Abstract
Objectives The aim of this study was to determine the clinical characteristics and cephalometric risk factors associated with decreased postoperative olfaction in patients in whom the transplanum and transtuberculum expanded endonasal approach (EEA) was performed.
Methods A retrospective cohort of 41 patients treated with the transplanum and transtuberculum EEA was divided into two groups based on the maximum change in the postoperative 22-item Sino-Nasal Outcome Test (SNOT22) olfaction score: prolonged olfactory loss group ( n = 5) with a ΔSNOT22 olfaction score of ≥ 4 without a return to baseline and a preserved olfaction group ( n = 36) with a ΔSNOT22 olfaction score ≤ 3 with return to baseline on follow-up of at least 3 months. Demographics, operative details, and cephalometric measurements were compared between the two groups.
Results There were no differences in terms of the type of surgical approach (transplanum and transtuberculum), resection of turbinates (middle and superior), use of reconstructive flap (nasoseptal flap and reverse flap), or tumor pathology between the two groups. In the prolonged olfactory loss group, there was a smaller angle between the planum and the face of the sella (89.75 ± 9.18 vs. 107.17 ± 16.57 degrees, p = 0.05) and a smaller angle between the anterior nasal spine and the sphenoid sinus face (21.20 ± 2.49 vs. 25.89 ± 4.90 degrees, p = 0.047) compared with the preserved olfaction group.
Conclusion Patients with a narrow angle between the planum and the face of the sella or that between the anterior nasal spine and the sphenoid sinus face are at a higher risk of prolonged olfactory dysfunction with the transplanum and transtuberculum approaches.
Level of Evidence IV.
Keywords: transplanum, transtuberculum, endoscopic approach, olfactory outcome
Introduction
The use of expanded endonasal approaches (EEAs) for the resection of suprasellar pathologies offers advantages over transcranial skull-based approaches such as early identification of the optic apparatus and less manipulation of the surrounding neural structures and vasculature. 1 Despite its advantages, endoscopic access to the planum sphenoidale can compromise postoperative olfaction, significantly impacting the quality of life of patients. 2 3
The olfactory mucosa distribution at the nasal septum, lateral nasal wall, and turbinates is variable and may impact postoperative olfactory outcomes if disrupted in the operative field. 4 In addition to intraoperative trauma, several other factors may contribute to postoperative olfactory dysfunction including inflammation or obstructive problems caused by packing, crusting, or scar formation, which may restrict odorants from reaching the olfactory receptors. 5 6 Lee et al assessed the olfactory outcomes after endoscopic transsphenoidal hypophysectomy and analyzed the risk factors for postoperative olfactory dysfunction based on several factors including demographics, intraoperative variables, and cephalometric measurements in the coronal and sagittal planes. 7 However, the transplanum and transtuberculum EEAs may be at a significantly greater risk of olfactory injury as these approaches require removal of the bony skull base anterior to the sella turcica, and the olfactory mucosa of the septum lies immediately anterior to this necessary bony removal from an endonasal anterior approach. The objective of this study was to determine the risk factors associated with the postoperative olfactory dysfunction in the transplanum and transtuberculum EEAs with an emphasis on cephalometric measurements, extent of sinonasal tissue manipulation, and patient demographics.
Materials and Methods
Institutional review board approval was obtained (STUDY 20040320). A retrospective chart review was performed to query all patients who underwent a transplanum or transtuberculum EEA at a tertiary academic center from 2016 to 2021. The exclusion criteria included age younger than 18 years, use of EEAs not involving the transplanum or the transtuberculum, follow-up less than 3 months, and missing 22-item Sino-Nasal Outcome Test (SNOT-22) data on follow-up clinic visits.
All the patients completed a SNOT-22 survey preoperatively and at each postoperative visit. The primary measured olfactory outcome was the change in SNOT-22 olfaction score (ΔSNOT-22), based on item no. 21 in the survey. Patients were divided into two groups based on the worst ΔSNOT-22 olfaction score in follow-up: (1) prolonged olfactory loss ( n = 5) with ΔSNOT-22 olfaction score ≥4 (“severe problem” or “problem as bad as it can be”) post-op without a return to baseline on follow-up after 3 months and (2) preserved olfaction group ( n = 36) with ΔSNOT-22 olfaction score ≤3 (“no problem,” “very mild problem,” “mild problem,” or “moderate problem”). The first postoperative visit for packing removal was excluded. Patients were seen in clinic for postoperative debridement every few weeks and maintained on either nasal saline spray or irrigations. Demographics, clinical history, and intraoperative details including the extent of surgical resection, resection of the middle and superior turbinates, use of reconstructive flaps and grafts, and tumor details were collected. Preoperative computed tomography (CT) and magnetic resonance imaging (MRI) were assessed by two radiologists independently to determine the cephalometric distances and angles at the midsagittal and coronal cuts at the anterior skull base ( Figs. 1 and 2 ). The cephalometric angles were defined as follows: angle of the overall anterior skull base from the 0-degree reference line (angle A), angle between the cribriform plate and the planum (angle B), angle between the planum and the face of the sella (angle C), angle between the planum and the posterior aspect of the clivus (angle D), and angle between the anterior nasal spine and the superior and inferior aspect of the sphenoid sinus face (angle E).
Fig. 1.
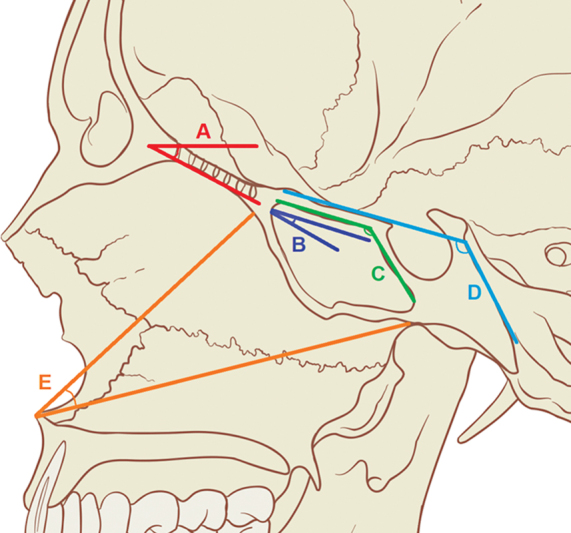
Cephalometric angles. Reference diagram for cephalometric angles. ( A ) Angle of overall anterior skull base (re: 0-degree reference line). ( B ) Angle between the cribriform plate and the planum. ( C ) Angle between the planum and the face of the sella. ( D ) Angle between the planum and the posterior aspect of the clivus. ( E ) Angle between the anterior nasal spine and the superior and inferior aspect of the sphenoid sinus face.
Fig. 2.
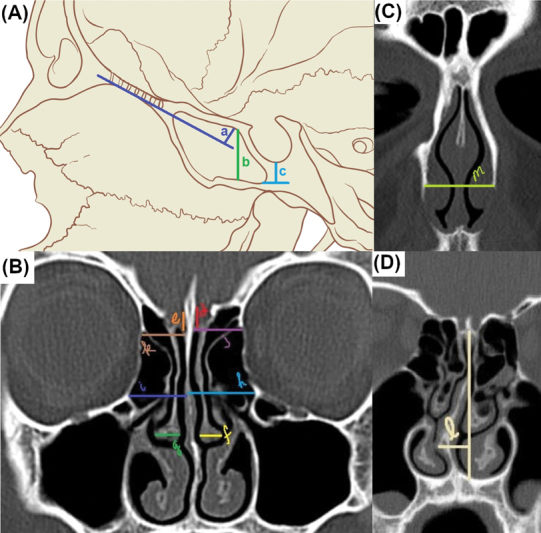
Cephalometric distances. Reference diagram for cephalometric distances at the skull base and sinonasal cavity. ( A ) a: Planum height; b: sphenoid sinus height—between the sphenoid floor and the planum; c: distance between the sphenoid floor to the base of the sella turcica. ( B ) d, e: Depth of the olfactory fossa; f, g: width of the middle turbinates; h, i: distance between the orbital struts and the septum; j, k: distance between the lamina papyracea and the midline cribriform. ( C ) m: Maximum width of the pyriform aperture. ( D ) l: Maximum septal deflection from the midline at the deepest part of the cribriform plate.
Statistical Analysis
Quantitative variables such as SNOT-22 olfaction scores, cephalometric angles, and distances were analyzed using a comparison of means tests. Relative risks (RRs) along with 95% confidence intervals (CI) were calculated for categorical variables such as gender, ethnicity, and other clinical variables. A p -value of ≤0.05 was considered statistically significant.
Results
Demographics and Baseline Information
A total of 41 patients were enrolled in the study ( Table 1 ). The majority of the patient cohorts were males (58% in preserved olfaction and 60% in prolonged olfactory loss) and Caucasians (97% in preserved olfaction and 60% in prolonged olfactory loss). The mean age of the patients in the preserved olfaction group was 56.98 ± 17.92 years and it was 53.34 ± 12.74 years for the prolonged olfactory loss group. Of those with preserved olfaction, the most common tumor pathology was pituitary adenoma (42%) followed by craniopharyngioma (22%), meningioma (17%), chordoma (8%), and Rathke's cleft cyst (6%). The five patients with prolonged olfactory loss had the following pathology: pituitary adenoma ( n = 3), meningioma ( n = 1), and craniopharyngioma ( n = 1). Non-Caucasian ethnicities were associated with higher risk of prolonged olfactory loss (RR: 14.40; 95% CI: 1.58–131.36). There were no significant differences in the baseline prevalence of chronic rhinosinusitis without polyps, chronic rhinosinusitis with polyps, septal deviation, prior endoscopic sinus surgery, asthma, and hypothyroidism between the two groups. Two patients in the preserved olfaction group were diagnosed with COVID-19 but had no reported olfactory changes: none of the patients in the prolonged olfactory loss group experienced COVID-19 infection.
Table 1. Demographic information and baseline characteristics.
| Preserved olfaction ( n = 36) | Prolonged olfactory loss ( n = 5) | RR (95% CI) | p -value | |
|---|---|---|---|---|
| Age, average (y) | 56.98 ± 17.92 | 53.34 ± 12.74 | –20.49 to 13.21 | 0.66 |
| Gender (%) | ||||
| Female | 42 | 40 | 0.96 (0.31–3.00) | 0.94 |
| Male | 58 | 60 | ||
| Ethnicity (%) | ||||
| Other | 3 | 40 | 14.40 (1.58–131.36) | 0.02 |
| Caucasian | 97 | 60 | ||
| CRSsNP (%) | 0 | 0 | 6.17 (0.13–282.33) | 0.35 |
| CRSwNP (%) | 6 | 0 | 1.23 (0.07–22.67) | 0.89 |
| Septal deviation (%) | 47 | 40 | 0.85 (0.27–2.62) | 0.77 |
| Prior FESS (%) | 39 | 40 | 1.03 (0.33–3.25) | 0.96 |
| Diabetes (%) | 42 | 40 | 0.96 (0.31–3.00) | 0.94 |
| Hypothyroidism (%) | 42 | 40 | 0.96 (0.31–3.00) | 0.94 |
| Asthma (%) | 11 | 20 | 1.80 (0.25–13.06) | 0.56 |
| Presence of neurologic disorders (%) | 33 | 40 | 1.20 (0.37–3.86) | 0.76 |
| Follow-up time frame (d) | 271.00 ± 240.41 | 143.80 ± 49.65 | –347.59 to 93.19 | 0.25 |
Abbreviations: CI, confidence interval; CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; FESS, functional endoscopic sinus surgery; RR, relative risk.
Intraoperative Findings
There were no differences between the preserved olfaction and prolonged olfactory loss groups in terms of the type of surgical approach (transplanum and transtuberculum), extent of tumor resection (complete and partial), resection of turbinates (middle and superior), use of reconstructive flap (nasoseptal flap and reverse flap), or tumor pathology ( Table 2 ).
Table 2. Intraoperative findings.
| Preserved olfaction ( n = 36) | Prolonged olfactory loss ( n = 5) | RR (95% CI) | p -value | |
|---|---|---|---|---|
| Partial tumor resection (%) | 15 | 0 | 0.53 (0.03–8.40) | 0.65 |
| Transplanum approach (excluding transtuberculum), % | 31 | 0 | 0.27 (0.02–3.98) | 0.34 |
| Transtuberculum approach (excluding transplanum), % | 25 | 60 | 2.40 (0.96–5.98) | 0.06 |
| Both transplanum and transtuberculum approaches (%) | 44 | 40 | 0.90 (0.29–2.80) | 0.86 |
| Resection of one or both middle turbinates (%) | 58 | 80 | 1.37 (0.82–2.30) | 0.23 |
| Partial resection of the superior turbinates (%) | 22 | 20 | 0.90 (0.14–5.76) | 0.91 |
| Septoplasty (%) | 11 | 0 | 0.69 (0.04–11.17) | 0.79 |
| Nasoseptal flap (%) | 86 | 60 | 0.70 (0.34–1.44) | 0.33 |
| Reverse flap (%) | 61 | 40 | 0.65 (0.22–1.98) | 0.45 |
| Optic canal or nerve involvement (%) | 46 | 40 | 0.85 (0.27–2.62) | 0.77 |
Abbreviations: CI, confidence interval; RR, relative risk.
Olfaction Measures
There was no significant difference in the baseline SNOT-22 olfaction survey between the two groups ( Table 3 ). In the preserved olfaction group, 58.33% of patients experienced no change or an improvement in olfaction post-op (ΔSNOT-22 olfaction score ≤0), whereas 41.67% had some worsening olfaction post-op that resolved on follow-up. In the prolonged olfactory loss group, three patients (60%) had some olfactory improvement after initially reporting severe olfactory loss albeit not returning to baseline. Patients reported attaining their best post-op olfaction at 68.47 ± 47.49 days for the preserved olfaction group and 88.00 ± 67.55 for the prolonged olfactory loss group. None of the patients in this study used olfactory training in the post-op period.
Table 3. Olfaction measures.
| Preserved olfaction ( n = 36) | Prolonged olfactory loss ( n = 5) | RR (95% CI) | p -value | |
|---|---|---|---|---|
| Baseline SNOT-22 olfaction score | 0.69 ± 1.45 | 0 ± 0 | –2.02 to 0.64 | 0.30 |
| Worst ΔSNOT-22 olfaction score post-op | 0.50 ± 1.61 | 4.40 ± 0.55 | 2.42 to 5.38 | <0.0001 |
| Best ΔSNOT-22 olfaction score post-op | –0.53 ± 1.21 | 2.60 ± 1.52 | 1.93 to 4.33 | <0.0001 |
| Time to worst ΔSNOT-22 olfaction score (d) | 49.69 ± 47.79 | 61.80 ± 64.75 | –35.96 to 60.18 | 0.61 |
| Time to best ΔSNOT-22 olfaction score (d) | 68.47 ± 47.49 | 88.00 ± 67.55 | –28.66 to 67.72 | 0.42 |
| Use of olfactory training (%) | 0 | 0 | 6.00 (0.13–274.59) | 0.36 |
Abbreviations: CI, confidence interval; RR, relative risk; SNOT-22, 22-item Sino-Nasal Outcome Test.
Cephalometric Measures
There was no significant difference between the preserved olfaction and prolonged olfactory loss groups with regard to the following metrics: supraplanum tumor height, suprasellar tumor width, depth of tumor involvement in the sella, planum height, sphenoid sinus height, distance between the sphenoid sinus floor and the base of the sella, width of the middle turbinates, distance between the orbital strut and the septum, and distance between the lamina papyracea and the cribriform plate ( Table 4 ). The preserved olfaction group had a larger angle between the planum and the face of the sella (angle C, 107.17 ± 16.57 vs. 89.75 ± 9.18 degrees; p = 0.050) and a larger angle between the anterior nasal spine and the sphenoid sinus face (angle E, 25.89 ± 4.90 vs. 21.20 ± 2.49 degrees; p = 0.047; Fig. 3 ). The prolonged olfactory loss group had a greater depth of the olfactory fossa (left: 0.70 ± 0.16 vs. 0.55 ± 0.16 cm, p = 0.059; right: 0.74 ± 0.21 vs. 0.58 ± 0.16 cm, p = 0.053) as compared with the preserved olfaction group.
Table 4. Cephalometric data.
| Cephalometric measure | Preserved olfaction ( n = 36) | Prolonged olfactory loss ( n = 5) | p -value |
|---|---|---|---|
| Supraplanum tumor height (cm) | 0.88 ± 0.56 | 0.7 ± 0.61 | 0.545 |
| Suprasellar tumor width (cm) | 1.81 ± 0.95 | 2.14 ± 0.41 | 0.447 |
| Angle of the overall anterior skull base (angle of the cribriform plate to 0-degree reference line) (A) | 12.22 ± 5.03 | 11.25 ± 6.95 | 0.729 |
| Angle between the cribriform plate and the planum (B) | 15.63 ± 7.48 | 22.25 ± 6.55 | 0.101 |
| Angle between the planum and the face of the sella (C) | 107.17 ± 16.57 | 89.75 ± 9.18 | 0.050 |
| Angle between the planum and the posterior aspect of the clivus (D) | 112.07 ± 7.83 | 106.75 ± 9.11 | 0.218 |
| Angle between the anterior nasal spine and the superior and inferior aspect of the sphenoid sinus face (E) | 25.89 ± 4.90 | 21.20 ± 2.49 | 0.047 |
| Depth of tumor involvement in the sella (length from the tuberculum to the deepest portion of the sella involved by tumor), cm | 1.26 ± 0.64 | 1.60 ± 0.22 | 0.308 |
| Planum height (distance from the frontobasal line to the top of the planum), (a, cm) | 0.61 ± 0.32 | 0.63 ± 0.13 | 0.903 |
| Sphenoid sinus height (between the sphenoid floor and the planum) (b, cm) | 2.53 ± 0.40 | 2.53 ± 0.13 | 1.000 |
| Distance between the sphenoid floor to the base of the sella turcica (c, cm) | 1.33 ± 0.41 | 1.00 ± 0.32 | 0.135 |
| Depth of the left olfactory fossa (d, cm) | 0.55 ± 0.16 | 0.70 ± 0.16 | 0.059 |
| Depth of the right olfactory fossa (e, cm) | 0.58 ± 0.16 | 0.74 ± 0.21 | 0.053 |
| Width of the left middle turbinate (f, cm) | 0.79 ± 0.31 | 0.85 ± 0.24 | 0.713 |
| Width of the right middle turbinate (g, cm) | 0.77 ± 0.24 | 0.78 ± 0.15 | 0.937 |
| Distance between the left orbital strut and the septum (h, cm) | 1.43 ± 0.22 | 1.50 ± 0.23 | 0.515 |
| Distance between the right orbital strut and the septum (i, cm) | 1.41 ± 0.20 | 1.48 ± 0.36 | 0.520 |
| Distance between the left lamina papyracea and the midline cribriform plate (j, cm) | 1.25 ± 0.17 | 1.30 ± 0.16 | 0.541 |
| Distance between the right lamina papyracea and the midline cribriform plate (k, cm) | 1.23 ± 0.17 | 1.20 ± 0.14 | 0.710 |
| Maximum septal deflection from midline at the deepest part of the cribriform plate (l, cm) | 0.59 ± 0.27 | 0.36 ± 0.15 | 0.075 |
| Maximum width of the pyriform aperture (m, cm) | 2.35 ± 0.19 | 2.38 ± 0.16 | 0.740 |
Fig. 3.
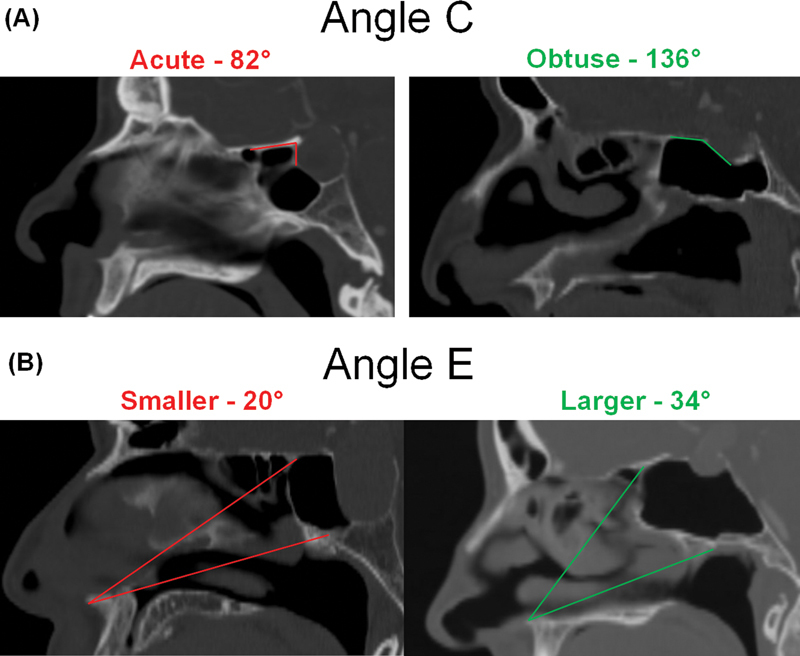
Cephalometric angles of significance. Examples of cephalometric angles that are protective against or predispose to prolonged postoperative olfactory dysfunction in the transplanum and transtuberculum approaches. ( A ) An acute Angle C, between the planum and the face of the sella, is associated with increased risk of post-op olfactory dysfunction. ( B ) A smaller Angle E, between the anterior nasal spine and the superior and inferior aspect of the sphenoid sinus face, is associated with increased risk of post-op olfactory dysfunction.
Discussion
Twelve percent of the patients undergoing the transtuberculum and transplanum approach did not return to their subjective level of olfaction postoperatively. Our study demonstrates that patients with prolonged olfactory loss postoperatively after these two approaches tend to have a smaller angle between the planum and the face of the sella (angle C) and between the anterior nasal spine and the sphenoid sinus face (angle E) as described in Figures 1 and 3 . Smaller angles C and E may predispose to greater trauma in the region of the olfactory neuroepithelium with repeat instrumentation during the transtuberculum and transplanum EEAs. With a smaller angle C, there is less visualization from an endonasal approach as the skull base is flatter. This predisposes to more manipulation of the olfactory mucosa and may cause difficulty with bony removal. In patients with smaller angle E, there may be a narrower sagittal endonasal working corridor that may, in turn, increase the risk of trauma to the olfactory mucosa.
There are exceptionally few studies assessing the olfactory outcomes from the transplanum and transtuberculum approaches. Riva et al utilized the Sino-Nasal Outcome Test for Neurosurgery (SNOT-NC) for 300 patients with anterior skull base disease and demonstrated that those undergoing transtuberculum and transplanum approaches had greater olfactory disturbances compared with other approaches such as the transsphenoidal, transclival, or transpterygoid approach. 8 However, the authors did not delineate which specific risk factors are associated with worse olfactory outcomes with the two approaches. Rioja et al used the Barcelona Smell Test 24 (BAST-24) to compare olfactory outcomes 1 year after the transsphenoidal approach versus EEAs such as the transplanum and transtuberculum. 9 In the expanded endonasal group, there was no significant difference in smell detection, memory/recognition, and forced identification categories compared with the preoperative time frame; however, this group did demonstrate greater decrease in the mucociliary clearance time compared with the standard transsphenoidal approach. 9 In another case series of craniopharyngioma patients who underwent the transtuberculum approaches, the prevalence of persistent and transient anosmia was reported to be 5.5 and 1.4%, respectively. 10
Lee et al analyzed the risk factors associated with olfactory dysfunction after endoscopic transsphenoidal approaches. 7 Although EEAs were excluded, the authors determined that use of abdominal fat grafting, smoking history, and a smaller angle between the planum sphenoidale and the face of the sella turcica increased the risk of postoperative olfactory dysfunction. 7 This corroborates the cephalometric data in our transtuberculum and transplanum case series where the prolonged olfactory loss group had a more acute angle between the planum and the face of the sella, nearly a right angle. However, our cephalometric analysis also revealed that a larger working angle between the anterior nasal spine and the face of the sphenoid is a protective factor against post-op olfactory dysfunction. This finding was not present in the Lee et al series. The use of nasoseptal flap, reverse flap, middle turbinate removal, and partial resection of the superior turbinates did not appear to increase the risk of postoperative olfactory dysfunction in our study. This is consistent with reports in the literature regarding the safety of the nasoseptal flap and partial resection of the superior turbinates from an olfactory standpoint. 11 12 13
There was a significantly higher percentage of non-Caucasian ethnicities in our prolonged olfactory loss group compared with the preserved olfaction group. The underlying reason for this difference in demographics is unclear. Given that the prolonged olfactory loss group had a small sample size ( n = 5), this could be a result of sampling bias. Our olfaction data are based on the SNOT-22 olfactory domain score, which has been utilized in prior studies for tracking outcomes in chronic rhinosinusitis and endoscopic pituitary surgeries. 14 15 While the SNOT-22 survey has been validated for various populations including Italian, Greek, and Brazilian Portuguese with good internal reliability, it is unclear whether differences in survey interpretation across ethnicities may have contributed to these results. 16 17 18 19 Further studies using cross-culturally applicable olfaction testing, such as the modified University of Pennsylvania Smell Identification Test (UPSIT), may clarify the role of ethnicity as an independent risk factor for postoperative olfactory dysfunction in EEAs. 20 21
There are a few limitations to this study. The patient cohort is small, and there was no standardized follow-up time frame for both the preserved olfaction and prolonged olfactory loss groups given the retrospective nature of the study. Olfaction was assessed via patient-reported outcomes measures, SNOT-22 olfaction domain, rather than objective olfactory testing such as UPSIT or Sniffin' sticks.
Conclusion
This is one of the first studies to review olfactory outcomes in the transplanum and transtuberculum EEAs. Only 12% of patients experienced prolonged olfactory loss greater than 3 months. Patients with a narrow angle between the planum and the face of the sella or a narrow angle between the anterior nasal spine and the sphenoid sinus face were at a higher risk of prolonged olfactory dysfunction. Measurement of these angles can help stratify patients at the greatest risk for prolonged olfactory dysfunction and perhaps necessitate the use of angled endoscopy to avoid manipulation of the olfactory mucosa. Surgeons should exercise additional caution to avoid inadvertent trauma to the olfactory mucosa in these patients.
Conflict of Interest None declared.
Author's Contributions
Z.B. and H.D. contributed to data collection and manuscript preparation and editing. A.K. were responsible for data collection and manuscript editing. P.G., G.Z., C.H.S., and K.T. contributed to manuscript editing and review. E.W.W. contributed to the project design and manuscript editing and review.
References
- 1.Bander E D, Singh H, Ogilvie C B et al. Endoscopic endonasal versus transcranial approach to tuberculum sellae and planum sphenoidale meningiomas in a similar cohort of patients. J Neurosurg. 2018;128(01):40–48. doi: 10.3171/2016.9.JNS16823. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho A CM, Dolci R LL, Rickli J CK et al. Evaluation of olfactory function in patients undergoing endoscopic skull base surgery with nasoseptal flap. Rev Bras Otorrinolaringol (Engl Ed) 2022;88(01):15–21. doi: 10.1016/j.bjorl.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolci R LL, de Carvalho A CM, Rickli J CK et al. Relationship between the bilateral removal of the middle nasal turbinate and the olfactory function in endoscopic skull base surgery. World Neurosurg. 2020;142:e337–e343. doi: 10.1016/j.wneu.2020.06.240. [DOI] [PubMed] [Google Scholar]
- 4.Escada P. Localização e distribuição da mucosa olfactiva humana nas fossas nasais [Localization and distribution of human olfactory mucosa in the nasal cavities] Acta Med Port. 2013;26(03):200–207. [PubMed] [Google Scholar]
- 5.Thompson C F, Kern R C, Conley D B. Olfaction in endoscopic sinus and skull base surgery. Otolaryngol Clin North Am. 2015;48(05):795–804. doi: 10.1016/j.otc.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Patel Z M, DelGaudio J M. Olfaction following endoscopic skull base surgery. Curr Opin Otolaryngol Head Neck Surg. 2016;24(01):70–74. doi: 10.1097/MOO.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 7.Lee J J, Thompson Z S, Piccirillo J F et al. Risk factors for patient-reported olfactory dysfunction after endoscopic transsphenoidal hypophysectomy. JAMA Otolaryngol Head Neck Surg. 2020;146(07):621–629. doi: 10.1001/jamaoto.2020.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riva G, Zenga F, Motatto G M et al. Quality of life after endoscopic skull base surgery: validation and reliability of the Italian version of the Sino-Nasal Outcome Test for Neurosurgery (SNOT-NC) World Neurosurg. 2022;163:e426–e434. doi: 10.1016/j.wneu.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Rioja E, Bernal-Sprekelsen M, Enriquez K et al. Long-term outcomes of endoscopic endonasal approach for skull base surgery: a prospective study. Eur Arch Otorhinolaryngol. 2016;273(07):1809–1817. doi: 10.1007/s00405-015-3853-9. [DOI] [PubMed] [Google Scholar]
- 10.Park H R, Kshettry V R, Farrell C J et al. Clinical outcome after extended endoscopic endonasal resection of craniopharyngiomas: two-institution experience. World Neurosurg. 2017;103:465–474. doi: 10.1016/j.wneu.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 11.Upadhyay S, Buohliqah L, Dolci R LL, Otto B A, Prevedello D M, Carrau R L. Periodic olfactory assessment in patients undergoing skull base surgery with preservation of the olfactory strip. Laryngoscope. 2017;127(09):1970–1975. doi: 10.1002/lary.26546. [DOI] [PubMed] [Google Scholar]
- 12.Friedman M, Caldarelli D D, Venkatesan T K, Pandit R, Lee Y. Endoscopic sinus surgery with partial middle turbinate resection: effects on olfaction. Laryngoscope. 1996;106(08):977–981. doi: 10.1097/00005537-199608000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Gong S W, Ahmadi S, Blackburn S L et al. Sniffin' sticks to measure olfactory function and recovery following bilateral superior turbinate resection as part of endoscopic transsphenoidal approach. Ann Otol Rhinol Laryngol. 2021;130(06):636–642. doi: 10.1177/0003489420965621. [DOI] [PubMed] [Google Scholar]
- 14.DeConde A S, Mace J C, Alt J A, Schlosser R J, Smith T L, Soler Z M. Comparative effectiveness of medical and surgical therapy on olfaction in chronic rhinosinusitis: a prospective, multi-institutional study. Int Forum Allergy Rhinol. 2014;4(09):725–733. doi: 10.1002/alr.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmer L A, Shah O, Theodosopoulos P V. Short-term quality-of-life changes after endoscopic pituitary surgery rated with SNOT-22. J Neurol Surg B Skull Base. 2014;75(04):288–292. doi: 10.1055/s-0034-1372464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozzanica F, Preti A, Gera R et al. Cross-cultural adaptation and validation of the SNOT-22 into Italian. Eur Arch Otorhinolaryngol. 2017;274(02):887–895. doi: 10.1007/s00405-016-4313-x. [DOI] [PubMed] [Google Scholar]
- 17.Lachanas V A, Tsea M, Tsiouvaka S, Hajiioannou J K, Skoulakis C E, Bizakis J G. The Sino-Nasal Outcome Test (SNOT)-22: validation for Greek patients. Eur Arch Otorhinolaryngol. 2014;271(10):2723–2728. doi: 10.1007/s00405-014-2969-7. [DOI] [PubMed] [Google Scholar]
- 18.Kosugi E M, Chen V G, Fonseca V M, Cursino M M, Mendes Neto J A, Gregório L C. Translation, cross-cultural adaptation and validation of SinoNasal Outcome Test (SNOT): 22 to Brazilian Portuguese. Rev Bras Otorrinolaringol (Engl Ed) 2011;77(05):663–669. doi: 10.1590/S1808-86942011000500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudmik L, Hopkins C, Peters A, Smith T L, Schlosser R J, Soler Z M. Patient-reported outcome measures for adult chronic rhinosinusitis: a systematic review and quality assessment. J Allergy Clin Immunol. 2015;136(06):1532–154000. doi: 10.1016/j.jaci.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Ogihara H, Kobayashi M, Nishida K, Kitano M, Takeuchi K. Applicability of the cross-culturally modified University of Pennsylvania Smell Identification Test in a Japanese population. Am J Rhinol Allergy. 2011;25(06):404–410. doi: 10.2500/ajra.2011.25.3658. [DOI] [PubMed] [Google Scholar]
- 21.Altundag A, Tekeli H, Salihoglu M et al. Cross-culturally modified University of Pennsylvania Smell Identification Test for a Turkish population. Am J Rhinol Allergy. 2015;29(05):e138–e141. doi: 10.2500/ajra.2015.29.4212. [DOI] [PubMed] [Google Scholar]


