Abstract
Objectives The lateral supraorbital (LSO) approach is a less-invasive alternative to the pterional craniotomy that provides rapid transsylvian access. Establishing familiarity with the LSO technique and its features as compared with other anterolateral approaches is an important component of advanced skull base training. We present a step-by-step demonstration of the LSO approach using cadaveric dissection in a manner that is digestible for trainees at various levels.
Design This is anatomic step-by-step dissection and representative case series.
Setting This study was carried out in the cadaveric dissection laboratory.
Participants A formalin-fixed, latex-injected cadaveric head specimen was dissected under microscopic magnification by a neurosurgery resident under faculty supervision. Following dissection, representative case applications were reviewed.
Main Outcome Measures Dissection and case illustration were the main outcome measures.
Results A single-layer myocutaneous flap is developed, and a single-burr-hole technique is used, followed by extensive drilling of the sphenoid wing. The dura is opened in a C-shaped fashion centered on the Sylvian fissure, exposing the inferior frontal and superior temporal lobes. Labeled photographs of dissections with pertinent anatomical structures are presented. Three case examples illustrating the versatility of the LSO approach, including the resection of a large pituitary adenoma, an inferior frontal melanoma metastasis presenting to the Sylvian surface, and a frontoinsular low-grade glioma, are reviewed.
Conclusion As compared with the pterional craniotomy, the LSO approach involves a shorter incision, smaller craniotomy, and faster exposure; it can be conveniently tailored to various indications. Understanding the step-by-step dissection and indications of the LSO approach is of paramount importance to neurosurgery trainees.
Keywords: awake craniotomy, cadaver, complex, craniotomy, lateral supraorbital, low-grade glioma, pituitary adenoma, skull base, trainee
Introduction
The lateral supraorbital (LSO) approach is a less-invasive alternative to the classical pterional craniotomy that provides rapid transsylvian access, initially popularized by Juha Hernesniemi et al as a preferred technique for select cerebrovascular operations. 1 2 Compared with the pterional craniotomy, the LSO approach involves a shorter incision, smaller craniotomy, and faster exposure. 3 It may also be associated with fewer complications and favorable aesthetic outcomes, given the more extensive preservation of temporalis muscle insertion, and the avoidance of frontotemporal facial nerve branches. 3 4 5 Although predominantly described in the setting of anterior circulation aneurysms, it is a versatile technique with wide application across a range of lesions involving the anterior fossa, middle fossa, parasellar space, and insula. 3 6 7
Establishing familiarity with the LSO technique and its features as compared with other anterolateral approaches is an important component of advanced skull base training, which in turn is facilitated by laboratory dissection. Although this approach has been demonstrated with intraoperative images and videos, there remains a need for a detailed, operatively centered, independently reproducible tutorial for trainees learning and mastering the technique. 1 8 9 We report a step-by-step anatomic demonstration of the LSO approach using cadaveric dissection, tailored to the educational needs of neurosurgical trainees. Additionally, we highlight three illustrative case examples that demonstrate the broad utility of this approach beyond conventional cerebrovascular indications.
Methods
All aspects of this study were approved by our institutional review board and biospecimens committee, as required by standard protocols. A formalin-fixed, latex-injected cadaveric head specimen was dissected under microscopic magnification by a neurosurgery resident (X.Z.) under faculty supervision (C.S.G.). Critical operative elements were photographed and documented in a stepwise fashion. Following dissection, representative case examples were reviewed (IRB 10195).
Results
Step-by-Step Surgical Approach
Positioning and Incision Planning
Following anesthesia induction, intubation, and preparation of cranial nerve (CN) monitoring, the patient is positioned supine with the head placed in three-point pinion fixation in slight extension and contralateral rotation of approximately 15 degrees along each axis. Given the relatively narrow corridor, optimal positioning is critical in the anticipation of an LSO approach. Some variability in positioning may be warranted for certain operative targets; however, these conventional maneuvers center the Sylvian fissure, facilitate gravity-based dissection, minimize retraction of either the frontal or temporal lobe, and reduce the risk of the orbital roof obstructing visualization of the skull base and proximal internal carotid artery (ICA) due to overextension. In particular, if a clinoidectomy is planned, care should be undertaken not to overextend the neck, so as to avoid positioning the anterior clinoid past the perpendicular line (relative to the floor). Overextension could cause the anterior clinoid to be directed away from the surgeon, which complicates the clinoidectomy. Similarly, although rotational configurations closer to anatomic positioning (≤15 degrees) favor access to midline structures such as the anterior communicating artery (ACoA) complex, and more pronounced rotation (≥45 degrees) favors distal ipsilateral access including the middle cerebral artery (MCA) candelabra and insula, each hazards unintentional obstruction, and most cases are best served by an intermediate positioning (15 degrees extension and rotation). 10
A curvilinear incision is planned from the level of the lateral canthus to the level of the midpupillary line along the hairline ( Fig. 1A ). The incision of an LSO approach is shorter and more anterior than the conventional incision for a pterional craniotomy, preserving sylvian and subfrontal access while minimizing temporal exposure. Certain LSO operations may benefit from extension or limitation of the incision in either trajectory, for example, to maximize pretemporal or cavernous sinus access. Although most operations involve a more limited incision, the current dissection presents a maximal iteration for illustrative purposes.
Fig. 1.
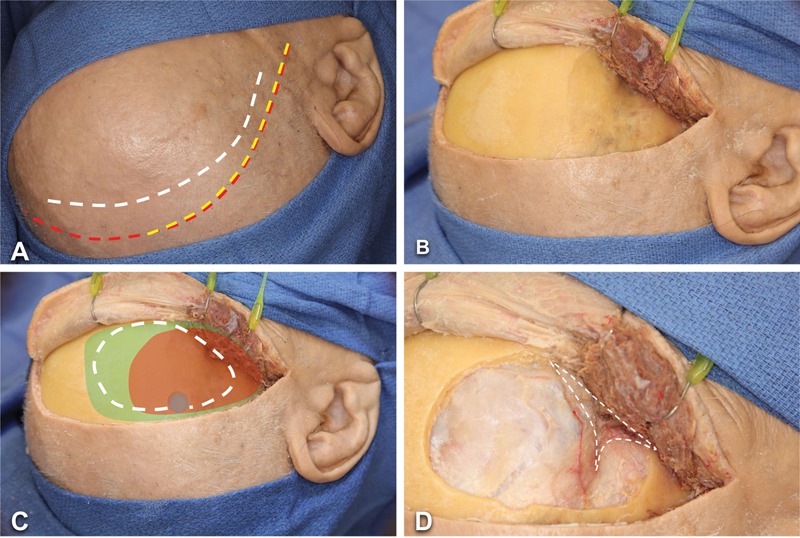
Step-by-step LSO approach in an anatomical specimen (right side). ( A ) A curvilinear incision (white dashed line) extends from the level of the lateral canthus to midline behind the hairline. A pterional (red dashed line) and a mini-pterional (yellow dashed line) incisions are demonstrated as comparison. ( B ) A myocutaneous flap is developed and secured as anteriorly as possible with fishhooks, exposing the zygomatic process of the frontal bone. ( C ) A burr hole and craniotomy (white dashed line) is created. A pterional (green shaded area) and a mini-pterional (orange shaded area) craniotomies are illustrated for comparison. ( D ) The sphenoid wing is drilled extensively (white dashed line indicates area covered by the right sphenoid wing). Abbreviation: LSO, lateral supraorbital.
Scalp and Muscle Flaps
The skin is incised and dissected off the pericranium. Usually, a single-layer myocutaneous flap is developed ( Fig. 1B ), as only the superior part of the temporalis is involved. The flap should be retracted as anteriorly as possible and secured with fishhooks so that the zygomatic process of the frontal bone (the “keyhole” area) is exposed.
Burr Hole, Craniotomy, and Bone Flap Elevation
A single burr hole is placed at the posterior portion of the exposure ( Fig. 1C ). This technique has been advocated by Hernesniemi et al given its efficiency and efficacy. 1 A craniotomy flap is then fashioned using a B1 bit and footplate attachment, followed by the extensive drilling of the sphenoid wing with an M8 or 4-mm diamond burr. The lesser sphenoid is drilled until the orbital roof has been flattened down to the inner cortical bone, and the meningo-orbital band has been exposed, which provides maximal exposure at depth and represents the most critical step in ensuring successful application of the LSO approach ( Fig. 1D ). Where indicated, an extradural anterior clinoidectomy can be readily added to the approach at this phase by continuing the dissection along the lesser sphenoid beyond the meningo-orbital band to expose and drill the optic canal roof.
Dural Opening and Intracranial Exposure
The dura is opened in a C-shaped fashion centered on the Sylvian fissure and pedicled anteriorly, exposing the inferior frontal and superior temporal lobes ( Fig. 2A ). A wide proximal Sylvian fissure split is completed, providing access to the MCA and insula ( Fig. 2B ). Taking a subfrontal trajectory, the ipsilateral ICA, optic nerve, and anterior clinoid process are exposed and provide essential landmarks for intracranial navigation ( Fig. 2C ). If the dissection is carried across the midline in the subfrontal plane, the contralateral optic nerve and anterior cerebral artery (ACA, A1 segment) are readily exposed ( Fig. 2D ); however, given the relatively narrow LSO corridor, access to superiorly or posteriorly projecting ACoA aneurysms within the interhemispheric fissure may be challenging, and gyrus rectus resection is recommended ( Fig. 2E ). The ICA and MCA bifurcation can be fully exposed and accessed ( Fig. 2F ). The pituitary stalk can be accessed via the optico-carotid window ( Fig. 2G ). The ipsilateral posterior communicating artery (PCoA) and anterior choroidal artery (AChA) branches and associated perforators are visualized as branches arising from the supraclinoid ICA segment ( Fig. 2G ). The lateral extension of the dissection provides access to the carotid-oculomotor triangle, oculomotor nerve, and oculomotor-tentorial triangle, which may require additional distal Sylvian fissure dissection to minimize the need for extensive temporal lobe retraction ( Fig. 2H ). Alternative approaches such as the pretemporal-transcavernous technique may be preferred for lesions involving the cavernous sinus or access lateral to the oculomotor nerve.
Fig. 2.
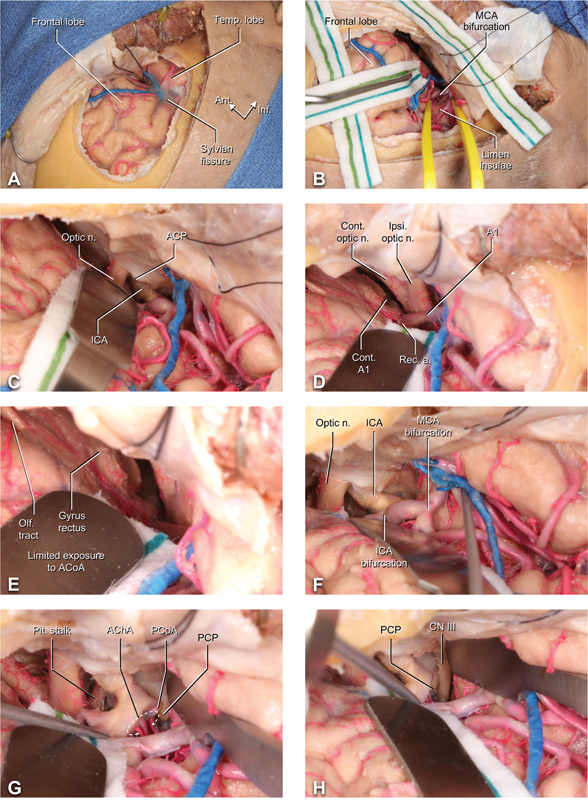
Dural opening and intradural exposure in the LSO approach. ( A ) The dura is opened in a C-shaped fashion, exposing the frontal lobe, the Sylvian fissure and the superior temporal lobe. ( B ) With proximal dissection of the Sylvian fissure, the MCA and insula are subsequently exposed. ( C ) The ICA, optic nerve, and anterior clinoid process are exposed subfrontally with minimal retraction. ( D ) The contra- and ipsilateral optic nerves and A1 segments of the ACA are further exposed; the recurrent artery of Heubner can be seen branching off A1. ( E ) The ACoA and bilateral A2 are not well visualized in this approach due to obstruction by the gyrus rectus. The ipsilateral olfactory tract can be seen on the inferior surface of the frontal lobe. ( F ) Further intrasylvian dissection reveals the ipsilateral ICA and MCA bifurcation. ( G ) The pituitary stalk can be accessed via the window between the ipsilateral optic nerve and ICA. The PCoA and AChA perforators are seen branching off the supraclinoidal ICA. ( H ) Further lateral dissection with temporal lobe retraction can expose the oculomotor nerve. Abbreviations: ACA, anterior cerebral artery; ACoA, anterior communicating artery; AChA, anterior choroidal artery; ACP, anterior clinoid process; A1, A1 segment of the anterior cerebral artery; A2, A2 segment of the anterior cerebral artery; CN, cranial nerve; cont., contralateral; ICA, internal carotid artery; ipsi, ipsilateral; LSO, lateral supraorbital; MCA, middle cerebral artery; olf., olfactory; optic n., optic nerve; PCoA, posterior communicating artery; PCP., posterior clinoid process; pit., pituitary; rec. a., recurrent artery (of Heubner); temp, temporal.
Illustrative Case Examples
Case One: Pituitary Macroadenoma
A 63-year-old man with a history of polysubstance abuse presented with 2 weeks of worsening visual acuity. No focal neurologic deficit was appreciated on exam. MRI identified a very large contrast-enhancing sellar and suprasellar mass with extension into and through the right cavernous sinus, as well as the third ventricle, consistent with pituitary macroadenoma ( Fig. 3A–C ). CT angiography demonstrated complete tumor encasement of the right cavernous ICA and bilateral ACA. The patient underwent a left-sided LSO craniotomy for resection of the mass. To optimize inferior-superior angulation, seller access, and optic nerve decompression, the standard LSO technique was modified slightly to include an orbital-optic osteotomy and anterior clinoidectomy.
Fig. 3.
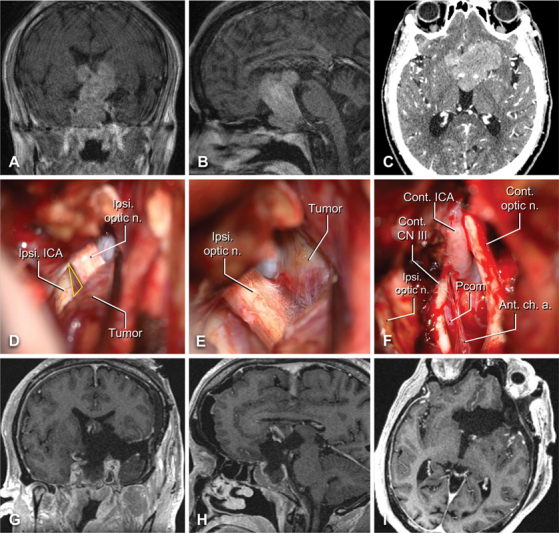
Illustrative case one – pituitary adenoma. Preoperative contrast-enhanced T1-weighted coronal ( A ) and sagittal ( B ) MRI slices are shown alongside axial CT angiogram ( C ). Intraoperative images demonstrating the optico-carotid triangle ( D ), yellow triangle), the ipsilateral optic nerve with tumor attached ( E ), and the contralateral anatomical structures after tumor removal ( F ) . ( G–I ) Comparable postoperative contrast-enhanced T1-weighted MRI demonstrating resection of the lesion with minor residual in the sphenoid sinus and right cavernous sinus. Abbreviations: AChA, anterior choroidal artery; CN, cranial nerve; cont., contralateral; ICA, internal carotid artery; ipsi, ipsilateral; optic n., optic nerve; PCoA, posterior communicating artery.
Exposure was followed by a wide Sylvian fissure split, during which cortical M4 branches of the left MCA identified and traced back to identify the ipsilateral MCA, ICA, PCoA, and posterior cerebral artery, as well as the bilateral A1 and A2 segments. The tumor was readily visualized and resected via multiple LSO-based trajectories including the optico-carotid, carotid-oculomotor, and inter-optic triangles; subfrontal and pretemporal corridors; lamina terminalis; and those basal subarachnoid planes that had been expanded by tumor growth ( Fig. 3D–E ). The tumor was predominantly soft and amenable to aspiration, facilitation extensive resected in a piecemeal fashion with careful sharp dissection technique used to remove tumor from along the critical parasellar neurovascular structures ( Fig. 3F ). Given that the superior boundary of the tumor is located relatively high, the LSO is somewhat limited here in terms of an upward angle; however, the tumor was soft enough to deliver itself throughout the debulking. Postoperative MRI confirmed a near-total resection, with a small residuum noted in the anterior-inferior aspect of the right cavernous sinus and underlying sphenoid ( Fig. 3G–I ). Final pathology confirmed gonadotroph adenoma, and the patient recovered well from surgery, with transient diabetes insipidus, stable panhypopituitarism, and a partial left oculomotor palsy that recovered in follow-up.
Case Two: Left Frontal Metastasis
A 69-year-old man with a history of stage 4 melanoma was found to have multiple hemorrhagic, enhancing lesions in the brain parenchyma, consistent with melanoma metastases ( Fig. 4A–C ), as well as an incidental left vestibular schwannoma. A left-sided LSO craniotomy was performed under general anesthesia for resection of the dominant lesion, located within the left Sylvian fissure along the inferior aspect of the frontal operculum. Following completion of a conventional LSO craniotomy and C-shaped dural exposure, the Sylvian arachnoid and venous complex presented to the center of the surgical field ( Fig. 4D ). A focused Sylvian fissure split overlying the anterior insula was performed using sharp dissection techniques, revealing the tumor presenting to the surface of the frontal operculum ( Fig. 4E ). The tumor was centrally debulked, allowing for a complete circumferential dissection of the capsule and gross total removal of the lesion ( Fig. 4F ). The patient was neurologically intact after surgery and discharged home on postoperative day 1. Postoperative MRI demonstrated gross total resection of the mass ( Fig. 4G–I ). He subsequently underwent stereotactic radiosurgery to the cavity and unresected brain metastases and has stable oncologic control as of his last postoperative follow-up at 3 months.
Fig. 4.
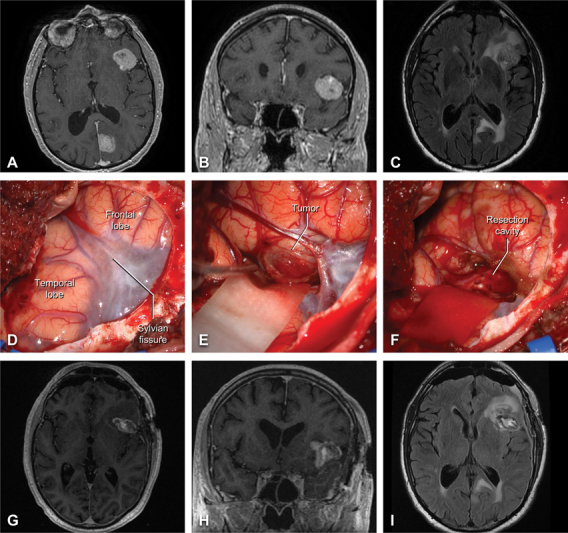
Illustrative case two – frontal melanoma metastasis. Preoperative contrast-enhanced T1-weighted axial ( A ) and coronal ( B ) MRI slices are shown alongside axial T2/FLAIR MRI ( C ). Intraoperative images demonstrating the exposure ( D ), the hemorrhagic tumor inferior to the frontal operculum ( E ), and the resection cavity after tumor removal ( F ). Comparable postoperative contrast-enhanced T1-weighted ( G–H ) and T2/FLAIR (I) MRI demonstrating gross total resection of the intrasylvian lesion.
Case Three: Awake Left Frontal Low Grade Glioma
A 31-year-old woman with several months of worsening left frontal headache and increased fatigue underwent a brain MRI that revealed a nonenhancing FLAIR-hyperintense intrinsic lesion of the left inferior frontal gyrus, consistent with low-grade glioma ( Fig. 5A–C ). She underwent an awake LSO craniotomy for resection of the tumor with intraoperative speech mapping given the eloquent location. Under moderate sedation and extensive local anesthesia, an awake left-sided LSO craniotomy was performed using the conventional technique. Following dura opening, the inferior frontal gyrus was identified, and the Sylvian fissure was focally dissected for access to the anterior insula ( Fig. 5D–E ). Intraoperative neuromonitoring techniques were used to map superficial speech areas, and an inferiorly based corticectomy was used to enter and aggressively debulk the lesion ( Fig. 5F ). At the posterior aspect of the cavity, low-threshold stimulation yielded transient speech arrest, thus tumor removal was halted along the margin. A near-total removal of the tumor was completed ( Fig. 5G–I ); the patient recovered with normal speech and was discharged home in neurologically intact condition on postoperative day 1. Pathology was consistent with World Health Organization Grade II, isocitrate dehydrogenase-mutant diffuse glioma. Protocol-based neuro-oncologic therapies were instituted, and she remains neurologically intact with stable tumor control as of the last follow-up at 3 months.
Fig. 5.
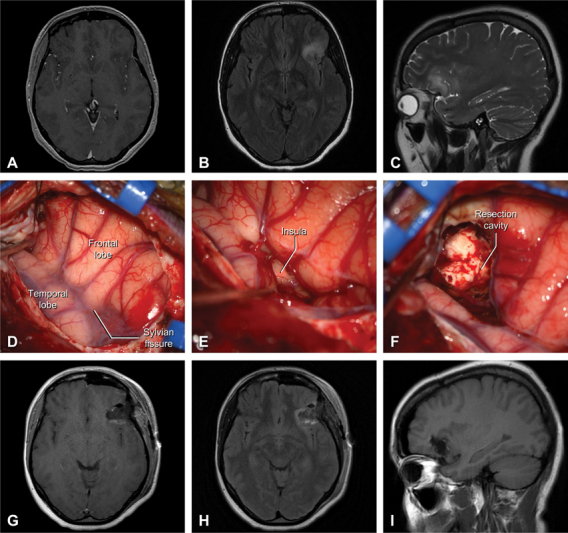
Illustrative case three – awake resection of frontoinsular low grade glioma. Preoperative contrast-enhanced T1-weighted ( A ) and T2/FLAIR ( B ) axial MRI slices are shown alongside sagittal T2-weighted MRI ( C ). Intraoperative images demonstrating the exposure ( D ), dissection of the Sylvian fissure revealing the underlying short insular gyri ( E ), and the resection cavity after tumor removal ( F ). Comparable postoperative contrast-enhanced T1-weighted axial ( G ), T2/FLAIR axial ( H ), and T1-weighted sagittal ( I ) MRI slices demonstrating gross total resection of the left frontoinsular lesion.
Discussion
Approach Overview, Indications, and Comparison to Common Alternatives
The LSO approach has been widely applied in managing a variety of pathologies in the anterior fossa, parasellar region, and Sylvian fissure. The most commonly encountered conventional approaches for these neuroanatomic locations include the standard pterional, frontotemporal, orbitozygomatic, supraorbital, their major variants such as the minipterional, and less common minimally invasive or keyhole techniques such as the eyebrow craniotomy. Although each approach has a particular range of strengths, weaknesses, and accepted indications, within this group of approaches, the LSO benefits from an efficiency-oriented posture that provides rapid and stereotyped access to the Sylvian fissure, basal cisterns, sellar and parasellar spaces, offering an appealing option for a wide range of appropriately select targets.
A significant advantage of the LSO compared with other frontotemporal craniotomy techniques is the protection of the temporal branch of the facial nerve, which courses within the superficial temporal fascia just underneath the loose alveolar layer. The LSO utilizes a single myocutaneous flap which avoids exposure of the nerve, thus minimizing the risk of injury.
In its conventional deployment, the LSO approach centers the lateral subfrontal trajectory and minimizes temporal lobe exposure without sacrificing Sylvian fissure access. By comparison, the pterional exposes most of the lateral temporal lobe and the frontotemporal and OZ approaches expose large regions of both the frontal and temporal lobes. For pathologies accessible via two central corridors, the LSO balances technical efficiency and parsimonious exposure without compromising on the dominant working trajectory. Notwithstanding, the relatively modest size of the craniotomy has two major limitations.
The first is circumstances where a lobar pathology either extends beyond the superficial exposure of the LSO craniotomy margins or would result in brain swelling that does. For example, a large temporal glioblastoma would be poorly served by the LSO and should preferentially be resected via a larger and more inferiorly biased exposure, such as a pterional or frontotemporal craniotomy. Similarly, although the LSO is very dexterous in the setting of all unruptured aneurysms at or ventral to the basilar apex and warrants consideration for many subarachnoid hemorrhage operations, those involving a very large intraparenchymal hematoma may result in excessive brain herniation into the smaller surgical field afforded by the LSO may be safer and more straightforward to manage via a larger exposure such as standard pterional craniotomy.
The second common limitation of the LSO is shared by the most of the adjacent approaches, which is diseases with marked extension in the superior-inferior plane—most commonly above the floor of the third ventricle or below the anterior aspect of the middle fossa floor. Although some cases such as the pituitary adenoma described in the current study may be amenable to an LSO-based resection due to their more favorable consistency, a tumor that has potentially the require nuanced dissection along the superior margin in the third ventricle such as a craniopharyngioma would be better suited to an OZ or interhemispheric technique. Similarly, although midposition basilar apex aneurysms can be clipped via the LSO, high-riding lesions almost universally require an OZ or transcavernous technique for adequate exposure, while lower lesions may be better managed using a subtemporal corridor, although consideration may be given to an expanded LSO that includes both an anterior and a posterior clinoidectomy. Cerebrovascular indications requiring intracranial–intracranial bypass are poorly suited to the LSO, given the need for a wide corridor that maximizes light entry and available sewing angles.
Although not decisive from a treatment planning perspective, the incision LSO also offers certain cosmetic and healing benefits. As compared with the pterional incision, the LSO is planned at a more anterior location, which ensures adequate subfrontal and Sylvian access while shortening the overall scalp flap size and decreasing the need for retraction. Similarly, the superior aspect of the temporalis muscle is managed via a single myocutaneous flap technique or simple disarticulation at the superior temporal line; when compared with an interfascial or subfascial technique more commonly employed for the pterional and OZ approaches, the muscle dissection required for LSO application is faster, less painful, and eliminates the potential for frontalis palsy due to dissection injury.
Clinical Pearls and Expanded Indications for Optimal LSO Application
Classically the LSO is considered a workhorse alternative to the pterional or minipterional for common cerebrovascular pathologies, most notably aneurysms involving the anterior circulation, as has been well described. 1 6 Aneurysms that are considered optimal for clip reconstruction via LSO include those arising from the paraclinoid or supraclinoid ICA, MCA bifurcation, PCoA, or AChA. Lesions arising from the distal ICA, MCA bifurcation, PCoA, or AChA are straightforward to access via the LSO, with proximal Sylvian fissure dissection providing the needed frontal lobe release to optimize their exposure. 11 These aneurysms are usually accessed via the carotid-oculomotor triangle; a more lateral angle of view can provide exposure to the posterior wall of the ICA, where the neck of the aneurysm is usually found. Complete visualization of the aneurysmal neck facilitates clip obliteration.
Paraclinoid ICA aneurysms including those arising from the ophthalmic or superior hypophyseal arteries rarely require open treatment in the contemporary landscape, but where indications are encountered, these lesions require additional dissection to establish proximal control. Numerous techniques have been described; we typically prefer the orbital-optic osteotomy with anterior clinoidectomy demonstrated in Case One . Large paraclinoid aneurysms represent a complex disease entity, which require a particular set of management considerations that have been well described elsewhere. 12 In medially directed paraclinoid aneurysms (e.g., superior hypophyseal artery and carotid cave aneurysms), if the size is sufficiently small, the LSO approach provides adequate access via a contralateral interoptic route; however, neck dissection should be considered to ensure proximal control. 13
ACoA represents a heterogeneous and at-times challenging collection of aneurysms, many of which are very well managed via the LSO. Notwithstanding, disease-specific features such as the lie of the neck and dome, as well as the configuration of the recurrent arteries of Huebner and other major perforators, may influence approach decision-making. Given the narrow LSO corridor and the relationship between the aneurysm dome and the surrounding structures, some amount of gyrus rectus resection is almost universally required for a complete exposure and distal control at the bilateral A2 segments—a fact common to most ACoA approaches. High-riding ACoA aneurysms may also benefit from an orbitotomy to maximize superior angulation; large, complex, or ruptured lesions may warrant consideration for a larger exposure.
Given the extensive preceding clinical literature regarding LSO applications for cerebrovascular indications, we emphasized in the current study several diverse examples of nonvascular pathologies for which the LSO represents a compelling treatment strategy. These included a complex sellar and parasellar pathology, as well as two different intra-axial diseases that were optimal for a Sylvian-based resection. Still, other relevant indications include dural-based mass lesions in the anterior fossa and the ventral aspect of the middle fossa, lesions extending anterolaterally from the cavernous sinus, and orbital diseases with superior or lateral extension into the intracranial compartment. Modular inclusion of additional bony exposure at the posterolateral orbit, anterior clinoid, or elsewhere may profoundly enhance the LSO for certain disease entities. Combining the LSO with an orbitotomy in a manner similar to the modified orbitozygomatic approach will allow access to subfrontal pathologies such as planum or tuberculum meningioma in addition to ACoA aneurysms. Lesions requiring more extensive access to the cavernous sinus wall or middle fossa floor are suboptimal for an LSO-based approach; however, as with all skull base operations, tailoring the exposure to the individual patient and disease features is critical.
Conclusion
The LSO approach is an efficient, versatile tool that provides deep exposure, marked maneuverability, and minimal approach-related morbidity, rendering it an excellent technique across a wide range of appropriately selected patients. We report a novel, cadaver-based, step-by-step dissection guide targeting neurosurgical trainees to learn the principles and pearls of this approach in laboratory and OR settings. We also highlight three case examples demonstrating the broad applications of the LSO beyond anterior circulation aneurysms. Finally, through these case discussions and a detailed presentation of the major approach modifications, we emphasize avenues for adapting the LSO to the demands of particular disease subtypes, for example, by including an orbital-optic osteotomy and anterior clinoidectomy. Although the LSO does not replace the pterional, frontotemporal, or OZ craniotomies, it offers a compelling alternative for numerous routine and complex cases. Given the favorable incision size, muscle dissection, and overall case efficiency, it warrants incorporation into neurosurgery training and skull base laboratory curricula, as well as clinical consideration across a range of indications.
Acknowledgment
We honor the memory of Dr. Juha Hernesniemi (1947–2023), who refined and popularized the lateral supraorbital craniotomy as the cornerstone of an elegant approach to neurosurgery that he characterized as “Simple, clean, while preserving normal anatomy. Clean is fast and effective. Surgery is art—you should be one of the artists.”
Footnotes
Conflict of Interest None declared.
References
- 1.Hernesniemi J, Ishii K, Niemelä Met al. Lateral supraorbital approach as an alternative to the classical pterional approach Acta Neurochir Suppl (Wien) 200594(Supplements.):17–21. [DOI] [PubMed] [Google Scholar]
- 2.Yasargil M G, Fox J L. The microsurgical approach to intracranial aneurysms. Surg Neurol. 1975;3(01):7–14. [PubMed] [Google Scholar]
- 3.Cha K C, Hong S C, Kim J S. Comparison between lateral supraorbital approach and pterional approach in the surgical treatment of unruptured intracranial aneurysms. J Korean Neurosurg Soc. 2012;51(06):334–337. doi: 10.3340/jkns.2012.51.6.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.La Rocca G, Della Pepa G M, Sturiale C L et al. Lateral supraorbital versus pterional approach: analysis of surgical, functional, and patient-oriented outcomes. World Neurosurg. 2018;119:e192–e199. doi: 10.1016/j.wneu.2018.07.091. [DOI] [PubMed] [Google Scholar]
- 5.Park H H, Sung K S, Moon J H et al. Lateral supraorbital versus pterional approach for parachiasmal meningiomas: surgical indications and esthetic benefits. Neurosurg Rev. 2020;43(01):313–322. doi: 10.1007/s10143-019-01147-8. [DOI] [PubMed] [Google Scholar]
- 6.Raygor K P, Garcia J, Rutledge C, Tonetti D A, Raper D MS, Abla A A. The lateral supraorbital craniotomy approach for anterior circulation aneurysms: a modern surgical case series in the endovascular era. World Neurosurg. 2022;166:e799–e807. doi: 10.1016/j.wneu.2022.07.107. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Al-Shaar H, Patel K P, Mallela A N, Sekula R F., JrLateral supraorbital approach for resection of large and giant olfactory groove meningiomas: a single center experienceBr J Neurosurg2022 [DOI] [PubMed]
- 8.Choque-Velasquez J, Hernesniemi J. One burr-hole craniotomy: Lateral supraorbital approach in Helsinki Neurosurgery. Surg Neurol Int. 2018;9:156. doi: 10.4103/sni.sni_185_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernesniemi J, Andrade-Barazarte H, Duarte R, Serrone J, Hijazy F. 2019. Lateral supraorbital approach. [Google Scholar]
- 10.Chaddad-Neto F, Doria-Netto H L, Campos-Filho J M, Ribas E SC, Ribas G C, Oliveira Ed. Head positioning for anterior circulation aneurysms microsurgery. Arq Neuropsiquiatr. 2014;72(11):832–840. doi: 10.1590/0004-282x20140156. [DOI] [PubMed] [Google Scholar]
- 11.Lawton M T. Thieme; 2011. Seven Aneurysms: Tenets and Techniques for Clipping. [DOI] [PubMed] [Google Scholar]
- 12.Krisht A F, Hsu S PC. Paraclinoid aneurysms: part 1: superior (true ophthalmic) aneurysms. Contemp Neurosurg. 2008;30(15):1. [Google Scholar]
- 13.Zhao X, Tayebi Meybodi A, Labib M A et al. Contralateral interoptic approach to paraclinoid aneurysms: a patient-selection algorithm based on anatomical investigation and clinical validation. J Neurosurg. 2020;134(06):1852–1860. doi: 10.3171/2020.3.JNS193205. [DOI] [PubMed] [Google Scholar]


