Abstract
Objective The aim of this study was to investigate the safety of induction chemotherapy (IC) for patients with sinonasal malignancies with brain invasion or a neurological deficit.
Methods We conducted a retrospective analysis of patients who underwent IC for sinonasal malignancies with intracranial invasion or a neurological deficit at a single tertiary cancer center from 1992 to 2020.
Results In total, 460 patients with sinonasal malignancies were included in the study. Of the patients reviewed, 341 underwent IC and within this group 40 had brain invasion (BI) and 31 had a neurological deficit (ND) at presentation. The most prevalent malignancy was sinonasal undifferentiated carcinoma (BI 40%, ND 41.9%), followed by esthesioneuroblastoma (BI 27.5%, ND 9.7%). All tumors were stage T4 with the majority lacking nodal metastases (BI N0: 72.5%, ND N0: 77.5%). All patients completed at least two cycles of IC. Partial or complete response to IC was seen in 80% of BI and 71% of ND patients. No patients had cessation of treatment due to neurologic decline and none required urgent surgery. Five patients (12.5%) with BI and 2 (6.5%) with ND had interruption of IC for reasons other than neurological decline. In patients with ND, IC led to improvement of 54.5% NDs.
Conclusion In patients with sinonasal malignancies with BI or ND who underwent IC, no patients had cessation of treatment due to neurologic decline. In contrast, most patients had improvement of neurologic symptoms with IC. IC was safely administered without interruption due to neurological decline or symptom progression.
Keywords: induction chemotherapy, sinonasal, esthesioneuroblastoma, squamous cell carcinoma, sinonasal undifferentiated carcinoma, brain invasion, neurological deficits
Introduction
Sinonasal malignancies represent a histologically diverse group of pathologies with varying risks of local recurrence, regional spread, systemic metastases, and overall disease-related mortality. Despite the heterogenous diagnoses within this disease category, local invasion beyond the paranasal sinuses into the orbit and intracranial compartment is common. 1 2 3 4 While surgery plays an important role in achieving local control, histology-specific multimodal treatment incorporating systemic therapy and radiation therapy is associated with improved long-term outcomes. 5 6 7 8 9 10 11
For biologically aggressive malignancies that are either locally invasive or at high risk for metastatic spread, induction chemotherapy (IC) has emerged as an integral part of the multimodal treatment strategy. Examples of malignancies where neoadjuvant therapy is often considered includes high-grade neuroendocrine carcinomas, squamous cell carcinoma, sinonasal undifferentiated carcinomas (SNUCs), and higher grade esthesioneuroblastomas. 6 8 12 13 14 15 16 The use of IC for response-adjusted local therapy has been associated with improved survival, higher rate of organ preservation, and negative surgical margins. 6 12 13 17 18
While IC plays an important role in the histology-specific treatment of patients with sinonasal malignancies, the treatment of patients who present with neurological deficits or brain invasion (BI) remains a challenge. 19 20 21 The specific questions in this patient population include whether IC exacerbates neurological decline, whether its use delays surgical resection leading to neurological decline, or whether up-front surgical resection is necessary to alleviate tumor mass effect. The aim of this study was to determine the neurological safety and efficacy of IC in patients with sinonasal malignancies who present with BI or neurological deficits.
Methods
This was an institutional review board-approved study for the retrospective analysis of patients with sinonasal pathologies treated at the University of Texas MD Anderson Cancer Center (protocol RCR04–0636). The database was queried for all patients with biopsy-proven sinonasal malignancies treated between 1992 and 2020. Our analytic cohort was composed of patients with BI or neurologic deficits (NDs), who were stratified by whether they received IC. The presence of BI with or without mass effect was identified based on radiologic screening with magnetic resonance imaging. Presentation with ND was identified based on documented neurologic exams prior to IC. All neurological deficits as well as symptoms attributed to frontal lobe or brain dysfunction were recorded. However, for the purpose of this study, subjective diplopia (without neuropathy), anosmia, and trigeminal dysfunction were not included under clinically relevant neurological deficits. For all patients, treatment was offered in a histology-specific manner at the discretion of the treating providers after multidisciplinary review; the treatment protocols used have been previously reported. 6 To account for potential selection bias in this study, during the same time period of this study, patients with similar histologic diagnoses who underwent upfront surgical resection without IC were identified. The data from this patient population were analyzed and characterized in the same manner.
A review of patients' charts was performed for key demographics. Pathology was reviewed and verified, along with tumor staging as outlined by the American Joint Committee of Cancer (AJCC) Staging Manual, seventh edition. 22 Anatomic invasion was further divided into violation of skull base bone, orbit, extension to the dura, subdural space, BI, and BI with radiographic evidence of mass effect. A note was made of previous treatments, including radiation, chemotherapy, or surgery. For the cohort with NDs prior to IC, neurological deficits were classified as “frontal” denoting symptoms of personality changes, affect alterations, or seizures. If neurologic defects were due to an optic neuropathy, they were categorized in the “optic” group.
Details regarding IC (agents used, number of cycles, grade 3 and 4 hematologic and nonhematologic adverse events, and response to treatment) were recorded. 23 Based on Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria, radiographic response to IC was scored in four classifications: complete response, partial response, stable disease, and progressive disease. 13 24 Information regarding management of disease post-IC was collected. For patients where IC was interrupted, the rationale for treatment interruption was noted. The reasoning for and approach used during surgery was noted.
The primary outcome assessed for each cohort was interruption of IC secondary to neurologic decline, with or without progression of intracranial disease. We used descriptive statistics, and presented findings as a mean ± standard deviation (SD), for patient demographic data, tumor characteristics, histologic features, and treatment-related variables and outcomes. Statistical analysis was performed utilizing two-tailed t -test, significance set at 0.05 α (Microsoft Excel, Microsoft Corporation v16.65, 2019).
Results
Patient Demographics and Tumor Characteristics
A total of 460 patients were reviewed ( Fig. 1 ). Of the 341 patients undergoing IC, 40 patients were found to have BI and 31 with a ND at presentation, prior to IC. Within this cohort of patients, seven experienced both BI and ND. Of the 119 patients who underwent upfront surgery without IC, 16 patients had BI and mass effect, 8 patients with a ND, and a total of 3 patients presenting with both.
Fig. 1.
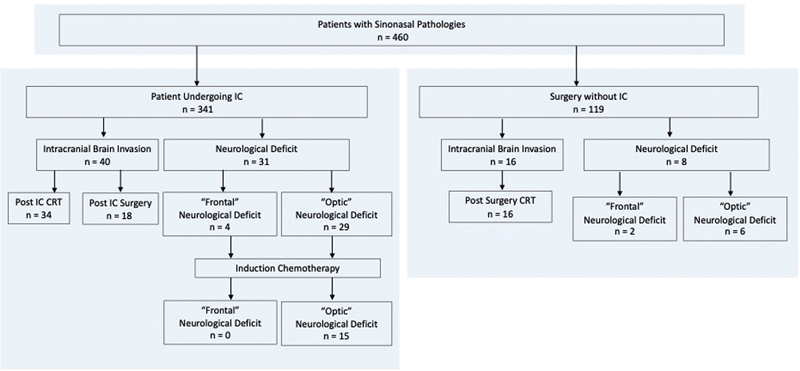
Flow chart of the study population.
Induction Chemotherapy Cohort
The demographics for all patients with BI or ND are shown in Table 1 . In the IC cohort with BI, the most prevalent histologic diagnoses were SNUC in 16 patients (40%), followed by esthesioneuroblastoma (Hyams 2–4) in 11 patients (27.5%), squamous cell carcinoma in 3 patients (7.5%), poorly differentiated carcinoma in 1 patient (2.5%), and endodermal sinus tumor in 1 patient (2.5%). All tumors were stage T4, a majority of which presented without nodal metastasis (N0 72.5%). Mass effect was found in 24 patients (60.0%). In the cohort with ND, the most prevalent pathology was SNUC in 12 patients (38.7%), followed by squamous cell carcinoma in 8 patients (25.8%), neuroendocrine carcinoma in 6 patients (19.4%), esthesioneuroblastoma (Hyams 2–4) in 3 patients (9.7%), and poorly differentiated carcinoma in 2 patients (6.5%). All tumors were stage T4 without nodal metastases in 24 patients (N0 77.4%). All tumors invaded the bony skull base and 26/31 patients (83.9%) showed orbital invasion. BI with mass effect was noted in seven patients (22.6%). Patients had previous treatment in 12.5% BI and 6.5% of the ND cohort.
Table 1. Patient demographics and tumor characteristics.
| Induction chemotherapy | Upfront surgery | |||
|---|---|---|---|---|
| Brain invasion cohort | Induction cohort with neurological deficit | Brain invasion cohort | Neurologic deficit cohort | |
| Total patients | 40 | 31 | 16 | 8 |
| Sex | 26 (65%) male | 18 (58.0%) male | 11 (68.8%) male | 5 (62.5%) male |
| 14 (35%) female | 13 (42.0%) female | 5 (31.2%) female | 3 (37.5%) female | |
| Age | 48.6, SD: 17.0 y | 55.1, SD: 12.8 y | 59.2, SD: 11.4 y | 69.1, SD: 14.0 y |
| Pathology | ||||
| Sinonasal undifferentiated carcinoma (SNUC) | 16 (40%) | 12 (38.7%) | 1 (6.25%) | – |
| Esthesioneuroblastoma | 11 (27.5%) | 3 (9.7%) | 11 (68.8%) | 2 (25.0%) |
| Squamous cell carcinoma | 3 (7.5%) | 8 (25.8%) | 1 (6.25%) | 2 (25.0%) |
| Neuroendocrine carcinoma | – | 6 (19.4%) | – | |
| Adenocarcinoma | – | – | 3 (18.8%) | 3 (37.5%) |
| Poorly differentiated carcinoma | 1 (2.5%) | 2 (6.5%) | – | – |
| Endodermal sinus tumor | 1 (2.5%) | – | – | – |
| Mucoepidermoid carcinoma | – | – | – | 1 (12.5%) |
| Stage | ||||
| T4 | 40 (100%) | 31 (100%) | 16 (100%) | 8 (100%) |
| N0 | 29 (72.5%) | 24 (77.4%) | 16 (100%) | 8 (100%) |
| N1–3 | 11 (27.5%) | 7 (22.6%%) | – | – |
| Tumor origin | ||||
| Nasal | 17 (42.5%) | 18 (58.0%) | 4 (25.0%) | 2 (25.0%) |
| Maxillary | 2 (5%) | 4 (12.9%) | – | 1 (12.5%) |
| Ethmoid | 20 (50%) | 6 (19.4%) | 12 (75%) | 5 (62.5%) |
| Sphenoid | – | 1 (3.2%) | – | – |
| Frontal | – | 2 (6.5%) | – | – |
| Tumor extension | ||||
| Bone | 40 (100%) | 31 (100%) | 16 (100%) | 8 (100%) |
| Orbital | 33 (82.5%) | 26 (83.9%) | 8 (50.0%) | 5 (62.5%) |
| Dural | 40 (100%) | 25 (80.6%) | 16 (100%) | 5 (62.5%) |
| Subdural | 40 (100%) | 15 (48.4%) | 16 (100%) | 4 (50.0%) |
| Brain invasion | 40 (100%) | 7 (22.6%) | 16 (100%) | 3 (37.5%) |
| Brain invasion w/ mass effect | 24 (60%) | 7 (22.6%) | 4 (25.0%) | 1 (12.5%) |
| Previous treatment | ||||
| Initial treatment | 35 (87.5%) | 29 (93.5%) | 12 (75%) | 6 (75.0%) |
| Previous treatment | 5 (12.5%) | 2 (6.5%) | 4 (25%) | 2 (25.0%) |
Abbreviation: SD, standard deviation.
Up-front Surgery Cohort
Of the 16 patients who underwent upfront surgery, the most common pathology in the BI group was esthesioneuroblastoma (Hyams 1–3) in 11 patients (68.8%) and the most common pathology in the ND group was adenocarcinoma in 3 patients (37.3%). Within the cohort of eight patients who presented with a ND, there was a nearly equal distribution of pathologies as outlined in Table 1 . Among patients with ND, 6 patients had an “optic” ND, while 2 had “frontal” ND, including personality deficit/confusion in 1 patient and seizures in 1 patient. In both groups, all tumors were stage T4 without nodal metastases. Mass effect was seen in 2/16 patients (12.5%) of the BI group and 1/8 patients (12.5%) of the ND group. A quarter of patients who underwent upfront surgery had previous treatment.
Induction Chemotherapy Details
Consistent with clinical practice, the majority of patients were treated with platinum-based IC. Platinum plus etoposide was used in 23/40 patients (57.5%) and 15/31 patients (48.4%) of the BI and ND groups, respectively ( Table 2 ). The average number of treatment cycles was 3.2 (SD: 1.4) and 3.1 (SD: 0.9) in the BI and ND groups, respectively. Complete response to IC was observed in 5 (12.5%) BI and 3 (9.7%) ND patients. The majority of patients achieved a partial response to IC in both groups (67.5% BI, 61.3% ND), followed by patients who achieved stable disease (17.5% BI and 19.4% ND). Representative images of tumor response to chemotherapy of patients in the study are shown in Fig. 2 . IC was interrupted in 5/40 BI patients (12.5%) and 2/31 ND patients (6.5%), but all patients were able to complete at least two cycles. The cause for interruption of IC was adverse effects of chemotherapy in six patients, and one patient with BI elected to switch her care to Chinese herbal therapy. There were no instances of neurologic decline that merited interruption of IC in any patient in either group. Hematologic adverse events were experienced in 9/40 BI patients (22.5%) and 4/31 ND patients (12.9%), while nonhematologic symptoms were seen in 12/40 BI patients (30.0%) and 7/31 ND patients (22.9%).
Table 2. Induction chemotherapy in patients with sinonasal malignancies with brain invasion and neurological deficits (5-FU: - 5-fluorouracil; IC: induction chemotherapy).
| Induction cohort with brain invasion | Induction cohort with neurological deficits | |
|---|---|---|
| Induction chemotherapy agents | ||
| Platinum + t axane | 6 (15%) | 4 (12.9%) |
| Platinum + 5-FU | 1 (2.5%) | 3 (9.7%) |
| Platinum + t axane + 5-FU | 3 (7.5%) | 2 (6.5%) |
| Platinum + t axane + ifosfamide | 3 (7.5%) | 4 (12.9%) |
| Platinum + e toposide | 23 (57.5%) | 15 (48.4%) |
| Platinum + e toposide + ifosfamide | 1 (2.5%) | 1 (3.2%) |
| Platinum + e toposide + vincristine | 1 (2.5%) | – |
| Adriamycin + ifosfamide + vincristine | 1 (2.5%) | – |
| Irinotecan | 1 (2.5%) | |
| Platinum + t axane + cetuximab | – | 1 (3.2%) |
| Platinum + t axane + gemcitabine | – | 1 (3.2%) |
| Cycles of IC | Avg: 3.2 (SD: 1.4), median: 3 | Avg: 3.1 (SD: 0.9), median: 3 |
| Radiographic response to induction chemotherapy | ||
| Complete r esponse | 5 (12.5%) | 3 (9.7%) |
| Partial r esponse | 27 (67.5%) | 19 (61.3%) |
| Stable d isease | 7 (17.5%) | 6 (19.4%) |
| Disease p rogression | 1 (2.5%) | 3 (9.7%) |
| Interruption in i nduction c hemotherapy | 5 (12.5%) | 2 (6.5%) |
| Adverse events to systemic therapy | ||
| Hematologic | 9 (22.5%) | 4 (12.9%) |
| Nonhematologic | 12 (30%) | 7 (22.6%) |
Abbreviation: SD, standard deviation.
Fig. 2.
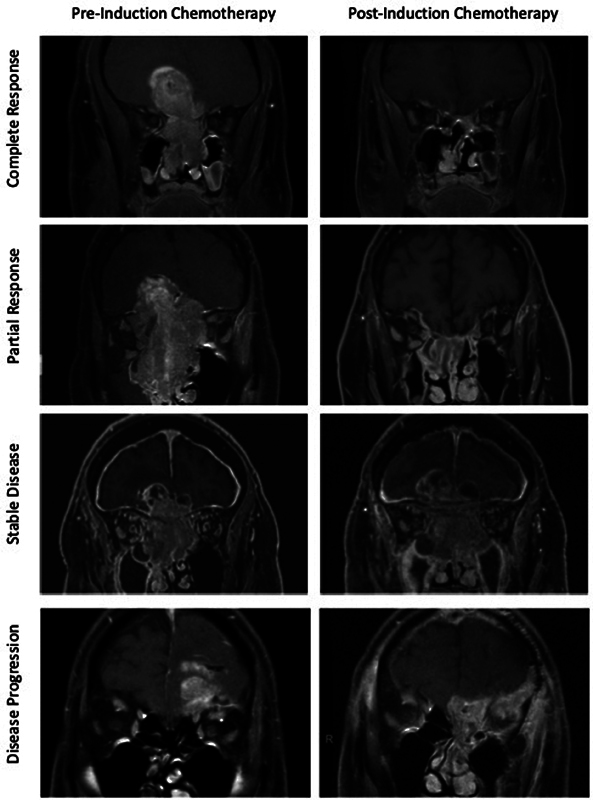
Examples of sinonasal malignancies and their radiographic response to induction chemotherapy.
Cohort Undergoing Induction Chemotherapy: Outcomes and Post-induction Chemotherapy Treatment
Consolidative radiation with or without concurrent chemotherapy was pursued post-induction in 34/40 patients (85.0%) with BI and 28/31 patients (90.3%) with ND ( Table 3 ). In the BI group, this was simultaneous chemotherapy and radiation (CRT) in 28/40 patients (70.0%). In total, 31 patients (77.5%) underwent chemotherapy and 30 patients (75%) underwent radiation, with an average dose of 66.3 Gy. Surgery was undertaken in 18/40 BI patients (45.0%) who underwent IC. The most common indication for surgery was residual disease after IC in 10/18 patients (55.5%), followed by poor or no response to IC in 5/18 patients (27.8%) and, lastly, recurrent disease after definitive CRT in 3/18 patients (16.7%). Gross total resection was achieved in 16/18 patients (88.9%), with negative margins in 13/18 patients (72.2%). Within this cohort with BI, the rate of disease recurrence was 16/40 patients (40%). The median progression-free survival was 24.8 months, and the overall survival was 55.7 months.
Table 3. Post-induction chemotherapy management and outcomes in patients with brain invasion and neurological deficit.
| Brain invasion cohort | Neurologic deficit cohort | |
|---|---|---|
| Post-induction chemotherapy treatment | 34/40 (85%) | 28/31 (90.3%) |
| Simultaneous CRT | 28 (70.0%) | 21/31 (67.7%) |
| Chemotherapy | 31 (77.5%) | 23/31 (74.2%) |
| Radiation | 30 (75.0%) (Avg: 66.3 Gy) | 25/31 (80.6%) (Avg: 61.1 Gy) |
| Post-induction chemotherapy surgery | 18/40 (45.0%)_ | 11/31 (35.5%) |
| Indication | ||
| Poor or no response to IC | 5/18 (27.8%) | 7/11 (63.6%) |
| Residual disease after IC | 10/18 (55.6%) | 3/11 (27.3%) |
| Recurrent disease after IC and CRT | 3/18 (16.7%) | 1/11 (9.10%) |
| Surgical approach | ||
| Transcranial | 3/18 (16.7%) | 3/11 (27.3%) |
| Transcranial + transfacial | 4/18 (22.2%) | 2/11 (18.2%) |
| Transcranial + endoscopic | 6/18 (33.3%) | 3/11 (27.3%) |
| Endoscopic | 4/18 (22.2%) | 1/11 (9.10%) |
| transfacial | 1/18 (5.60%) | 2/11 (18.2%) |
| Extent of resection | ||
| Gross total resection | 16/18 (88.9%) | 10/11 (90.9%) |
| Negative margins | 13/18 (72.2%) | 9/11 (81.8%) |
| Long-term disease control outcomes | ||
| Recurrence | 16/40 (40.0%) | 16/31 (51.6%) |
| PFS (mo) | 24.8 (SD: 27.4) | 25.9 (SD: 32.9) |
| OS (mo) | 55.7 (SD: 47.3) | 34.9 (SD: 38.2) |
Abbreviations: CRT, chemoradiation therapy; IC, induction chemotherapy; PFS, progression-free survival; OS, overall survival; SD, standard deviation.
Of the patients who presented with ND, 21/31 (67.7%) underwent CRT. Again, the most common reason for surgery, which was performed in 11/31 patients (35.5%), was poor or no response to IC, or residual disease after treatment. The recurrence rate in this group was 51.6%, with median progression-free and overall survival of 25.9 and 39.4 months, respectively.
Cohort Undergoing Induction Chemotherapy with Neurologic Deficits: Neurologic Outcomes
For patients presenting with ND, no patients experienced neurologic decline requiring interruption of IC. Of the 31 patients that presented with a ND, 2 had both an optic and a frontal ND. In total, 33 neurological deficits were analyzed. Eighteen of the 33 (54.5%) NDs improved after IC ( p < 0.05) ( Table 4 ). Of all patients with ND, 29/33 (87.9%) patients had optic neuropathy prior to IC, compared with 15/29 (51.7%) after IC ( p < 0.05). In addition, 4/33 (12.1%) NDs with a frontal etiology resolved after IC.
Table 4. Neurological deficits and response to induction chemotherapy.
| Prior to induction chemotherapy | Post-induction chemotherapy | ||
|---|---|---|---|
| Total | 33 (100%) | 15 (45.5%) | p < 0.05 |
| Frontal ND | 4 (12.1%) | 0 | n/a |
| Optic ND | 29 (87.9%) | 15 (51.7%) | p < 0.05 |
Abbreviations: IC, induction chemotherapy; ND, neurological deficit.
Note: Of the 31 total patients that had a neurological deficit, 2 had both a frontal and optic deficits, thus a total of 33 neurological deficits included. Neurological frontal deficit (personality changes, impulsivity, seizures), optic neuropathy, or CN III, IV, VI deficit.
Cohort with Upfront Surgery: Outcomes and Postsurgical Treatment
Within the BI group, gross total resection was achieved in 11/16 patients (68.7%), with negative margins in 7/16 patients (43.7%). A transcranial surgical approach was used in 56.3% of patients and a combined transcranial, endoscopic, endonasal approach was used in 31.3%. The recurrence rate was 56.3%, with a median progression-free survival of 39.1 months and an overall survival of 84.9 months. The majority of these patients (69.7%) went on to undergo radiation, with 25% treated with CRT. In the ND group, the predominant surgical approach was a combined transcranial and transfacial approach (50.0%), followed by a transcranial approach alone (37.5%). A gross total resection was achieved in 6/8 patients (75%) with an equal number achieving negative margins. In this cohort, the recurrence rate was 62.5%, with a median progression-free survival of 34.2 months and overall survival of 87.1 months ( Table 5 ).
Table 5. Surgical approach and outcome of patients with sinonasal malignancy patients with brain invasion and neurological deficits who underwent upfront surgical resection without induction chemotherapy.
| Surgical cohort with brain invasion | Surgical cohort with neurological deficit | |
|---|---|---|
| Total patients | 16 | 8 |
| Surgical approach | ||
| Anterior transcranial | 9 (56.3%) | 3 (37.5%) |
| Ant transcranial, transfacial | 1 (6.25% | 4 (50.0%) |
| Ant transcranial, endoscopic | 5 (31.3%) | 1 (12.5%) |
| Endoscopic | 1 (6.25%) | – |
| Extent of resection | ||
| GTR | 11 (68.7%) | 6 (75.0%) |
| Negative margins | 7 (43.7%) | 6 (75.0%) |
| Post-op adjuvant treatment | ||
| Chemotherapy | 5 (31.3%) | 1 (12.5%) |
| Radiation | 11 (68.7%) | 3 (37.5%) |
| Chemotherapy + radiation | 4 (25.0%) | 0 |
| Long-term disease control outcomes | ||
| Recurrence rate | 9 (56.3%) | 5 (62.5%) |
| PFS (mo) | 39.1, SD 35.7 mo | 34.2, SD: 71.6 mo |
| OS (mo) | 84.9, SD 69.5 mo | 87.1, SD: 115 mo |
Abbreviations: GTR, gross total resection; OS, overall survival; PFS, progression-free survival; SD, standard deviation.
Discussion
Sinonasal malignancies present a challenge to clinicians due to their often-advanced stage at disease presentation. Further, their advanced stage not only denotes histopathological aggressiveness, but also a diffusely invasive tumor, extending beyond the nasal and paranasal sinuses, into the orbit and intracranial compartments. 1 2 25 26 Additionally, particular diagnoses carry elevated risks of regional and metastatic spread. A growing body of literature supports the role of IC for select diagnoses as an initial treatment as part of a larger multimodal strategy. 6 12 13 The current study demonstrates that upfront surgical resection may not be required for patients presenting with significant intracranial invasion with mass effect or a neurological deficit at presentation. Alternatively, IC is safe from a neurologic standpoint.
In a prior study, 123 patients with biopsy-proven squamous cell carcinomas who were treated with curative intent, response to neoadjuvant chemotherapy was associated with better outcomes and organ preservation. 12 Among the patients who showed at least a partial radiographic response to IC (56.9%), overall survival at 2 years was 68.2% compared with 33.3% in nonresponders. This study further provided support that IC could be utilized as an organ-sparing strategy, as a higher rate of orbital preservation was also observed. Response to neoadjuvant systemic therapy provides insight into the chemosensitivity of the tumor, and thus can aid in post-IC treatment planning. A prior study by Amit et al retrospectively reviewed data from patients diagnosed with SNUC who were treated with IC and provides further support for the use of IC; they found that a favorable response to IC was associated with better disease-specific and overall survival. A poor response to IC in this study, indicating a chemoresistant tumor, had a more surgically aggressive post-induction treatment course. 13 The multimodality management of sinonasal malignancies also includes that of esthesioneuroblastomas. While most authors reserve neoadjuvant chemotherapy for higher grade Hyams 2/3 or Kadish C tumors, a benefit has been shown in this patient population without an increase in complication rates. 16 27
The relative safety and efficacy of IC in patients with advanced local disease at presentation with either ND or BI has not previously been established. In our study, we screened 460 patients in a prospectively maintained database of sinonasal malignancies to answer this question. The overall incidence of BI was 40/341 (11.7%), while the incidence of a ND at presentation was 31/341 (9.1%). To our knowledge, this is the first study to quantify these two important patient subsets within the sinonasal malignancy patient population. What remains unanswered in the literature, and a question that often arises within the treatment team, is that if there is a ND, or BI at presentation, should up-front surgical resection be pursued to address compressive etiologies causing the symptoms.
Our results show that our patient population matches well with previously published cohorts of patients with advanced locoregional sinonasal malignancies. 3 While this study was not designed to assess a specific pathology, SNUC, esthesioneuroblastoma, and squamous cell carcinoma together made up 75 and 77.4% of the BI and ND groups, respectively. All patients had stage T4 tumors, and a majority did not present with nodal metastases. Further, we extended our inclusion criteria for BI beyond simple dural, or extradural involvement to include intraparenchymal invasion, because in our opinion, this is most clinically significant. The neurological deficits included within the study were mainly composed of optic nerve neuropathies, and to a lesser extent, seizures and frontal lobe dysfunction. A platinum-based IC was utilized in the majority of patients in our study. All patients underwent at least two cycles of chemotherapy which is in keeping with the current standards of IC at our institution. The majority of patients showed a complete or partial response to IC (80% in the BI group and 71% in the ND group) on a radiographic assessment.
Within the group of patients with ND, there were 33 total deficits in 31 patients. Two patients had both what we quantified as an “optic” and a “frontal” deficit. All of the frontal symptoms resolved after IC, prior to the initiation of further definitive treatment. Optic neuropathies improved in 14 patients, a 48.3% improvement ( p < 0.05). Further, there were no patients in either the ND or BI cohort that experienced a neurological decline which necessitated the early termination of IC or surgical intervention to address progression of ND. Thus, our data not only support the safety of IC in patients presenting with a ND or BI, but also show a benefit in terms of improvement of neurological deficits, prior to any further definitive chemotherapy, radiation, or surgery.
During the same study period, we also report patients who underwent upfront surgical resection who had a ND or BI at presentation. Of the 119 patients who underwent direct surgical intervention, 16 presented with BI (13.4%), and 8 with a ND (6.7%). There was a higher proportion of esthesioneuroblastomas and adenocarcinomas within this patient cohort overall, which are less aggressive pathologies than squamous cell and SNUC. It is likely that the higher number of these etiologies in the surgical cohort is due to the current treatment practice that define sinonasal malignancies as a spectrum of pathologic aggressiveness, where Hyams 1/2 esthesioneuroblastomas and adenocarcinomas are typically considered less aggressive or less chemoresponsive and are more favorable surgical targets. 4 6 28 Further, the gross total resection rates were higher in the patients who underwent surgical resection after IC as compared with upfront surgical resection, (88.9 vs. 68.7%, respectively), highlighting another potential benefit of neoadjuvant chemotherapy in sinonasal malignancies.
Limitations of our study include those inherent in a retrospective study design. This is not a pathology-specific analysis due to the relative rarity of the pathologies discussed, thus our results are represented in aggregate. Given this heterogeneity, we did not feel it was appropriate for a direct comparison of up-front surgery versus IC, as the distribution of pathologies was also different between these groups. However, the strength of our work is that it provides important information for clinicians and surgeons faced with patients presenting with BI or ND. The study supports the continued use and safety of IC in patients with select sinonasal malignancies with BI or ND without the need for urgent upfront surgical resection and allows patients to benefit from neoadjuvant treatment.
Conclusion
Sinonasal malignancies represent an aggressive and clinically challenging problem for multidisciplinary treatment teams. In recent years, retrospective studies suggest IC is associated with an increased disease-free and overall survival. Our study further delves into the safety and efficacy of sinonasal malignancies in patients who present with BI or ND. We conclude that undergoing IC in this patient population is both safe and efficacious. This may allow patients to obtain the previously published benefits of neoadjuvant systemic therapy toward improved clinical outcomes.
Acknowledgment
The authors are in gratitude to Preeti Ramadoss for her careful review and editing of this manuscript.
Conflict of Interest None declared.
Previous Presentation
Podium presentation at the North American Skull Base Society Annual Meeting, Tampa Florida, United States, February 17, 2023.
References
- 1.Levine P A, Frierson H F, Jr, Stewart F M, Mills S E, Fechner R E, Cantrell R W.Sinonasal undifferentiated carcinoma: a distinctive and highly aggressive neoplasm Laryngoscope 198797(8, Pt 1):905–908. [PubMed] [Google Scholar]
- 2.Dutta R, Dubal P M, Svider P F, Liu J K, Baredes S, Eloy J A. Sinonasal malignancies: a population-based analysis of site-specific incidence and survival. Laryngoscope. 2015;125(11):2491–2497. doi: 10.1002/lary.25465. [DOI] [PubMed] [Google Scholar]
- 3.Turner J H, Reh D D. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34(06):877–885. doi: 10.1002/hed.21830. [DOI] [PubMed] [Google Scholar]
- 4.König M, Osnes T, Bratland Å, Jebsen P, Meling T R. Treatment of sinonasal adenocarcinoma: a population-based prospective cohort study. J Neurol Surg B Skull Base. 2020;81(06):627–637. doi: 10.1055/s-0039-1694050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Mamgani A, van Rooij P, Mehilal R, Tans L, Levendag P C. Combined-modality treatment improved outcome in sinonasal undifferentiated carcinoma: single-institutional experience of 21 patients and review of the literature. Eur Arch Otorhinolaryngol. 2013;270(01):293–299. doi: 10.1007/s00405-012-2008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta G U, Raza S M, Su S Y, Hanna E Y, DeMonte F. Management of olfactory neuroblastoma, neuroendocrine carcinoma, and sinonasal undifferentiated carcinoma involving the skullbase. J Neurooncol. 2020;150(03):367–375. doi: 10.1007/s11060-020-03537-1. [DOI] [PubMed] [Google Scholar]
- 7.Gamez M E, Lal D, Halyard M Y et al. Outcomes and patterns of failure for sinonasal undifferentiated carcinoma (SNUC): the Mayo Clinic experience. Head Neck. 2017;39(09):1819–1824. doi: 10.1002/hed.24834. [DOI] [PubMed] [Google Scholar]
- 8.Paré A, Blanchard P, Rosellini S et al. Outcomes of multimodal management for sinonasal squamous cell carcinoma. J Craniomaxillofac Surg. 2017;45(08):1124–1132. doi: 10.1016/j.jcms.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Chatelet F, Simon F, Bedarida V et al. Surgical management of sinonasal cancers: a comprehensive review. Cancers (Basel) 2021;13(16):3995. doi: 10.3390/cancers13163995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thawani R, Kim M S, Arastu A et al. The contemporary management of cancers of the sinonasal tract in adults. CA Cancer J Clin. 2023;73(01):72–112. doi: 10.3322/caac.21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su S Y, Bell D, Hanna E Y. Esthesioneuroblastoma, neuroendocrine carcinoma, and sinonasal undifferentiated carcinoma: differentiation in diagnosis and treatment. Int Arch Otorhinolaryngol. 2014;18 02:S149–S156. doi: 10.1055/s-0034-1390014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelmeguid A S, Teeramatwanich W, Roberts D B et al. Neoadjuvant chemotherapy for locoregionally advanced squamous cell carcinoma of the paranasal sinuses. Cancer. 2021;127(11):1788–1795. doi: 10.1002/cncr.33452. [DOI] [PubMed] [Google Scholar]
- 13.Amit M, Abdelmeguid A S, Watcherporn T et al. Induction chemotherapy response as a guide for treatment optimization in sinonasal undifferentiated carcinoma. J Clin Oncol. 2019;37(06):504–512. doi: 10.1200/JCO.18.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartel R, Gonzalez-Compta X, Cisa E et al. Importance of neoadjuvant chemotherapy in olfactory neuroblastoma treatment: series report and literature review. Acta Otorrinolaringol Esp (Engl Ed) 2018;69(04):208–213. doi: 10.1016/j.otorri.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Aljumaily R M, Nystrom J S, Wein R O. Neoadjuvant chemotherapy in the setting of locally advanced olfactory neuroblastoma with intracranial extension. Rare Tumors. 2011;3(01):e1. doi: 10.4081/rt.2011.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loy A H, Reibel J F, Read P W et al. Esthesioneuroblastoma: continued follow-up of a single institution's experience. Arch Otolaryngol Head Neck Surg. 2006;132(02):134–138. doi: 10.1001/archotol.132.2.134. [DOI] [PubMed] [Google Scholar]
- 17.Hanna E Y, Cardenas A D, DeMonte F et al. Induction chemotherapy for advanced squamous cell carcinoma of the paranasal sinuses. Arch Otolaryngol Head Neck Surg. 2011;137(01):78–81. doi: 10.1001/archoto.2010.231. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell E H, Diaz A, Yilmaz T et al. Multimodality treatment for sinonasal neuroendocrine carcinoma. Head Neck. 2012;34(10):1372–1376. doi: 10.1002/hed.21940. [DOI] [PubMed] [Google Scholar]
- 19.Yaniv D, Su S Y. Updates in management strategies of locally advanced sinonasal malignancy. Curr Opin Otolaryngol Head Neck Surg. 2023;31(01):39–44. doi: 10.1097/MOO.0000000000000866. [DOI] [PubMed] [Google Scholar]
- 20.Kong K A, Thorp B D, Sheth S H. The role of induction therapy for sinonasal cancers. Curr Treat Options Oncol. 2023;24(03):162–169. doi: 10.1007/s11864-022-01046-z. [DOI] [PubMed] [Google Scholar]
- 21.London N R, Jr, Mohyeldin A, Daoud G et al. Sinonasal undifferentiated carcinoma: Institutional trend toward induction chemotherapy followed by definitive chemoradiation. Head Neck. 2020;42(11):3197–3205. doi: 10.1002/hed.26357. [DOI] [PubMed] [Google Scholar]
- 22.Edge S B, Compton C C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(06):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 23.Common Terminology Criteria for Adverse Events (CTCAE) . Version 5. US Department of Health and Human Services. National Institutes of Health. National Cancer Institute. Published online November 27, 2017 at:https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- 24.Eisenhauer E A, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(02):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Chambers K J, Lehmann A E, Remenschneider A et al. Incidence and survival patterns of sinonasal undifferentiated carcinoma in the United States. J Neurol Surg B Skull Base. 2015;76(02):94–100. doi: 10.1055/s-0034-1390016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin E M, Sparano A, Spalding A et al. Sinonasal undifferentiated carcinoma: a 13-year experience at a single institution. Skull Base. 2010;20(02):61–67. doi: 10.1055/s-0029-1236165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McElroy E A, Jr, Buckner J C, Lewis J E.Chemotherapy for advanced esthesioneuroblastoma: the Mayo Clinic experience Neurosurgery 199842051023–1027., discussion 1027–1028 [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Wang L, He H, Li Y, Song X. The treatment outcomes of olfactory neuroblastoma patients with frontal lobe invasion. Front Oncol. 2021;11:640892. doi: 10.3389/fonc.2021.640892. [DOI] [PMC free article] [PubMed] [Google Scholar]


