M1 trunk of the middle cerebral artery, (3) stroke classification of subtypes classified as large-artery atherosclerosis, and (4) preoperative National Institutes of Health Stroke Scale score (NIHSS) ≥6 and Alberta Stroke Project Early CT Score (ASPECTS) ≥6. The exclusion criteria were as follows: (1) Previous modified Rankin Scale (mRS) score ≥2, (2) Tendency to bleed, such as platelets less than 100*109/l, and (3) incomplete follow-up data.
Data Collection and follow-up
Clinical data of patients were collected through an electronic medical record system. All patients completed the 3-month follow-up period. Primary end point: mRS score at 90 days; secondary end point: early neurological improvement (ENI) (defined as 24h NIHSS ≤1 or ≥8 points decrease from baseline),8 intracranial hemorrhage, and successful reperfusion defined as modified Thrombolysis in Cerebral Infarction (mTICI) 2b-3. Data were collected and followed up by the Brain Heart Health Manager, who was unaware of the subgroups.
Methods of ICAS Determination
ICAS was determined by 2 experienced neurologists. The criteria for patient selection were based on objective factors, including the presence of intracranial stenosis on prior imaging, fluctuating or progressive exacerbation of symptoms, intracranial arterial calcification on CT or MRI, occlusive proximal conical stenosis on CTA or DSA, and the “stenting effect” or “microcatheter first-pass effect” before thrombectomy.9,10
Definition of the “microcatheter first-pass effect”: When angiography showed an acute occlusion of the middle cerebral artery, the following procedure was performed before thrombectomy. A microcatheter and microwire were navigated through the occluded area to the distal patent artery. The microcatheter was then retrieved proximal to the thrombus. An angiogram was performed using a guide catheter or microcatheter to determine whether blood was flowing through the vessel at the site of the occlusion. This blood flow was recorded as the microcatheter first-pass effect.
Definition of the “stenting effect”: When angiography showed an acute occlusion of the middle cerebral artery, residual stenosis occurred after the stent retriever was released in the occluded area. An angiogram was performed using a guide catheter to determine whether blood flow had been restored (stenting effect), whether blood flow had been partially restored, and whether an artery trunk was missing (non-stenting effect).
Endovascular Treatment
All patients were treated according to the 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke.11 Cerebral angiography was performed to assess the location of the diseased vessel, collateral circulation, and vascular pathways. We punctured the femoral artery and placed a sheath using a guide catheter or a long sheath for support. The intracranial pathway catheter was delivered to the end of the internal carotid artery, and the microcatheter was guided through the occluded segment of the lesion. The distal, proximal, and length of the lesion were clarified by double contrast within the intracranial pathway catheter and the microcatheter. “Microcatheter first-pass effect” was performed before thrombectomy, and preferred angioplasty was performed if there was “microcatheter first-pass effect,” and preferred stent thrombectomy was performed if there was no “microcatheter first-pass effect.”
Preferred angioplasty group: We used an exchange-length guidewire and exited the microcatheter. We delivered an intracranial balloon dilatation catheter that was slowly inflated, held for 10 to 30 seconds, and slowly withdrawn. If residual stenosis was <50%, the procedure was completed by observing 15 minutes of flow maintenance. If residual stenosis is >50% or if blood flow could not be maintained, repeat balloon angioplasty or stenting may be indicated. (Fig. 1)
FIGURE 1.
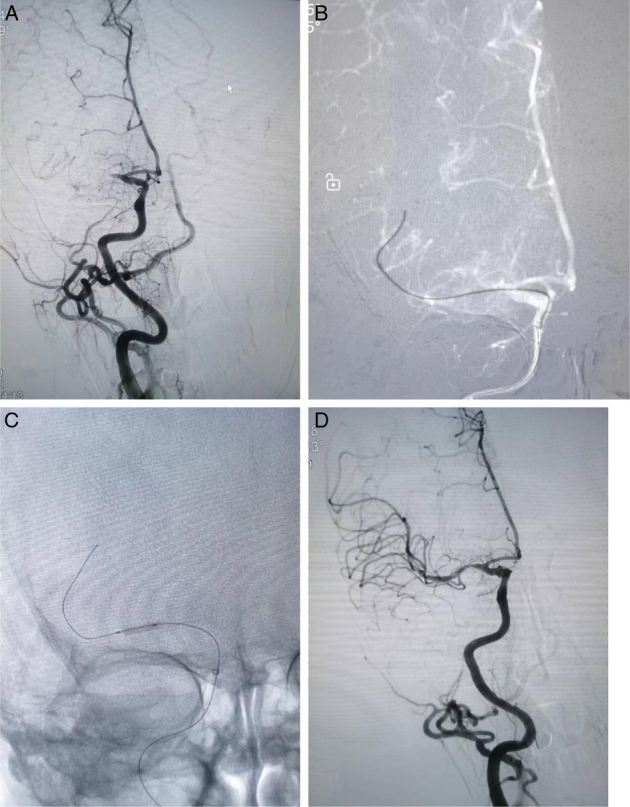
A stroke patient with acute occlusion of the right middle cerebral artery (A). A microcatheter and microwire were navigated through the occluded area to the distal patent artery. The microcatheter was then retrieved proximal to the thrombus. The angiogram showed partial recanalization of the blood flow, which was recorded as the microcatheter first-pass effect (B). An intracranial balloon was delivered for slow inflation and dilatation (2.0*10 mm) (C). Flow was maintained after angioplasty with residual stenosis <50% (D).
Preferred stent thrombectomy group: We placed a stent retriever in the occluded arterial segment and used a combination of pump and pull techniques for thrombectomy no more than three times. If the occluded artery was recanalized and the residual stenosis was <50%, the procedure was completed by observing 15 minutes of flow maintenance. If residual stenosis was >50% or re-occluded, a micro-guidewire should be advanced through the occluded segment for intracranial balloon dilatation (Fig. 2).
FIGURE 2.
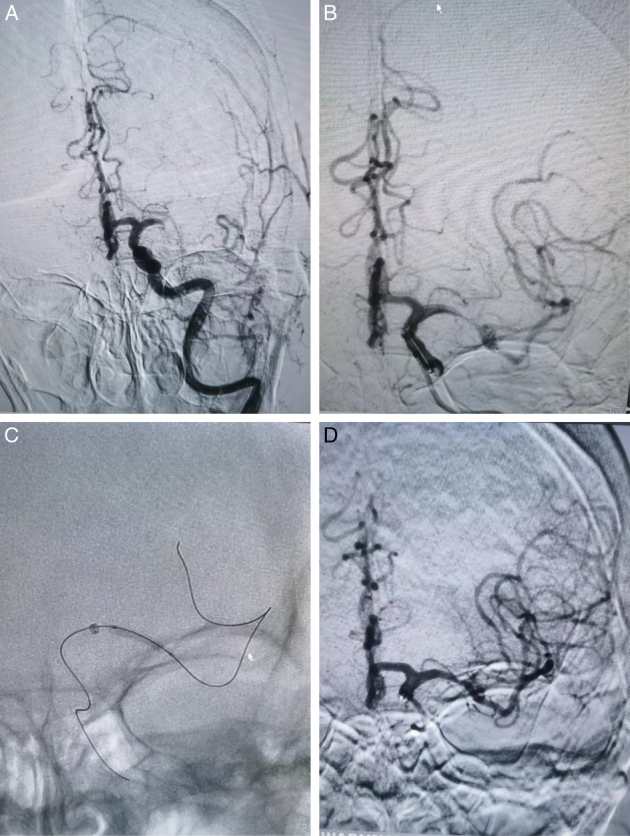
A stroke patient with acute occlusion of the left middle cerebral artery (A). The stent retriever was deployed at the lesion, and angiography showed flow recanalization combined with severe stenosis, the “stenting effect” (B). An intracranial balloon was delivered for slow inflation and dilatation (2.0*10 mm) (C). Flow was maintained after angioplasty with residual stenosis <50% (D).
We used the intracranial balloon dilatation catheter (Neuro RXTM, CHN) (2.0*10 mm) and the Solitaire ABTM (ev3 Neurovascular, USA) stent retriever(4.0*20 mm or 6.0*30 mm). During the perioperative period, tirofiban was administered through the arterial vein to reduce the risk of secondary thrombosis and arterial occlusion during plasty.12 The time to puncture, final recanalization, and mTICI were recorded.
Statistical Analysis
Independent sample t-tests or Mann-Whitney U-test were used for continuous variables, and the χ2 test or Fisher exact test was used for binary variables to perform univariable analyses as appropriate. The relationship between the two treatment groups and prognosis was calculated using a one-way and corrected multifactorial logistic regression model, with correctors as clinically significant variables, and odds ratio (OR) and 95% CI were calculated. All data were analyzed using the statistical package SPSS (version 27.0; SPSS, Chicago, IL, USA). P<0.05 was considered a statistically significant difference. The study was approved by the ethical committee of our institution (ZJGYYLL-2023-05-005), and individual informed consent was not required; however, all study patients’ data were kept strictly confidential.
RESULTS
Baseline Characteristics and Outcomes
A total of 62 patients, with a mean age of 60.66±13.21 y, 14 females and 48 males were included. Of the 32 patients who preferred stent thrombectomy, 15 underwent rescue angioplasty. Twenty-seven achieved mTICI 2b-3. All 30 patients in the preferred angioplasty group underwent angioplasty, and 28 achieved mTICI 2b-3. There were no statistically significant differences in baseline characteristics (age, gender, stroke risk factors, baseline NIHSS, baseline ASPECTS, ASITN/SIR 0-1 ratio, baseline leukocyte count, baseline blood pressure, fasting blood glucose, and D-dimer) and outcomes (time from puncture to recanalization and successful reperfusion of mTICI 2b-3) between patients in the 2 treatment groups (P>0.05). However, the first-pass recanalization was higher in the preferred angioplasty group than in the preferred stent thrombectomy group (P<0.001). The incidence of hyperdensity after EVT was higher in the preferred stent thrombectomy group than in the preferred preferred angioplasty group (P<0.05) (Table 1).
TABLE 1.
Comparison of Baseline Characteristics and Outcomes between the Preferred Stent Thrombectomy Group and the Preferred Angioplasty Group
| Variables | All (N=62) | Preferred stent thrombectomy (N=32) | Preferred angioplasty (N=30) | t/X 2 /Z value | P |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Age, (y, ±s) | 60.66±13.21 | 62.00±12.64 | 59.23±13.86 | 0.822 | 0.414 |
| Gender (femel, n%) | 14 (22.6) | 6 (18.8) | 8 (26.7) | 0.555 | 0.456 |
| NIHSS at admission, median (M, IQR) | 12 (9,15) | 13 (11,16) | 11 (8,14) | −0.808 | 0.419 |
| ASPECTS at admission, median (M, IQR) | 9 (8,9) | 8 (7.5,9) | 9 (8,9) | −0.221 | 0.825 |
| ASITN/SIR 0-1 (n %) | 14 (22.6) | 10 (31.3) | 4 (13.3) | 2.843 | 0.092 |
| Stroke risk factors n (%) | |||||
| Hypertension | 51 (82.3) | 25 (78.1) | 26 (86.7) | 0.774 | 0.379 |
| Diabetes | 11 (17.7) | 7 (21.9) | 4 (13.3) | 0.774 | 0.379 |
| Smoking | 35 (56.5) | 18 (56.3) | 17 (56.7) | 0.001 | 0.974 |
| Drinking | 18 (29.0) | 11 (34.4) | 7 (23.3) | 0.916 | 0.338 |
| Baseline BP (mm Hg, ± s ) | |||||
| SBP | 150.03±17.81 | 149.72±18.70 | 150.37±17.13 | −0.142 | 0.888 |
| DBP | 87.6±12.18 | 85.91±13.26 | 89.40±10.84 | −1.132 | 0.262 |
| Baseline laboratory tests | |||||
| FBG (mmol/L, M, IQR) | 6.73 (5.49,7.96) | 6.99 (5.89,8.26) | 6.60 (5.47,7.65) | −0.008 | 0.994 |
| LDL-C (mmol/L, ±s) | 2.80±0.74 | 2.79±0.70 | 2.85±0.77 | −0.161 | 0.873 |
| Leukocyte (*109/L, ±s) | 7.96±2.33 | 8.30±2.49 | 7.72±2.04 | 1.243 | 0.219 |
| D-dimer (mmol/L, M, IQR) | 0.37 (0.19,0.67) | 0.45 (0.25,0.86) | 0.35 (0.19,0.64) | −0.468 | 0.640 |
| Surgical outcomes | |||||
| Hyperdense after EVT (n %) | 32 (51.6) | 21 (65.6) | 11 (36.7) | 5.199 | 0.023 |
| Puncture to recanalization time (min, M, IQR) | 60 (50,80) | 70 (60,90) | 57.5 (45,70) | −0.909 | 0.363 |
| First-pass recanalization (n %) | 26 (41.9) | 7 (21.9) | 19 (61.3) | 10.930 | <0.001 |
| mTICI(2b-3) (n %) | 55 (88.7) | 27 (84.4) | 28 (93.3) | 1.241 | 0.265 |
ASITN/SIR indicates American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology; ASPECTS, Alberta Stroke Project Early CT Score; DBP, diastolic blood pressure; EVT, Endovascular Therapy; FBG, fasting blood glucose; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; mTICI, modified Thrombolysis in Cerebral Infarction; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood pressure.
Prognosis
The prognosis in the 2 groups was corrected by NIHSS at admission and ASTIN/SIR 0-1 ratio. We found that the proportion of functionally independent patients (mRS 0-3) at 90 days was higher in the preferred angioplasty group than in the preferred stent thrombectomy group (OR=3.681, 95% CI:1.009-13.428, P=0.048) (Fig. 3). However, there was no statistically significant difference between the 2 groups in terms of ENI (P>0.05) (Table 2).
FIGURE 3.
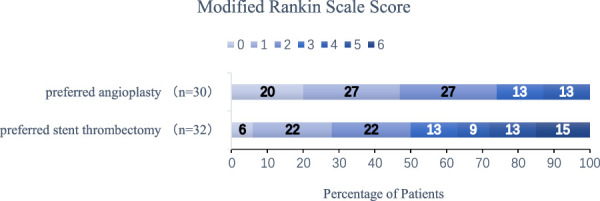
Distribution of functional scores at 90 days: A score of 0 on the modified Rankin scale indicates no symptoms; a score of 1, no clinically significant disability; and a score of 2, slight disability (patients are able to look after their affairs without assistance but are unable to carry out all previous activities); a score of 3, moderate disability (patients require some help but can walk unassisted); a score of 4, moderately severe disability (patients are unable to attend to bodily needs without assistance and are unable to walk unassisted); a score of 5, severe disability (patients require constant nursing care and attention); and a score of 6, death. We found that the proportion of functionally independent patients (mRS 0-3) at 90 days was higher in the preferred angioplasty group than in the preferred stent thrombectomy group (OR=3.681, 95% CI:1.009-13.428, P=0.048).
TABLE 2.
Prognosis in the 2 Treatment Groups by Comparative Logistic Analysis
| Outcomes | Preferred stent thrombectomy (N=32) | Preferred angioplasty (N=30) | OR (95% CI) | P |
|---|---|---|---|---|
| mRS 0-2 | 16 (50.0) | 22 (73.3) | 2.756 (0.926-8.204) | 0.068 |
| mRS 0-3 | 20 (62.5) | 26 (86.7) | 3.681 (1.009-13.428) | 0.048 |
| ENI | 13 (40.6) | 17 (56.7) | 1.748 (0.613-4.987) | 0.296 |
| ICH | 8 (25.0) | 2 (6.7) | 0.245 (0.046-1.301) | 0.099 |
Correction factors: NIHSS at admission and ASITN/SIR 0-1 ratios.
ENI indicates early neurological improvement; ICH, intracranial hemorrhage; mRS, modified Rankin Scale.
DISCUSSION
In this study, we analyzed data from consecutive patients admitted to our hospital with acute middle cerebral artery occlusion due to ICAS who were treated with endovascular therapy. The study found that the preferred angioplasty group had a higher rate of first-pass recanalization and functional independence (mRS 0-3) at 90 days compared with the preferred stent thrombectomy group. However, there was no significant difference in the rate of successful reperfusion and time from a puncture to recanalization between the 2 groups.
No technical strategy for urgent endovascular treatment of large-vessel occlusion in ICAS is currently recommended by the guidelines. A registry study of ICAS-associated large-vessel occlusions in which mechanical thrombectomy was ineffective showed that performing rescue angioplasty significantly improved patient prognosis.13 Endovascular angioplasty treatments for ICAS include balloon dilatation alone, direct stenting, and balloon predilatation followed by stenting. However, there is a lack of trials comparing the advantages and disadvantages of these treatments.14 Previously, the difficulty of endovascular treatment in ICAS was thought to lie in the length of the lesion and the nature of the plaque.15 Therefore, the core of modern reperfusion therapy for mechanical thrombectomy is the quest for rapid and effective recanalization with reduced endothelial damage and embolus escape.16 The study demonstrated that the angioplasty group of choice had a higher rate of first-pass recanalization and improved procedural efficiency. The key to accurately identifying ICAS preoperatively and intraoperatively is crucial for the success of preferred angioplasty. This is achieved through various methods such as identifying watershed infarcts on preoperative cranial DWI, Flair high signal vascular sign,17 and observing the intraoperative microcatheter first-pass effect. ICAS with a few thrombin tends to be rapidly recanalized by arterial lavage techniques in combination with tirofiban during angioplasty. Based on our experience, if there are only a few thrombi and ICAS-LVO is clearly assessed preoperatively, angioplasty is the preferred option. The advantage of preferred angioplasty is the relative simplicity of endovascular manipulation and avoidance of excessive manipulation leading to endothelial damage and perforating lesions. For patients without a microcatheter first-pass effect with ICAS-LVO combined with a significant amount of thrombi, stent thrombectomy is the recommended approach as it reduces thrombi escape. This study also showed that the preferred angioplasty group had a lower incidence of high postoperative CT density and a lower incidence of intracranial hemorrhage (6.7% vs. 25.0%). The relative ease of endovascular manipulation may be associated with lower CT hyperdensity and therefore reduced the incidence of intracranial hemorrhage and poor prognosis, consistent with previous studies.18
Although postoperative stroke recurrence in patients with symptomatic ICAS who undergo angioplasty (stenting) is not superior to medical care alone,19,20 However, timely endovascular therapy can improve prognosis in patients with large mismatches.21 Endovascular treatment of ICAS carries the risk of acute thrombosis, occlusion of perforating arteries, and other serious challenges, including perioperative drug therapy. Acute large vessel occlusion has a high probability of reocclusion after stenting due to the absence of a well-established pre-procedural antithrombotic drug preparation, so angioplasty is chosen over stenting. The study’s advantages include the use of balloon dilatation alone for angioplasty, without routine stenting, resulting in good short-term efficacy. However, the long-term efficacy remains to be determined.
In summary, in patients with ICAS-LVO, the preferred angioplasty group had a higher proportion of first-pass recanalization and a functionally independent (mRS 0-3) at 90 days, although there was no significant effect of the 2 treatment groups on the rate of successful reperfusion and time to recanalization. A limitation of this study was the small sample size. The specification of the stent retriever and the number of thrombectomies were not standardized in this study. Residual stenosis after successful mechanical thrombectomy, which was not followed by rescue angioplasty in some patients, leads to a poor prognosis due to activation of the coagulation system and possible reocclusion. Final infarct volumes and reocclusion rates were not available due to a lack of statistical data. In practice, patients with a “microcatheter first-pass effect” had fewer thrombi, and therefore, angioplasty was preferred, which may result in a higher proportion of functionally independent patients. Therefore, angioplasty may be preferred in ICAS-LVO patients with few thrombi, but as this was an observational cohort study, our results should be interpreted with caution. A larger-sample, multicenter, prospective study is expected.
Footnotes
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
The study protocol was approved by the Ethics Committee of Zhangjiagang Hospital, affiliated with Soochow University (No. ZJGYYLL-2023-05-005), informed consent was waived as this was a retrospective study, and all patient information was strictly confidential.
K.C. and Y.Z. contributed to the design and implementation of the research, K.C. analyzed the results and wrote the manuscript. G.G., Q.W., and K.C. conceived the original and supervised the project.
This study was supported by the Suzhou Science Programme Project Fund (SKY2023019).
The authors declare no conflict of interest.
Contributor Information
Kechun Chen, Email: chenkechun0108@sina.com.cn.
Yin Zhou, Email: zhouyin8605@126.com.
Gang Guo, Email: 38112455@qq.com.
Qiuyi Wu, Email: qiuyi1028@163.com.
REFERENCES
- 1.Nath M, Swarnkar P, Sharma R, et al. Association of modifiable risk factors with ischaemic stroke subtypes in Asian versus Caucasian populations: A systematic review and meta-analysis. Eur J Clin Invest. 2022;52:e13849. [DOI] [PubMed] [Google Scholar]
- 2.Jia B, Ren Z, Mokin M, et al. Current status of endovascular treatment for acute large vessel occlusion in China: A real-world nationwide registry. Stroke. 2021;52:1203–1212. [DOI] [PubMed] [Google Scholar]
- 3.Yoo J, Lee SJ, Hong JH, et al. Immediate effects of first-line thrombectomy devices for intracranial atherosclerosis-related occlusion: Stent retriever versus contact aspiration. BMC Neurol. 2020;20:283.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliveira R, Correia MA, Marto JP, et al. Reocclusion after successful endovascular treatment in acute ischemic stroke: Systematic review and meta-analysis. J Neurointerv Surg. 2022;15:964–970. [DOI] [PubMed] [Google Scholar]
- 5.Marto JP, Strambo D, Hajdu SD, et al. Twenty-four-hour reocclusion after successful mechanical thrombectomy: associated factors and long-term prognosis. Stroke. 2019;50:2960–2963. [DOI] [PubMed] [Google Scholar]
- 6.Psychogios M, Brehm A, López-Cancio E, et al. European stroke organisation guidelines on treatment of patients with intracranial atherosclerotic disease. Eur Stroke J. 2022;7:III–IV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobrocky T, Kaesmacher J, Bellwald S, et al. Stent-retriever thrombectomy and rescue treatment of M1 occlusions due to underlying intracranial atherosclerotic stenosis: Cohort analysis and review of the literature. Cardiovasc Intervent Radiol. 2019;42:863–872. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Farouki Y, Hulscher F, et al. Early neurological improvement predicts clinical outcome after thrombectomy for distal medium vessel occlusions. Front Neurol. 2022;13:809066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havenon AD, Zaidat OO, Amin-Hanjani S, et al. Large vessel occlusion stroke due to intracranial atherosclerotic disease: Identification, medical and interventional treatment, and outcomes. Stroke. 2023;54:1695–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi TY, Chen WH, Wu YM, et al. Microcatheter “first-pass effect” predicts acute intracranial artery atherosclerotic disease-related occlusion. Neurosurgery. 2019;84:1296–1305. [DOI] [PubMed] [Google Scholar]
- 11.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49:46–110.29203686 [Google Scholar]
- 12.Kim YW, Sohn SI, Yoo J, et al. Local tirofiban infusion for remnant stenosis in large vessel occlusion: Tirofiban ASSIST study. BMC Neurol. 2020;20:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baek JH, Kim BM, Ihm EH, et al. Clinical outcomes of rescue stenting for failed endovascular thrombectomy: A multicenter prospective registr. J Neurointerv Surg. 2022;14:1166–1172. [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Lee SJ, Hong JM, et al. Endovascular treatment of large vessel occlusion strokes due to Intracranial atherosclerotic disease. J Stroke. 2022;24:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yaghi S, Khatri P, Havenon A de, et al. Peri-procedural stroke or death in stenting of symptomatic severe intracranial stenosis. J NeuroInterventional Surg. 2020;12:374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadhav AP, Desai SM, Zaidat OO, et al. First pass effect with neuro thrombectomy for acute ischemic stroke: Analysis of the systematic evaluation of patients treated with stroke devices for acute ischemic stroke registry. Stroke. 2022;53:e30–e32. [DOI] [PubMed] [Google Scholar]
- 17.Wang E, Wu C, Yang D, et al. Association between fluid-attenuated inversion recovery vascular hyperintensity and outcome varies with different lesion patterns in patients with intravenous thrombolysis. Stroke Vasc Neurol. 2021;6:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Q, Hou J, Ge J, et al. Clinical significance of hyperdense area after endovascular therapy in patients with acute ischemic stroke: a systematic review and meta-analysis. Cerebrovasc Dis. 2021;50:500–509. [DOI] [PubMed] [Google Scholar]
- 19.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis trial investigators. aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014;383:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao P, Wang T, Wang D, et al. Effect of stenting plus medical therapy vs medical therapy alone on risk of stroke and death in patients with symptomatic intracranial stenosis: The CASSISS randomized clinical trial. JAMA. 2022;328:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]


