Abstract
HCC, the most common type of primary liver cancer, is a leading cause of cancer-related mortality worldwide. Although the advancement of immunotherapies by immune checkpoint inhibitors (ICIs) that target programmed cell death 1 or programmed cell death 1-ligand 1 has revolutionized the treatment for HCC, the majority is still not beneficial. Accumulating evidence has pointed out that the potent immunosuppressive tumor microenvironment in HCC poses a great challenge to ICI therapeutic efficacy. As a key component in tumor microenvironment, tumor-associated macrophages (TAMs) play vital roles in HCC development, progression, and ICI low responsiveness. Mechanistically, TAM can promote cancer invasion and metastasis, angiogenesis, epithelial-mesenchymal transition, maintenance of stemness, and most importantly, immunosuppression. Targeting TAMs, therefore, represents an opportunity to enhance the ICI therapeutic efficacy in patients with HCC. While previous research has primarily focused on biochemical cues influencing macrophages, emerging evidence highlights the critical role of biophysical signals, such as substrate stiffness, topography, and external forces. In this review, we summarize the influence of biophysical characteristics within the tumor microenvironment that regulate the phenotype and function of TAMs in HCC pathogenesis and progression. We also explore the possible mechanisms and discuss the potential of manipulating biophysical cues in regulating TAM for HCC therapy. By gaining a deeper understanding of how macrophages sense and respond to mechanical forces, we may potentially usher in a path toward a curative approach for combinatory cancer immunotherapies.
Keywords: biophysical cues, extracellular matrix, HCC, tumor microenvironment, tumor-associated macrophage
INTRODUCTION
Liver cancer remains a formidable global health challenge, ranking as the sixth most diagnosed cancer and the fourth leading cause of cancer-related mortality worldwide. HCC accounts for ~75%–85% of primary liver cancers.1 Despite notable scientific progress, the prognosis for most patients with HCC remains discouraging.2 For patients with advanced HCC who are ineligible for surgery, palliative approaches such as tyrosine kinase inhibitors like sorafenib, lenvatinib, regorafenib, or cabozantinib, anti-VEGF antibody ramucirumab as well as immunotherapy are currently available.3 Compared to targeted therapies, immunotherapies, in particular immune checkpoint inhibitors (ICIs), are rapidly establishing themselves as a promising therapeutic strategy in patients with HCC. Mechanistically, checkpoint molecules, including programmed death 1/programmed death-ligand 1, cytotoxic T-lymphocyte-associated protein 4, T-cell immunoglobulin mucin-3, lymphocyte activating gene 3, and T-cell immunoglobulin and ITIM domain, mediate immunosuppression on antitumor T-cell responses to support tumor progression.4,5 Release of this immunosuppression could, therefore, lead to T-cell reactivation to kill the tumor cells. Although ICI treatment, either alone or in combination with other systemic therapies, including anti-VEGF antibody, showed promising efficacy in patients with advanced HCC, the majority of patients remain unbeneficial.6 Accumulating evidence has proved that the immunosuppressive tumor microenvironment (TME) plays a key role in restricting the ICI efficacy in patients with HCC.5
TME refers to the complex ecosystem surrounding the tumor, which consists of various components, including stromal cells like fibroblasts, immune cells, extracellular matrix (ECM), blood vessels, and other soluble factors.7 Tumor-associated macrophages (TAMs) are differentiated from naïve macrophages in the presence of tumor, which are widely present in TME and function in promoting tumor growth, invasion, metastasis, and drug resistance.8,9 TAMs also play important roles in supporting the development and progression of HCC through promoting immunosuppression, enhancing cancer invasion and metastasis, angiogenesis, inducing epithelial-mesenchymal transition, and maintaining stemness.10,11,12 Thus, approaches that target TAMs hold great potential for HCC immunotherapies.
Besides biochemical features, recent studies have pinpointed that the biophysical cues within the TME may play important roles in regulating the recruitment and function of TAMs.13,14 For example, how biophysical cues of the ECM influence macrophage behaviors in the TME of breast cancer through direct and indirect factors has been well-reviewed.14 Nevertheless, due to the heterogeneity and diversity of TME and biophysical stimuli in different cancers, the impact of biomechanical contributions to TAM needs to be clarified and specified. From the perspective of liver cancers, there are few review papers that have summarized the current progress on the influence of biophysical cues, such as ECM architecture and chemical properties, topography, surface roughness, and pore size, on macrophages in liver physiology and cancer pathogenesis. Therefore, we summarized the current understanding of the importance of biophysical cues in regulating macrophages, and how such influences may be applied to macrophage-based immunotherapy in liver cancer.
TARGETING TAM IN HCC IMMUNOTHERAPY
Macrophages
The interactions among different components in the TME have been widely reported to function in cancer development and progression.7 Nonmalignant cells within the TME play critical roles in tumorigenesis by stimulating and facilitating uncontrolled cell proliferation.7,8,9,10,11,12,13,14,15 In contrast, malignant cells infiltrate healthy tissues and metastasize to other parts of the body through the lymphatic or circulatory system.16 In particular, multiple inflammation-related risk factors jointly contribute to the development of chronic inflammation in the liver. Chronic inflammation, in turn, leads to continuous cycles of destruction-regeneration in the liver, contributing to the development and progression of HCC.5 TAMs are a key component of the immune cell populations within HCC TME, which play crucial roles in cancer-related inflammation.10,11,12,13,14,15,16,17 TAMs are the main type of inflammatory cells in the TME that promote chronic inflammation and HCC progression.18,19 Hence, gaining a better understanding of how TAM infiltrate and function in the TME would facilitate the development of novel TAM-targeted immunotherapies.10
Macrophages are differentiated from myeloid lineage and characterized by phagocytic nature according to the mononuclear phagocytic system.20 Hematopoietic stem cells in the bone marrow give rise to myeloid progenitor cells and differentiation into circulating monocytes. Inflammatory monocytes mediate the inflammatory response, and the patrolling monocytes clear the damaged cells and debris intravascularly. After extravasation into the tissue through the endothelium, monocytes can differentiate into macrophages.21 Macrophages play crucial roles in the initiation, maintenance, and resolution of inflammation. They exert phagocytosis, antigen presentation capacity, and immune regulation effect by releasing multiple growth factors and cytokines.22,23 Macrophages are found in almost all tissues of adult mammals and display incredible anatomical plastic and functional diversity.24 Hepatic macrophages, consisting of KCs and monocyte-derived macrophages, are the largest population of innate immune cells in the liver.25 KCs are the liver-resident macrophages, which are self-sustaining, nonmigratory tissue-resident phagocytes and originate from yolk sac–derived precursors during embryogenesis. They are located at the luminal side of the hepatic sinusoidal endothelium and sense their microenvironment through long cytoplasmic expansions. Moreover, KCs do not seem to patrol the liver but rather occupy a fixed position over time.26 Monocyte-derived macrophages are detected in inflammatory sites, orchestrating the immune response to tissue injury or pathogens. Hepatic macrophages are involved in the initiation and progression of various liver diseases. They act as tolerogenic antigen-presenting cells to inhibit T-cell activation by producing distinct sets of cytokines, chemokines, and mediators to maintain or resolve inflammation. In addition, hepatic macrophages also promote tissue regeneration by releasing regenerative growth factors and matrix metalloproteinases.27
Macrophage polarization
Plasticity and flexibility are key features of mononuclear phagocytes, as influenced by their activation states.28,29,30 Macrophages can be generally induced into 2 distinct polarization phenotypes according to the range of their responses to different microenvironmental stimuli. These phenotypes are known as the classically activated M1 and alternatively activated M2 macrophages. It is now widely recognized that a spectrum of phenotypic states exists between these 2 extremes of macrophage polarization.31,32 M1 macrophage exhibits proinflammatory properties, possesses high antigen presentation ability, and promotes T-cell activation.33 On the other hand, M2 macrophage displays anti-inflammatory activities, possesses immunoregulatory functions, and contributes to tissue repair.34 The polarization of M1-M2 macrophages can, to some extent, be reversed both in vitro and in vivo.35,36
There is a consensus that macrophage polarization is strongly associated with tumor stages, with a dynamic switching that exists from the M1 phenotype during the early phases of chronic inflammation to an M2-like phenotype in established tumors.18 Classically activated M1-polarized macrophages have the potential to exhibit antitumor activity and disrupt tumor tissue.29 In certain mouse models of carcinogenesis, tumor progression is linked to a phenotype switch from M1 to M2 in TAM.37 Th1-driven macrophage activation has been found to mediate the elimination of senescent hepatocytes, which subsequently drives tumorigenesis. Therefore, it is likely that classically activated M1 macrophages contribute to the T-cell–mediated elimination phase during tumor progression.38 In later stages of progression in both mice and humans, TAMs generally display an M2-like phenotype characterized by low IL-12 but high IL-10 expression, reduced tumoricidal activity, and promotion of tissue remodeling and angiogenesis.39 Therefore, approaches that convert M2-like TAMs to M1-like macrophages hold great potential for cancer therapy.
Roles of TAMs in HCC
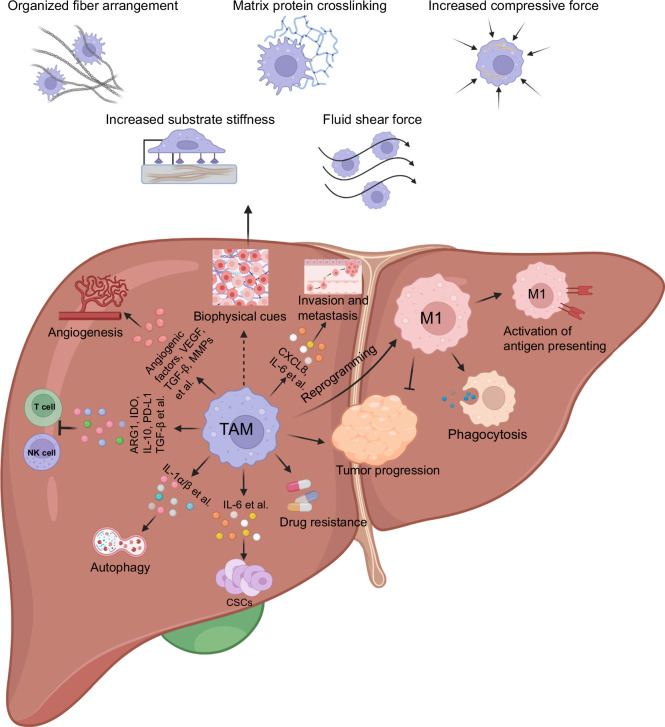
The TME of HCC consists of various components, including cancer-associated fibroblasts (CAFs), HSCs, endothelial cells and immune cells, and ECM.5,6,7,8,9,10,11,12,13,14,15,16,17 This complex environment influences the function of macrophages and shapes their behavior. Monocytes originating from the bone marrow can be recruited to the TME, primarily through chemokine (C-C motif) ligand 2 (CCL2) and macrophage (M)-CSF, ultimately differentiating into TAMs.19 TAMs constitute a significant portion of the immune cells in HCC TME.17 While the functions of TAMs are reported to be controversial in other types of cancers, they play an essential role in HCC pathogenesis.17,18,19,20,21,22,23,24 High levels of TAMs have been linked to poor prognosis in patients with HCC.40 Functionally, TAMs contribute to the establishment of a proinflammatory and protumorigenic environment through suppressing antitumor immune responses, promoting angiogenesis, facilitating tumor invasion and metastasis, providing metabolic support, inducing drug resistance, promoting autophagy, and contributing to malignant transformation of HCC stem cells17,18,19 (Figure 1). Abundant production of various cytokines and chemokines, including VEGF, TGF-β, and matrix metalloproteinases, by TAMs attracts or induces immunoregulatory/immunosuppressive cells within the TME.18 TAMs subvert local immune surveillance by reducing the activity of T cells and natural killer cells through the expression of cell surface proteins or the release of immunosuppressive factors like arginase 1, IL‑10, programmed death-ligand 1, and TGF-β.9 They also indirectly suppress T-cell activities by recruiting other immune-suppressive cells, such as regulatory T cells.9 In addition, pathological analysis reveals a significant positive correlation between tumor vascularity and macrophage count during the early stages of HCC.41 As angiogenesis is a key factor in cancer metastasis, TAMs are also reported to facilitate the invasion and metastasis of HCC. For example, TAMs enhance HCC metastasis by producing IL-6, a chemokine (C-X-C motif) ligand 8.42,43 Moreover, TAMs promote the cancer stem cell–like properties of tumor cells, contributing to the survival and expansion of cancer stem cells in HCC.44,45 Most importantly, the high density of TAMs is associated with low responsiveness toward targeted therapy and ICIs in HCC.46,47,48 Taken together, it is crucial to gain a better understanding of the mechanisms underlying TAM function to develop novel immunological interventions that target TAMs (Figure 1).
FIGURE 1.
TAM and biophysical cues in the TME of HCC. As one of the key components in the TME of HCC, TAM contributes to the establishment of the immunosuppressive microenvironment by suppressing antitumor immune responses, promoting angiogenesis and proinflammatory response to facilitate tumor invasion and metastasis, as well as drug resistance. In parallel, biophysical properties of the TME, such as increased organized fiber arrangement, matrix protein crosslinking, compressive force, substrate stiffness, and fluid shear force, show the potential to regulate the features and functional significance of TAMs. The figure is created by BioRender.com. Abbreviations: TAM, tumor-associated macrophage; TME, tumor microenvironment.
THE ROLE OF BIOPHYSICAL FEATURES OF HCC TME IN REGULATING MACROPHAGES
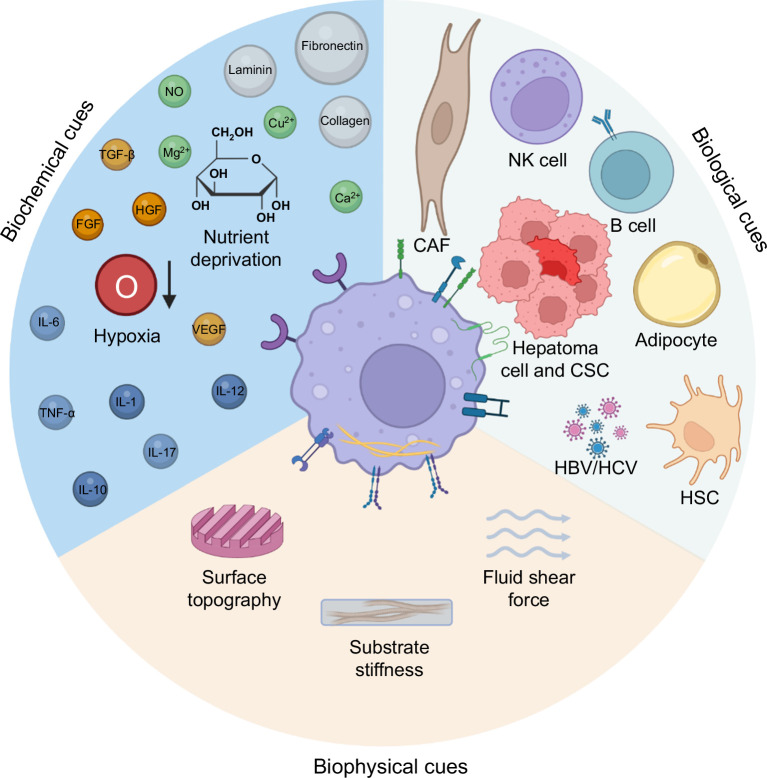
Previous studies have primarily focused on the effect of biological and biochemical cues (eg, cells, growth factors, chemokines, and metabolic factors) within the TME on macrophage behaviors, while the biophysical complexity of the TME has often been overlooked (Figure 2).14 Biophysical cues, including topography, porosity, stiffness, or external mechanical forces such as compression, tension, or shear stress, are equally important as biochemical cues in regulating cell behaviors (growth, migration, and metastasis etc.) and contributing to the TME maintenance.14 For instance, an increase in ECM protein crosslinking and linearization is often observed in the TME.49,50 Such dense and highly crosslinked ECM can have diverse consequences. It can promote cancer cell growth, migration, metabolism, and survival while also serving as a physical barrier that prevents the infiltration of drugs or immune cells, thus hindering therapeutic efficacy.51 Modulating the biophysical attributes of the TME to re-educate TAMs may be a promising therapeutic strategy for HCC treatment (Figure 1).
FIGURE 2.
Possible biological, biochemical, and biophysical cues in TAM regulation. The phenotype and function of macrophages in the TME may be influenced by biological, biochemical, and biophysical cues. The interactions of TAM toward CAF, NK cells, B cells, hepatoma cells, CSCs, adipocytes, HSC, or hepatitis virus (HBV/HCV) serve as biological cues. The levels of nutrient supply, oxygen, cytokines, and inorganic molecules may contribute to the biochemical cues in regulating TAM. Biophysical cues refer to the surface topography, substrate stiffness, and fluid shear force. The figure is created by BioRender.com. Abbreviations: CAF, cancer-associated fibroblast; CSC, cancer stem cell; NK, natural killer; TAM, tumor-associated macrophage; TME, tumor microenvironment.
Substrate stiffness
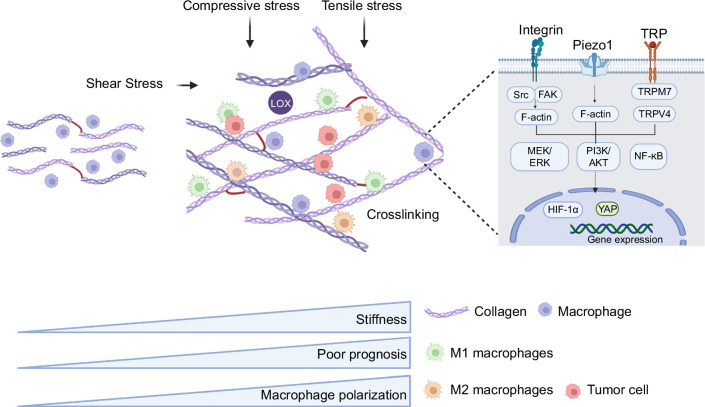
Stiffness, also known as “rigidity,” is a characteristic of a material or structure that denotes its ability to resist elastic deformation (Figure 3). Within the tissue microenvironment, the excessive secretion and crosslinking of collagens within the ECM can lead to an increase in the overall matrix stiffness.52,53 When the substrate stiffness increases, cells typically exhibit enhanced cell adhesion,54,55,56 increased cell spreading with well-defined actin organization,56,57,58,59 heightened cellular contractility, decreased migration speed,60,61 augmented cell proliferation,54,62,63,64 and improved cell differentiation.65,66,67 In particular, substrate stiffness has also been shown to influence the attachment, morphology, migration, and proliferation of macrophages,68,69 as well as their surface protein expressions and cytokine secretion. However, there is conflicting evidence regarding how stiffness affects macrophage polarization. Both M132,70,71,72,73,74,75 and M2 phenotypes68,76,77,78,79,80 have been reported to be favored by increasing substrate stiffness. Interestingly, macrophages could interact with resident fibroblasts to prompt their differentiation into myofibroblasts. These myofibroblasts, in turn, secrete large quantities of collagens, which promotes further matrix deposition. In addition, myofibroblasts actively remodel the ECM by regulating the balance of matrix metalloproteinases and their inhibitors.81 Therefore, matrix stiffness and macrophage mechanosensing capabilities become mutually regulated in a feedforward manner, ultimately contributing to tumor invasion and metastatic dissemination. Mechanistically, several signaling pathways have been identified to be involved in substrate stiffness–induced macrophage responses, including integrin-mediated focal adhesion signals and ion channels.80,82,83 In response to substrate stiffness in the TME, macrophages typically sense it through the activation of various types of integrins. This activation triggers downstream signaling pathways that not only regulate cytoskeletal dynamics but also control numerous cellular behaviors, including proliferation, migration, and differentiation.84,85,86 In addition, macrophages can be regulated by signaling pathways related to ion channels. Macrophages express transient receptor potential vanilloid 4 (TRPV4), TRPV2, TRPC6, and TRPM7 that are involved in inflammatory activation and phagocytosis87 (Figure 3).
FIGURE 3.
Stiffness regulates TAM features and functions in the TME of HCC. Increased ECM stiffness leads to enhanced ECM crosslinking, which is reported to regulate macrophage polarization into M2 type through mechanoreceptors such as Piezo1, TRP ion channels, and integrins. The external forces generated by stiffness could further enhance the interaction between tumor cells and TAM. The figure is created by BioRender.com. Abbreviations: ECM, extracellular matrix; TAM, tumor-associated macrophage; TME, tumor microenvironment.
In the context of HCC, increased stiffness is a prominent feature, which can be 5–10 times stiffer than normal liver, with a mean stiffness value of around 3.5–6.0 kPa.88,89,90,91,92,93,94,95,96,97,98,99,100,101 Specifically, the majority (80%) of HCCs develop in the context of advanced liver fibrosis or cirrhosis, one of the key risk factors for HCC.67 Liver cirrhosis and HCC are often associated with increased tissue stiffness (Table 1) due to extensive matrix deposition in the extracellular microenvironment. In addition to the macroscopic mechanical features, various strategies have been applied to measure microscopic mechanical properties, such as individual cells or subcellular levels, using techniques like atomic force microscopy (AFM), micropipette aspiration, and optical tweezers.102 Among these methods, AFM-based nanoindentation is one of the most widely used modalities for assessing cellular structures103 and mechanical properties.104,105 With its nanoscale imaging precision and real-time force measurement capabilities, AFM has emerged as a versatile tool that enables researchers to image and probe individual biological specimens in physiological solutions.106,107 During the progression of HCC, AFM can detect and record minute changes in the mechanical properties of cancer cells. By comparing these properties to those of normal cells, the pathogenesis stages of cancer cells can be evaluated. For instance, Gang and colleagues used AFM to measure the mechanical features of TME in diethylnitrosamine-induced HCC rat model. The results demonstrated a quantitative increase in tissue stiffness during hepatocarcinogenesis.99 Similarly, Tian and colleagues investigated Young’s modulus of surgically removed samples from patients with HCC using Indentation Type-AFM. They found that different stages of HCC exhibited specific mechanical signatures. The lowest elasticity peak in the mechanical profile of HCC tissues was directly correlated with the nanomechanics of cancer cells and could serve as a biophysical signal for cancer diagnosis. In addition, tumor tissues with lower elasticity were more prone to microvascular invasion.101 These studies illustrated that tumorigenic areas were softer compared with their counterparts.108 Given that the pathophysiological increases in matrix stiffness, as seen in fibrotic and cirrhotic livers, promote proliferation, migration, chemotherapeutic response, and progression in HCC cells,109,110,111 liver stiffness could serve as a clinical indicator for predicting HCC occurrence, progression, and prognosis.112,113
TABLE 1.
Biophysical features of liver cirrhosis and HCC
| Biophysical features | Species | Method | Healthy range | Pathological range | Ref. |
|---|---|---|---|---|---|
| Stiffness | H | Transient elastography | <6k Pa | >20k Pa in HCC | 93 |
| H | Transient elastography | — | >8.1k Pa in HCC | 94 | |
| H | Transient elastography | — | 10.4–28.4k Pa in HCC with severe complication (CCI >26.2) | 95 | |
| H | MRE | — | >4.7k Pa | 96 | |
| R | Rheometry | 400–600 Pa | 1400–1600 Pa | 97 | |
| M | AFM | 150 Pa | 1–6k Pa | 98 | |
| Elastic modulus | R | AFM | 0.18 ± 0.04 MPa | 0.42 ± 0.07 MPa | 99 |
| Fluid shear stress | H | Microfluidic traction force microscopy/intracellular tension sensors/confocal microscopy/optical coherence tomography/4-dimensional flow MRI | 0.1–0.5 dyn/cm2 in hepatic sinusoid, >2 dyn/cm2 in central vein | 100 | |
| H | — | 0.1–1 dyn/cm2 by interstitial flow | 101 | ||
Abbreviations: AFM, atomic force microscopy; CCI, comprehensive complication index; H, human; M, mouse; MRE, Magnetic resonance elastography; R, rat.
Increased TME stiffness has been associated with enhanced cell proliferation, chemotherapeutic resistance, and stem cell characteristics, which is also positively correlated with poor prognosis in patients with HCC109,114,115 (Figure 3). In addition, changes in TME stiffness also impact tumor immunity, including the behaviors of macrophages, which in turn significantly influence tumor development and outcomes68,69,70,71,72,80,116,117,118,119,120,121,122,123,124,125,126,127 (Table 2). For example, matrix stiffness could induce the polarization of monocytes into TAM.74 The TAMs interact with tumor cells, leading to improved migration and metastasis of HCC as well as tumor immunosuppression.128 The polarization state of macrophages is known to be associated with HCC progression (Figure 3). HCC cells communicate with TAMs and promote the M2 polarization, resulting in enhanced immunosuppression and HCC progression.128 Importantly, such effects of matrix stiffness on macrophage polarization occur regardless of the chemical inducers.74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129 Mechanistically, several signaling pathways have been implicated in matrix stiffness–strengthened macrophage M2 polarization. For instance, the work by Xing et al125 demonstrated that the activation of the integrin β5-FAK-MEK1/2-ERK1/2 pathway contributes to matrix stiffness–mediated upregulation of HIF-1α, leading to increased expression of lysyl oxidase–like 2 (LOXL2). In summary, the stiffness can regulate the features and functions of macrophages as well as their interactions with tumor cells in HCC TME, thereby further promoting TAM differentiation and immunosuppression.
TABLE 2.
Summary of relationships between matrix stiffness properties and macrophage features
| Material types | Material stiffness | Macrophage properties | Involved pathways | Species | Cell types | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Low stiffness (lower than mean liver stiffness value) | |||||||
| Poly (carboxybetaine) hydrogel | 2–19 kPa | Minimum adhesion (2 kPa hydrogen) | M | Raw 246.7 cell line | Not mentioned | 116 | |
| Polyacrylamide hydrogels | 1, 20 and 150 kPa | Increased secretion of TNFα on soft hydrogen (1 kPa) | ROCK1/2 | M | Raw 246.7 cell line | Not mentioned | 68 |
| Polyacrylamide hydrogels | 2.55 ± 0.32 kPa, 34.88 ± 4.22 kPa and 63.53 ± 5.65 kPa | M1 polarization (2.55 ± 0.32 kPa) | NF-κB | M | Macrophage | Inflammation, tissue regeneration, antitumor | 117 |
| GelMA hydrogel | 3.4, 14.2 and 31.4 kPa | Anti-inflammatory macrophage polarization (3.4 kPa) | MAPK, Hippo and AP1 | M | Bone marrow–derived macrophage | Stem cell–based therapy and tissue engineering strategy in regenerative medicine | 118 |
| Polyacrylamide gel | 0.2, 14.3 and 33.1 kPa | Increased sensitivity of BMDMs to proinflammatory stimuli (0.2 kPa) | ROCK1/2 | M | Bone marrow–derived macrophage | Biomaterials with immune regulatory properties in implantable medical devices | 119 |
| Agarose gel | 4,15 and 100 kPa | M2 polarization (4 kPa) | H | THP-1 | Not mentioned | 72 | |
| Naturally occurring biopolymers (collagen I, glycosaminoglycans [GAGs]) | Coll (Coll (27.1 ± 9.8 Pa), EDC (57.5 ± 25.9 Pa), HA (73.6 ± 27.5 Pa), and sHA (118.5 ± 34 Pa) | M2 phenotype in EDC matrices (57.5 ± 25.9 Pa). | H | Human-macrophage | Not mentioned | 120 | |
| High stiffness (higher than mean liver stiffness value) | |||||||
| PEG-RGD hydrogels | Cell morphology change; increased cell spreading | Integrin | M | Raw 246.7 cell line | Not mentioned | 70 | |
| Polyacrylamide gel | 0.2, 14.3 and 33.1 kPa | Increased cell spreading (33.1 kPa) | ROCK1/2 | M | Bone marrow–derived macrophage | Biomaterials with immune regulatory properties in implantable medical devices | 119 |
| Polyacrylamide gels | 1, 3, 5, and 280 kPa | Increased migration speed (280 kPa) | H | Human-macrophage | Biomaterials with immune regulatory properties for atherosclerosis | 69 | |
| Polydimethylsiloxane (PDMS) | 2.36 ± 0.14 Mpa, 149.53 ± 21.83 kPa and 589.99 ± 24.53 kPa | M1 polarization (149.53 kPa) | M | Raw 246.7 cell line | Tendon tissue engineering | 121 | |
| Polyacrylamide hydrogels | 10, 70, and 260 kPa | Pro-healing M2 phenotype (70 kPa) | TGF-β1 | M | Raw 246.7 cell line | Bone repair and regeneration | 122 |
| Polyacrylamide hydrogels | 2.55 ± 0.32 kPa, 34.88 ± 4.22 kPa and 63.53 ± 5.65 kPa | M2 polarization (34.88 ± 4.22 kPa) | NF-κB | M | Macrophage | Inflammation, tissue regeneration, antitumor | 117 |
| Skin tissue | 1 kPa, 50 kPa | M1 macrophage polarization (50 kPa) | M | Bone marrow–derived macrophage | Bio-competent implants and therapeutics | 80 | |
| GelMA cryogel scaffold | 20, 70, 190 kPa | M1 macrophage polarization (20 kPa) | M | Bone marrow–derived macrophage | Tailor-made scaffolds for injury | 123 | |
| GelMA cryogel scaffold | 20, 70, 190 kPa | M2 polarization (190 kPa) | M | Bone marrow–derived macrophage | Tailor-made scaffolds for injury | 123 | |
| Polyacrylamide gels | 0.3, 1, 6, 27, 47, 120, and 230 kPa | Increased proinflammatory mediator production (≥47 kPa) | NF-κB; TLR4 | M | Bone marrow–derived monocyte | Not mentioned | 71 |
| Collagen I | 0.2 and 64 kPa | M2 polarization and chemotaxis (64 kPa) | H | THP-1 | Bone repair | 124 | |
| Fibronectin | 6, 10, and 16 kPa | M2 macrophage polarization (with increased matrix stiffness) | integrin β5-FAK-MEK1/2-ERK1/2 | H | THP-1 | Not mentioned | 125 |
| PDMS | 0.61 and 3.17 MPa | Increased IL1B (0.61 MPa); reduced IL8 (3.17 MPa) | H | THP-1 | Tenogenic differentiation and regeneration | 126 | |
| Polyacrylamide gels | 11, 88, and 323 kPa | Proinflammatory phenotype with impaired phagocytosis (323 kPa); anti-inflammatory with phagocytic phenotype (11 kPa and 88 kPa). | H | THP-1 | Biomaterials with immune regulatory properties | 127 | |
| Polyacrylamide gels | 1, 3, 5, and 280 kPa | Increased migration (280 kPa) | H | Human-macrophage | atherosclerosis | 69 | |
Abbreviations: BMDM, bone marrow-derived macrophage; EDC, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride; H, human; M, mouse.
Similar to HCC, stiffness features of the ECM are also widely reported to regulate TAM in other cancers. For example, increased matrix stiffness is reported to facilitate the polarization of macrophages toward the M2 phenotype. In the context of metastatic breast cancer, the deposition of ECM components has been shown to contribute to macrophage M2 polarization.130 Moreover, single-cell RNA sequencing showed a significantly higher proportion of M2-like macrophages within the stiffer TME in an MMTV-PyMT mouse mammary tumor model.131 Similarly, Larsen and colleagues employed a 3D collagen matrix culture system, varying collagen densities to mimic healthy and tumorous tissues, to culture the murine macrophage cell line RAW 264.7. Their findings reveal that the collagen density within tumor ECM was found to modulate the immunosuppressive activity of TAMs. Comprehensive transcriptomic analysis exposed a profound TAM response to the local collagen density, impacting immune regulatory and chemokine-related gene expression.132
The ECM stiffening is largely contributed by the ECM deposition and remodeling activities of CAFs in the TME.53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133 CAFs possess the ability to facilitate monocyte migration into TME and subsequently polarize toward the M2 macrophages. For instance, in the context of pancreatic cancer, the CAF-derived factors, such as M-CSF1, IL-6, and CCL2, have been shown to enhance monocyte recruitment, leading to an increased M2/M1 ratio of TAM.134,135 In cases of female breast cancer, recent evidence indicated that CAFs contribute significantly to the population of TAMs, and these 2 cell types are engaged in reciprocal paracrine interactions.136 Collectively, these findings suggest that matrix stiffness may serve as a key driver in promoting the polarization of macrophages toward the immunosuppressive M2 phenotype within the TME.
In turn, TAMs could also influence the composition and structure of the ECM in the TME, thereby promoting tumor invasion and metastatic progression. TAMs have been observed to orchestrate the ECM remodeling, which induced the deposition and crosslinking of collagen fibers within the TME, particularly in regions characterized by heightened tumor invasiveness. This dynamic interplay between TAMs and the ECM has been documented in preclinical models of colorectal cancer, wherein TAMs were found to drive tumor progression concurrently with the remodeling of the ECM composition and structure.137 In addition, a recent study has demonstrated that TAMs possess the capacity to promote fibrosis in pancreatic cancer. This process is facilitated by a mechanism involving mannose receptor–mediated internalization of collagen followed by lysosomal degradation, which in turn induces metabolic reprogramming in TAMs.138 In diffuse large B-cell lymphoma, TAMs were reported to contribute to the tumor progression through a legumain-mediated remodeling of ECM deposition and angiogenesis. In parallel, they could fabricate an immunosuppressive network by secreting immunosuppressive factors, mediating the activation of CAFs, and ultimately leading to enhanced ECM deposition.139 These evidence indicate that TAMs also play a crucial role in ECM remodeling, thereby promoting tumor invasiveness and disease progression.
Taken together, the reciprocal relationships between ECM stiffness and TAM phenotype in different cancers shed light on the importance of how biophysical cues may regulate TAM to support tumor progression. The stiffness of ECM has the potential to impact the polarization of TAMs, while TAMs also contribute to the remodeling of ECM composition and structure by coordinating processes such as collagen fiber degradation, deposition, crosslinking, and alignment. The intricate interplay between TAMs and ECM significantly contributes to tumor invasion and metastasis, which has emerged as a critical determinant and provides a framework for targeting TAM through reprogramming stiffness in HCC TME.137
Substrate topography
In the context of biophysical cues, the term “topography” refers to the physical configuration of the substrate, including ECM architecture, organization, specific geometry, roughness, nanostructures, and the diameter and alignment of ECM fibers.140,141 Cells within living organisms are surrounded by and in contact with these topographic cues, which exert various influences on cell behaviors, including cell adhesion, orientation, and the production of growth factors and cytokines.140,141 Macrophage phenotypes have also been shown to be affected by topographic cues in recent studies.142,143,144,145 For example, the surface roughness,146,147,148 nanostructures,149,150,151,152 and nanofiber diameter and orientation153,154 of implant materials can modulate macrophage polarization and the expression of secreted cytokine expression in different ways, often correlating with the range of roughness present.
The topographic features exhibit significant differences between normal tissues and tumors. In addition to the increased stiffness of the desmoplastic ECM, the architecture and organization of collagen fibers undergo dynamic changes during tumor progression.155 In normal conditions, ECM fibers are randomly and isotropically arranged. During tumor growth, these fibers adopt an organized and anisotropic arrangement, resulting in a confined pore structure and distinct fiber alignment. Previous studies have demonstrated that cancer cells actively remodel the surrounding ECM fibers by exerting contractile forces to align them perpendicular to the tumor.155,156 These aligned collagen fibers can serve as a “highway” for malignant cell migration, thereby promoting tumor metastasis and progression.156,157 During the development of HCC, the components of the ECM, such as collagens, glycosaminoglycans, laminins, proteoglycans, and fibronectins, undergo various changes within TME, resulting in alterations in the overall topography and mechanical properties of the matrix.158,159 The increased deposition of matrix proteins and collagen crosslinking contributes to tumor progression by interfering with cell-cell adhesion and cell polarity and amplifying growth factor signaling through diverse signaling mechanisms.160,161 However, conflicting reports suggest that depletion of fibrillar collagens I and III can also promote malignance.162 Tumor cells control matrix stiffness and architecture by influencing the degree of fibrosis and controlling crosslinking and the expression of ECM proteins, as well as secreting certain enzymes such as lysyl oxidases (LOX).50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137
Numerous studies have investigated the impact of topography on macrophage behavior in different physiological and pathological contexts.163 Nevertheless, our understanding of how the topographic features of the TME, specifically in the TAM of HCC remains limited. We speculate that certain topographic features of HCC TME may also contribute to the polarization and function of TAMs, in which their modulation may, therefore, serve as additional approaches for HCC immunotherapy by targeting TAM in the future.
External forces
Biomechanical cues acting on cells are measured by the physical quantity known as stress, which is defined as force per unit area (pascal, Pa = N/m2). These stresses include but are not limited to (1) compressive stress, acting perpendicular to the cell surface leading to compression; (2) tensile stress, acting perpendicular to the cell surface causing extension; and (3) shear stress, acting parallel to the cell surface (Figure 3). Macrophages also experience such external forces, that is, compressive stress, tensile stress, and shear stress in the TME. In general, cell proliferation, matrix deposition, tissue swelling, and the increased volume of tumor tissue within a confined space result in interactions between the tumor and the surrounding normal tissues. This leads to elevated solid stress within the tumor and at the interface with the surrounding tissue. Macrophages, particularly those located in the inner or peripheral regions of the TME, are subjected to significant compressive forces exerted by the solid stress arising from increased ECM stiffness and cancer cell density. Conversely, to balance the compressive force within the TME, the outer regions of the tumor experience tensile stress.
To study how diverse mechanical cues regulate macrophages, various in vitro engineered systems have been developed. Initially, studies focused on biomedical applications and tissue repair. More recent investigations have shifted their focus toward immunotherapy targeting macrophages. Some studies have indicated that compression can activate macrophages and enhance their phagocytic capabilities.164 Specifically, when exposed to compressive force, macrophages tend to shift toward the M1 phenotype and exhibit increased expression of IL-6 and TNF-α.165,166,167 In the TME, strong compressive forces can directly or indirectly influence immune cells, including macrophages. For example, these forces can reduce the number of normal blood vessels, leading to decreased oxygen supply. The resulting hypoxic environment can, in turn, suppress macrophage activation and phagocytosis in the TME.168
Fluid shear stress is defined as the frictional force between moving layers in laminar flow. It is determined by the product of fluid viscosity and shear rate and is measured in newtons per square meter (N/m2) or dynes per square centimeter (dyn/cm2). Studies have shown that macrophages/monocytes respond to shear stress stimulation, resulting in functional alterations, such as paracrine signaling and cytokine secretion in macrophages.169 In solid tumors, the high permeability of tumor-associated vasculature can alter fluid movement, likely due to changes in hydrostatic and oncotic pressure, leading to the generation of shear stress within the tumors.170 HCC is generally characterized by hypervascularity, and blood flow in the vascular vessels promotes the development and metastasis of HCC. The interstitial fluid pressure gradient in healthy liver tissues is ~2.2 mm Hg, while in HCC, it ranges from 0 to 30 mm Hg.171 This increase in tumor interstitial fluid pressure results in a steep pressure gradient between the tumor and stroma, driving elevated interstitial fluid flow. Interstitial fluid flow can induce invasion of HCC cells through the formation of autologous transcellular gradients of CXCL12, and interstitial fluid flow–induced invasion of Huh7 cells requires MEK/ERK activity independent of CXCR4/CXCL12.172 Fluid shear stress has also been shown to induce changes in cell morphology and migration capacity of HCC HepG2 cells through the integrins-FAK-Rho GTPases signaling pathway.172 In addition, Lien et al173 reported that shear stress induced the formation of acidic vesicular organelle, the transformation of microtubule-associated protein light chain 3 (LC3B), and degradation of p62/SQSTM1 in HCC Hep3B cells, suggesting that shear stress induces autophagy in HCC cells.146 This finding is consistent with the report from Wang et al, which demonstrated that shear stress induces autophagy through the PI3K-FAK-Rho GTPase pathway.174 Yan et al175 suggested that shear stress induces autophagy to promote migration and invasion of HepG2 cells through integrin/cytoskeleton pathways in vitro. Recently, research findings from Yu et al176 revealed the molecular processes underlying shear stress–induced translocation of YAP from the cytomembrane to the nucleus, contributing to epithelial-mesenchymal transition and metastasis in HCC (Figure 3).
While direct evidence is currently lacking, it is plausible to infer from other reported studies that mechanical forces, such as compressive, tensile, or shear stress, play crucial roles in regulating macrophage behaviors and functions. Therefore, it is reasonable to anticipate that these mechanical forces may also exert significant influences on TAMs and contribute to HCC progression. Understanding the impact of mechanical forces on TAMs and HCC progression is invaluable for guiding the application of immunotherapy in the future.
Viscoelasticity
Viscoelasticity is the property of materials that exhibit both viscous and elastic properties when undergoing deformation. Most biological tissues are not only elastic but have viscous components.177 However, less attention is paid to the viscous components in understanding tissue or cell mechanics during disease progression. In HCC, tissue viscosity increases more than 2-fold, which suggests that the viscous dissipation could be highly associated with the diseased state.178
Recent research has additionally illustrated that those alterations in ECM viscoelasticity, irrespective of stiffness, influence cellular behaviors such as the proliferation and migration in breast cancer.179,180,181,182 In HCC, Fan et al183 reported that advanced glycation end-products promote changes in collagen architecture and enhance ECM viscoelasticity. High advanced glycation end-products and viscoelasticity combined with oncogenic β-catenin signaling promote HCC induction. Mechanistically, enhanced viscoelasticity promotes HCC cell proliferation and invasion through an integrin-β1-tensin-1-YAP mechanotransductive pathway.183 Viscoelasticity of the microenvironment may also have varied responses based on different cell types. Mandal and colleagues studied the mechanoresponsive of normal hepatocytes and HCC cells to elastic and viscoelastic substrates. They pointed out that the normal hepatocytes display reduced spread area and actin bundle assembly in reaction to viscoelastic substrates of similar stiffness in comparison to fully elastic substrates. Conversely, HCC cells spread more rapidly on viscoelastic substrates compared to purely elastic substrates, resulting in significantly larger spread areas.178 Vining et al184 reported that the mechanical properties of myelofibrosis, particularly the transition between liquid and solid states (viscoelasticity) of the bone marrow, play a role in the dysregulated differentiation of monocytes. Specifically, human monocytes cultured in stiff, elastic hydrogels show proinflammatory polarization and differentiation toward dendritic cells, as opposed to those cultured in a stiff viscous ECM. Moreover, transcriptional changes driven by viscoelasticity are consistent with transcriptional profiles of myeloid cells in other human fibrotic diseases, suggesting that viscoelasticity is an important factor in promoting the activation of inflammatory cells. Although viscoelasticity has been shown to regulate fundamental cell processes such as spreading and differentiation in adherent cells, the influence of viscoelasticity on macrophage behavior has not been explored until recent publication by Kalashnikov and Moraes.185 They used a tunable viscoelastic polyacrylamide hydrogel culture system to demonstrate that viscoelasticity is an important biophysical cue in regulating macrophage function. THP-1 cells cultured on more viscous polyacrylamide hydrogel substrates become smaller, rounder, and less efficient at phagocytosis.185 Since macrophages play key roles in mounting responses such as inflammation and fibrosis, these results indicate that viscoelasticity is an important parameter in the design of immunomodulatory biomaterials for better cancer therapy.
MODULATING BIOPHYSICAL FEATURES OF TME FOR TARGETING TAM IN HCC
Therapeutic approaches to target TAM in TME
TAMs are essential immune cells within the TME that exhibit high heterogeneity and complex roles as regulators of tumor immunity and immunotherapy. Given increased recognition of the profound impact of TAMs on immunotherapies, there is growing interest in targeting TAMs alone or in combination with current ICIs in HCC. The various approaches explored for targeting TAMs can be broadly categorized into 3 main categories: (1) Eliminating TAMs already present in the TME. One strategy involves depletion by chlorolipid or zoledronic acid. Studies in HCC have shown that the depletion of macrophages using these agents significantly inhibits tumor progression, angiogenesis, and metastasis.41,186 In addition, zoledronic acid treatment has been shown to enhance the effects of transarterial chemoembolization by suppressing the infiltration of TAMs in HCC.187 (2) Inhibition of monocyte/macrophage recruitment. The recruitment of circulating monocytes relies heavily on chemotaxis, such as CCL2/CCR2 signaling and SDF-1α/CXCL12 signaling. Therefore, blockade of chemotaxis using monoclonal antibodies or small molecule inhibitors could effectively impede TAM accumulation in the TME. (3) Reprogramming TAMs. Exploiting the plasticity of macrophages provides an opportunity to restore their antitumor properties. Shifting TAMs toward an “immune-effective” phenotype can help reshape the immune-suppressive or exclusionary TME and enhance the effectiveness of current ICIs. For instance, TAMs that differentiated into an M1 phenotype promote the elimination and degradation of tumor cells. Therapeutic strategies that promote macrophage polarization have shown promise. In a mouse model of HCC, compounds such as baicalin, 8-bromo-7-methoxychrysin, and the competitive CSF-1R inhibitor PLX3397 have been found to suppress tumor growth by shifting TAM polarization toward the M1 phenotype.188
Reconfiguring TME characteristics through biophysical cues for targeting TAM
Since biophysical cues show the potential to regulate macrophages, selectively reprogramming macrophages toward a restorative phenotype by biophysical modulations may, therefore, provide new approaches for macrophage-targeted therapies. In HCC, the tumor cells undergo changes such as increased stiffness and interstitial fluid pressure when growing, which have significant impacts on the surrounding cells, including TAMs. Therefore, strategies that can reduce tumor stiffness, release solid stress, and lower interstitial fluid pressure have the potential to be effective for HCC treatment.189 Most of the reported research focuses on targeting tumor stiffness, particularly by targeting major matrix components such as collagens in HCC. One approach is to eliminate the components (collagens) or cells (CAFs) to reduce the extent of collagens crosslinking in the TME.189 Direct depletion of collagen using recombinant collagenase has shown potential as a cancer therapeutic by increasing drug uptake and diffusion. However, the benefits of collagenase treatment are complicated by the risks of toxicity from collagen breakdown in healthy tissues.189 Similarly, CAFs are also attractive targets for HCC treatment as they secrete ECM proteins that reinforce fibrillar collagen deposition, leading to ECM stiffening. Targeting CAFs through “anti-CAFs” therapy has shown promising benefits for patients with tumors.37 As fibrillary collagen is a major contributor to increased ECM stiffness, another approach is to limit the synthesis or promote the breakdown of ECM tumor collagen. TGF-β plays a significant role in collagen synthesis, in which its inhibition has been shown to increase drug penetration and enhance the efficacy of anticancer therapeutics in HCC cell lines and patient-derived tumor spheroids.190 Moreover, inhibition of LOX, critical inducers of collagen crosslinking and ECM rigidity, is another promising strategy to reduce matrix stiffness and facilitate drug delivery and tumor therapy.191 Although clinical trials targeting LOX family members for HCC are still limited, several drugs are developing in the preclinical stages. For example, β-aminopropionitrile, an irreversible inhibitor of LOX and LOX1-4 catalytic activity, has been shown to block the proliferation and tube formation of endothelial cells in vitro and suppress angiogenesis and tumor growth in vivo.191 Ninomiya et al192 demonstrated that β-aminopropionitrile inhibits LOXL2 to impede the migration and invasion abilities of HCC cells.
The biophysical cues present in HCC TME extend beyond the ECM structure and stiffness predominantly influenced by collagen fibers. External forces such as solid stress, tumor interstitial fluid pressure, and vascular shear stress also contribute to the progression of HCC. For example, high interstitial fluid pressure dramatically affects cell behaviors due to mechanical shearing, leading to ECM remodeling and hindering effective drug delivery into the tumor.92 Thus, targeting ECM components such as collagen and hyaluronan can alter ECM configuration, reducing swelling pressure and interstitial fluid pressure. In addition, treatments aimed at normalizing tumor blood vessels and relieving vascular permeability can also contribute to the reduction of interstitial fluid pressure. Building upon this knowledge, future efforts can be directed toward the development of safer and more effective drugs that can interrupt the oncogenic signals induced by these biophysical cues present in TME. The ultimate goal is to prevent cancer progression and enhance the therapeutic efficacy of cancer immunotherapy.193
CONCLUDING REMARKS
Cells possess remarkable complexity as sensors capable of detecting and responding to diverse extracellular signals. Besides biochemical signals, a growing body of publications has recently highlighted the significant role of biophysical cues in modulating cell behaviors. This recognition extends to HCC, where the TME comprises various heterogeneous cell populations and ECM components that contribute to tumorigenesis. Biophysical cues such as ECM stiffness, topography, and external forces (eg, solid force, interstitial fluid pressure, and shear stress) in the TME now emerge as crucial factors in regulating HCC development.
Although a numbers of reports have investigated the impact of several biophysical cues in regulating macrophage, there remains a lack of comprehensive understanding regarding macrophage responses to multiple biophysical factors in HCC. It is now feasible to artificially engineer and manipulate the stiffness or external loading conditions of macrophages in vitro, leading to modulated behaviors and functions such as spreading area, phagocyte ability, migration, and cytokine release. In addition, there is still much work to be done in achieving controllable manipulation of macrophage polarization. Influenced by factors such as cell types, material properties (such as swelling, porosity, and degradation), dimensionality, and spatiotemporal configurations, macrophage polarization remains a complex phenomenon. Moreover, future research efforts aimed at elucidating the underlying molecular mechanisms will contribute to our understanding of how biophysical features regulate macrophage functions in HCC, as well as expanding to other cancers. By unraveling these mechanisms, we can explore ways to modulate biophysical cues to manipulate macrophages and improve cancer treatment. This can be achieved through interdisciplinary collaborations among clinical oncologists, cancer biologists, immunologists, tissue engineers, and bioinformaticians. By leveraging computational and mathematical modeling, multifactorial deep machine learning model, and using large quantities of imaging data, liquid biopsy data, and gene/single-cell sequencing data, we can comprehensively evaluate the characteristics of the patient’s physical TME. Such an approach will contribute to a thorough understanding of the synergistic effects of biochemical and biophysical features in the TME during carcinogenesis, ultimately leading to enhanced therapeutic outcomes for HCC.
Acknowledgments
FUNDING INFORMATION
This work was supported by The Chinese University of Hong Kong (Startup funds: Jingying Zhou and Dan Michelle Wang); Lin He’s Academician Workstation of New Medicine and Clinical Translation in Jining Medical University (JYHL2021MS29; Ying Zhang); and The Innovation and Technology Commission (Health@InnoHK, Dai Fei Elmer Ker and Dan Michelle Wang).
CONFLICTS OF INTEREST
The authors have no conflicts to report.
Footnotes
Ying Zhang, Ying Rao, and Jiahuan Lu share co-first authorship.
Abbreviations: AFM, atomic force microscopy; CAFs, cancer-associated fibroblasts; CCL, chemokine (C-C motif) ligand; CXCL, C-C-C motif ligand; ECM, extracellular matrix; ICI, immune checkpoint inhibitor; LC3B, light chain 3; LOX, lysyl oxidases; LOXL2, lysyl oxidase–like 2; TAMs, tumor-associated macrophages; TME, tumor microenvironment; TRPV4, transient receptor potential vanilloid 4.
Contributor Information
Ying Zhang, Email: zhangying831020@163.com.
Ying Rao, Email: 1155129743@link.cuhk.edu.hk.
Jiahuan Lu, Email: vikkilo@link.cuhk.edu.hk.
Jiyu Wang, Email: jakejywang@link.cuhk.edu.hk.
Dai Fei Elmer Ker, Email: Elmer.ker@polyu.edu.hk.
Jingying Zhou, Email: zhoujy@cuhk.edu.hk.
Dan Michelle Wang, Email: wangmd@cuhk.edu.hk.
REFERENCES
- 1.Bray FFJ, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Ren ZMX, Duan Z, Chen X. Diagnosis, therapy, and prognosis for hepatocellular carcinoma. Anal Cell Pathol (Amst). 2020;2020:8157406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koulouris A, Tsagkaris C, Spyrou V, Pappa E, Troullinou A, Nikolaou M. Hepatocellular carcinoma: An overview of the changing landscape of treatment options. J Hepatocell Carcinoma. 2021;8:387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Shao X, Zhang Y, Zhu M, Wang FXC, Mu J, et al. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023;12:11149–11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prieto JMI, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681–700. [DOI] [PubMed] [Google Scholar]
- 6.van Bömmel F, Berg T, Lordick F. Immune checkpoint inhibition (ICI) in current systemic therapies for hepatocellular carcinoma (HCC). ESMO Gastrointest Oncol. 2023;1:27–39. [Google Scholar]
- 7.B A. Tumor microenvironment. Medicina (Kaunas). 2019;56:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngambenjawong CGH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017;114:206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeNardo DGRB. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YGW, Zhou J, Gao B, Qian X, Wang W. The role of tumor associated macrophages in hepatocellular carcinoma. J Cancer. 2021;12:1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu SYM, Wu Y, Dong B, Wu K. Roles of tumor-associated macrophages in tumor progression: Implications on therapeutic strategies. Exp Hematol Oncol. 2021;10:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li MHL, Zhu J, Zhang P, Liang S. Targeting tumor-associated macrophages for cancer treatment. Cell Biosci. 2022;12:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spill FRD, Kamm RD, Zaman MH. Impact of the physical microenvironment on tumor progression and metastasis. Curr Opin Biotechnol. 2016;40:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann EJPS. Biomechanical contributions to macrophage activation in the tumor microenvironment. Front Oncol. 2020;10:787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanahan DCL. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. [DOI] [PubMed] [Google Scholar]
- 16.Leong SPNK, Keller L, Pantel K, Witte M. Molecular mechanisms of cancer metastasis via the lymphatic versus the blood vessels. Clin Exp Metastasis. 2022;39:159–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian ZHX, Liu W, Han Z, Wei L. Macrophages and hepatocellular carcinoma. Cell Biosci. 2019;9:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capece DFM, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F, et al. The inflammatory microenvironment in hepatocellular carcinoma: A pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arvanitakis KKT, Mitroulis I, Germanidis G. Tumor-associated macrophages in hepatocellular carcinoma pathogenesis, prognosis and therapy. Cancers (Basel). 2022;14:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon SPA. The mononuclear phagocytic system. Generation of diversity. Front Immunol. 2019;10:1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginhoux FJS. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. [DOI] [PubMed] [Google Scholar]
- 22.Wan SKN, Kryczek I, Zou W, Welling TH. Myeloid cells in hepatocellular carcinoma. Hepatology. 2015;62:1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S, Saeed A, Liu Q, Jiang Q, Xu H, Xiao GG, et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon SPA, Martinez Estrada F. Macrophage heterogeneity in tissues: Phenotypic diversity and functions. Immunol Rev. 2014;262:36–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon LJBM, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol. 2013;3:785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillot ATF. Liver macrophages: Old dogmas and new insights. Hepatol Commun. 2019;3:730–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dou LSX, He X, Gao Y. Macrophage phenotype and function in liver disorder. Front Immunol. 2020;10:3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biswas SKMA. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat Immunol. 2010;11:889–896. [DOI] [PubMed] [Google Scholar]
- 29.Mantovani ASS, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. [DOI] [PubMed] [Google Scholar]
- 30.Mosser DMEJ. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray PJAJ, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity. 2014;41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.PJ M. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566. [DOI] [PubMed] [Google Scholar]
- 33.Galli SJBN, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sica AIP, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034–2042. [DOI] [PubMed] [Google Scholar]
- 35.Guiducci CVA, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. [DOI] [PubMed] [Google Scholar]
- 36.Saccani AST, Porta C, Biswas SK, Nebuloni M, Vago L, Bottazzi B, et al. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006;66:11432–11440. [DOI] [PubMed] [Google Scholar]
- 37.Zhang JGC, Song Q, Zhu M, Xu Y, Xiao M, Zheng W. Identifying cancer-associated fibroblasts as emerging targets for hepatocellular carcinoma. Cell Biosci. 2020;10:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schreiber RDOL, Smyth MJ. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. [DOI] [PubMed] [Google Scholar]
- 39.Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: The multifaceted role of NF-kappaB. Blood. 2009;113:3139–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi YQ, Xiong F, Chen YJ. The correlation between tumor-associated macrophages and the prognosis of east Asian hepatocellular carcinoma patients: A systematic review and meta-analysis. Pathol Res Pract. 2023;252:154919. [DOI] [PubMed] [Google Scholar]
- 41.Zhou DYQJ, Huang J, Wang F, Xu GP, Lv YT, Zhang JB, et al. Zoledronic acid inhibits infiltration of tumor-associated macrophages and angiogenesis following transcatheter arterial chemoembolization in rat hepatocellular carcinoma models. Oncol Lett. 2017;14:4078–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang J, Wang GZ, Wang Y, Huang HZ, Li WT, Qu XD. Hypoxia-induced HMGB1 expression of HCC promotes tumor invasiveness and metastasis via regulating macrophage-derived IL-6. Exp Cell Res. 2018;367:81–88. [DOI] [PubMed] [Google Scholar]
- 43.Yin Z, Huang J, Ma T, Li D, Wu Z, Hou B, et al. Macrophages activating chemokine (C-X-C motif) ligand 8/miR-17 cluster modulate hepatocellular carcinoma cell growth and metastasis. Am J Transl Res. 2017;9:2403–2411. [PMC free article] [PubMed] [Google Scholar]
- 44.Fan QMJY, Yu GF, Kou XR, Ye F, Gao L, Li R, et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352:160–168. [DOI] [PubMed] [Google Scholar]
- 45.Li XFCC, Xiang DM, Qu L, Sun W, Lu XY, Zhou TF, et al. Chronic inflammation-elicited liver progenitor cell conversion to liver cancer stem cell with clinical significance. Hepatology. 2017;66:1934–1951. [DOI] [PubMed] [Google Scholar]
- 46.Dong NSX, Wang S, Gao Y, Kuang Z, Xie Q, Li Y, et al. M2 macrophages mediate sorafenib resistance by secreting HGF in a feed-forward manner in hepatocellular carcinoma. Br J Cancer. 2019;121:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu XTSK, Zhou J, Shi YH, Liu WR, Shi GM, Gao Q, et al. Tumor-associated macrophages modulate resistance to oxaliplatin via inducing autophagy in hepatocellular carcinoma. Cancer Cell Int. 2019;19:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu QZW, Yin S, Zhou Y, Chen T, Qian J, Su R, et al. Blocking triggering receptor expressed on myeloid cells-1-positive tumor-associated macrophages induced by hypoxia reverses immunosuppression and anti-programmed cell death ligand 1 resistance in liver cancer. Hepatology. 2019;70:198–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kong HJPT, Alsberg E, Mooney DJ. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proc Natl Acad Sci USA. 2005;102:4300–4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Høye AMEJ. Structural ECM components in the premetastatic and metastatic niche. Am J Physiol Cell Physiol. 2016;310:C955–C967. [DOI] [PubMed] [Google Scholar]
- 51.Kalli M, Poskus MD, Stylianopoulos T, Zervantonakis IK. Beyond matrix stiffness: Targeting force-induced cancer drug resistance. Trends Cancer. 2023;9:937–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong J, Xiao R, Zhao J, Zhao Q, Luo M, Li F, et al. Matrix stiffness affects tumor-associated macrophage functional polarization and its potential in tumor therapy. J Transl Med. 2024;22:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang JY, Zhu WW, Wang MY, Zhai RD, Wang Q, Shen WL, et al. Cancer-associated fibroblasts promote oral squamous cell carcinoma progression through LOX-mediated matrix stiffness. J Transl Med. 2021;19:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo WHFM, Burnham NA, Wang YL. Substrate rigidity regulates the formation and maintenance of tissues. Biophys J. 2006;90:2213–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solon JLI, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93:4453–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeung TGP, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. [DOI] [PubMed] [Google Scholar]
- 57.Collin OTP, Stephanou A, Usson Y, Clément-Lacroix J, Planus E. Spatiotemporal dynamics of actin-rich adhesion microdomains: Influence of substrate flexibility. J Cell Sci. 2006;119:1914–1925. [DOI] [PubMed] [Google Scholar]
- 58.Reinhart-King CADM, Hammer DA. Cell-cell mechanical communication through compliant substrates. Biophys J. 2008;95:6044–6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghosh KPZ, Guan E, Ge S, Liu Y, Nakamura T, Ren XD, et al. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials. 2007;28:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong Joyce Y, Velasco A, P Rajagopalan, Pham Q. Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir. 2003;19:1908–1913. [Google Scholar]
- 61.Mih JDMA, Liu F, Sharif AS, Tschumperlin DJ. Matrix stiffness reverses the effect of actomyosin tension on cell proliferation. J Cell Sci. 2012;2012:5974–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsiong SXCP, Kong HJ, Lee KY, Mooney DJ. Differentiation stage alters matrix control of stem cells. J Biomed Mater Res A. 2008;85:145–156. [DOI] [PubMed] [Google Scholar]
- 63.Wang YWG, Luo X, Qiu J, Tang C. Substrate stiffness regulates the proliferation, migration, and differentiation of epidermal cells. Burns. 2012;38:414–420. [DOI] [PubMed] [Google Scholar]
- 64.Engler AJSS, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. [DOI] [PubMed] [Google Scholar]
- 65.Saha SJL, de Pablo JJ, Palecek SP. TGFbeta/Activin/Nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophys J. 2008;94:4123–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang CTM, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13:645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fattovich GST, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology. 2004;127:S35–S50. [DOI] [PubMed] [Google Scholar]
- 68.Gruber EHC, Cameron J, Leifer C. Toll-like receptor signaling in macrophages is regulated by extracellular substrate stiffness and Rho-associated coiled-coil kinase (ROCK1/2). Int Immunol. 2018;30:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adlerz KMA-EH, Hayenga HN. Substrate elasticity regulates the behavior of human monocyte-derived macrophages. Eur Biophys J. 2016;45:301–309. [DOI] [PubMed] [Google Scholar]
- 70.Blakney AKSM, Bryant SJ. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res A. 2012;100:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Previtera MLSA. Substrate stiffness regulates proinflammatory mediator production through TLR4 activity in macrophages. PLoS One. 2015;10:e0145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okamoto TTY, Kawamoto E, Park EJ, Usuda H, Wada K, Shimaoka M. Reduced substrate stiffness promotes M2-like macrophage activation and enhances peroxisome proliferator-activated receptor γ expression. Exp Cell Res. 2018;367:264–273. [DOI] [PubMed] [Google Scholar]
- 73.Hsieh JYKM, Smith TD, Meli VS, Botvinick EL, Liu WF. Matrix crosslinking enhances macrophage adhesion, migration, and inflammatory activation. APL Bioeng. 2019;3:016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sridharan RRE, Kearney CJ, Kelly DJ, O’Brien FJ. Macrophage polarization in response to collagen scaffold stiffness is dependent on cross-linking agent used to modulate the stiffness. ACS Biomater Sci Eng. 2019;5:544–552. [DOI] [PubMed] [Google Scholar]
- 75.Meli VSAH, Veerasubramanian PK, Nagalla RR, Luu TU, Chen EY, Guerrero-Juarez CF, et al. YAP-mediated mechanotransduction tunes the macrophage inflammatory response. Sci Adv. 2020;6:eabb8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel NRBM, Chen C, Hardin CC, Kho AT, Mih J, Deng L, et al. Cell elasticity determines macrophage function. PLoS One. 2012;7:e41024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scheraga RGAS, Niese KA, Southern BD, Grove LM, Hite RD, McDonald C, et al. TRPV4 mechanosensitive ion channel regulates lipopolysaccharide-stimulated macrophage phagocytosis. J Immunol. 2016;196:428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carnicer-Lombarte A, Barone DG, Dimov IB, Hamilton RS, Prater M, Zhao X, et al. Mechanical matching of implant to host minimises foreign body reaction. bioRxiv. 2019:829648. [Google Scholar]
- 79.Xu WCY, Guo Y, Yao W, Qian H. Targeting tumor associated macrophages in hepatocellular carcinoma. Biochem Pharmacol. 2022;199:114990. [DOI] [PubMed] [Google Scholar]
- 80.Dutta BGR, Rahaman SO. TRPV4 plays a role in matrix stiffness-induced macrophage polarization. Front Immunol. 2020;11:570195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simoes FC, Cahill TJ, Kenyon A, Gavriouchkina D, Vieira JM, Sun X, et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat Commun. 2020;11:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Betriu NAA, Alonso A, Semino CE. Increased stiffness downregulates focal adhesion kinase expression in pancreatic cancer cells cultured in 3D self-assembling peptide scaffolds. Biomedicines. 2022;10:1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ni YQH, Zhang F, Jiang S, Tang Q, Cai W, Mo W, et al. Macrophages modulate stiffness-related foreign body responses through plasma membrane deformation. Proc Natl Acad Sci USA. 2023;120:e2213837120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schiller HBFR. Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep. 2013;14:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Katsumi AOA, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. [DOI] [PubMed] [Google Scholar]
- 86.Sun ZGS, Fässler R. Integrin-mediated mechanotransduction. J Cell Biol. 2016;215:445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu SHS, Ding Y, Wang W, Wang A, Lu Y. Transient receptor potential ion-channel subfamily V member 4: A potential target for cancer treatment. Cell Death Dis. 2019;10:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fung J, Lee CK, Chan M, Seto WK, Wong DK, Lai CL, et al. Defining normal liver stiffness range in a normal healthy Chinese population without liver disease. PLoS One. 2013;8:e85067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roulot D, Czernichow S, Le Clesiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: Influence of gender and metabolic syndrome. J Hepatol. 2008;48:606–613. [DOI] [PubMed] [Google Scholar]
- 90.Marginean CO, Melit LE, Ghiga DV, Sasaran MO. Reference values of normal liver stiffness in healthy children by two methods: 2D shear wave and transient elastography. Sci Rep. 2020;10:7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bazerbachi F, Haffar S, Wang Z, Cabezas J, Arias-Loste MT, Crespo J, et al. Range of normal liver stiffness and factors associated with increased stiffness measurements in apparently healthy individuals. Clin Gastroenterol Hepatol. 2019;17:54–64.e51. [DOI] [PubMed] [Google Scholar]
- 92.Nia HTML, Jain RK. Physical traits of cancer. Science. 2020;370:eaaz0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mueller S, Sandrin L. Liver stiffness: A novel parameter for the diagnosis of liver disease. Hepat Med. 2010;2:49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jung KS, Kim SU, Ahn SH, Park YN, Kim DY, Park JY, et al. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology. 2011;53:885–894. [DOI] [PubMed] [Google Scholar]
- 95.Serenari M, Han KH, Ravaioli F, Kim SU, Cucchetti A, Han DH, et al. A nomogram based on liver stiffness predicts postoperative complications in patients with hepatocellular carcinoma. J Hepatol. 2020;73:855–862. [DOI] [PubMed] [Google Scholar]
- 96.Cho HJ, Kim B, Kim HJ, Huh J, Kim JK, Lee JH, et al. Liver stiffness measured by MR elastography is a predictor of early HCC recurrence after treatment. Eur Radiol. 2020;30:4182–4192. [DOI] [PubMed] [Google Scholar]
- 97.Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, et al. Increased stiffness of the rat liver precedes matrix deposition: Implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147–G1154. [DOI] [PubMed] [Google Scholar]
- 98.Desai SS, Tung JC, Zhou VX, Grenert JP, Malato Y, Rezvani M, et al. Physiological ranges of matrix rigidity modulate primary mouse hepatocyte function in part through hepatocyte nuclear factor 4 alpha. Hepatology. 2016;64:261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gang Z, Qi Q, Jing C, Wang C. Measuring microenvironment mechanical stress of rat liver during diethylnitrosamine induced hepatocarcinogenesis by atomic force microscope. Microsc Res Tech. 2009;72:672–678. [DOI] [PubMed] [Google Scholar]
- 100.Huang Q, Hu X, He W, Zhao Y, Hao S, Wu Q, et al. Fluid shear stress and tumor metastasis. Am J Cancer Res. 2018;8:763–777. [PMC free article] [PubMed] [Google Scholar]
- 101.Yan Z, Guo D, Tao R, Yu X, Zhang J, He Y, et al. Fluid shear stress induces cell migration via RhoA-YAP1-autophagy pathway in liver cancer stem cells. Cell Adh Migr. 2022;16:94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang HDC, Shen H, Gu M, Wang Y, Liu J, Chen L, et al. Recent advances on the model, measurement technique, and application of single cell mechanics. Int J Mol Sci. 2020;21:6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reynolds NHMJ. Single cell active force generation under dynamic loading—Part II: Active modelling insights. Acta Biomater. 2015;27:251–263. [DOI] [PubMed] [Google Scholar]
- 104.Dufrêne YFAT, Garcia R, Alsteens D, Martinez-Martin D, Engel A, Gerber C, et al. Imaging modes of atomic force microscopy for application in molecular and cell biology. Nat Nanotechnol. 2017;12:295–307. [DOI] [PubMed] [Google Scholar]
- 105.L M. Discrimination between normal and cancerous cells using AFM. Bionanoscience. 2016;6:65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuznetsova TGSM, Yegorenkov NI, Chizhik SA, Zhdanov RI. Atomic force microscopy probing of cell elasticity. Micron. 2007;38:824–833. [DOI] [PubMed] [Google Scholar]
- 107.Rettler EHS, Sigusch BW, Schubert US. Mapping the mechanical properties of biomaterials on different length scales: Depth-sensing indentation and AFM based nanoindentation. J Mater Chem B. 2013;1:2789–2806. [DOI] [PubMed] [Google Scholar]
- 108.Tian MLY, Liu W, Jin L, Jiang X, Wang X, Ding Z, et al. The nanomechanical signature of liver cancer tissues and its molecular origin. Nanoscale. 2015;7:12998–13010. [DOI] [PubMed] [Google Scholar]
- 109.Schrader JG-WT, Aucott RL, van Deemter M, Quaas A, Walsh S, Benten D, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu PZX, Fu Q, Liu C, Luo Q, Yu P, Chen S, et al. LINC01419 promotes the proliferation of hepatoma cells by recruiting XRCC5 and regulating its phosphorylation to repair DNA damage. Dis Markers. 2022;2022:9313680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rinaldi LNR, Franci G, Perrella A, Corvino G, Marrone A, Berretta M, et al. Risk of hepatocellular carcinoma after HCV clearance by direct-acting antivirals treatment predictive factors and role of epigenetics. Cancers (Basel). 2020;12:1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Singh SFL, Murad MH, Wang Z, Asrani SK, Ehman RL, Kamath PS, et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:573–584.e571-572; quiz e588–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tachi YHT, Kojima Y, Ishizu Y, Honda T, Kuzuya T, Hayashi K, et al. Liver stiffness measurement predicts hepatocellular carcinoma development in patients treated with direct-acting antivirals. JGH Open. 2017;1:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang LSP, Zhao G, Xu J, Peng W, Zhang J, Zhang G, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bhattacharya AAM, Mukherjee R, Sen P, Sinha DK. 3D micro-environment regulates NF-κβ dependent adhesion to induce monocyte differentiation. Cell Death Dis. 2018;9:914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Trel’ova D, Salgarella AR, Ricotti L, Giudetti G, Cutrone A, Sramkova P, et al. Soft hydrogel Zwitterionic coatings minimize fibroblast and macrophage adhesion on polyimide substrates. Langmuir. 2019;35:1085–1099. [DOI] [PubMed] [Google Scholar]
- 117.Chen M, Zhang Y, Zhou P, Liu X, Zhao H, Zhou X, et al. Substrate stiffness modulates bone marrow-derived macrophage polarization through NF-kappaB signaling pathway. Bioact Mater. 2020;5:880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhuang Z, Zhang Y, Yang X, Yu T, Zhang Y, Sun K, et al. Matrix stiffness regulates the immunomodulatory effects of mesenchymal stem cells on macrophages via AP1/TSG-6 signaling pathways. Acta Biomater. 2022;149:69–81. [DOI] [PubMed] [Google Scholar]
- 119.Escolano JC, Taubenberger AV, Abuhattum S, Schweitzer C, Farrukh A, Del Campo A, et al. Compliant substrates enhance macrophage cytokine release and NLRP3 inflammasome formation during their pro-inflammatory response. Front Cell Dev Biol. 2021;9:639815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Friedemann M, Kalbitzer L, Franz S, Moeller S, Schnabelrauch M, Simon JC, et al. Instructing human macrophage polarization by stiffness and glycosaminoglycan functionalization in 3D collagen networks. Adv Healthc Mater. 2017;6. 10.1002/adhm.201600967. [DOI] [PubMed] [Google Scholar]
- 121.Sheng R, Liu J, Zhang W, Luo Y, Chen Z, Chi J, et al. Material stiffness in cooperation with macrophage paracrine signals determines the tenogenic differentiation of mesenchymal stem cells. Adv Sci (Weinh). 2023;10:e2206814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hang R, Wang Z, Wang H, Zhang Y, Zhao Y, Bai L, et al. Matrix stiffness-induced platelet activation determines immunomodulation of macrophages. Biomater Adv. 2023;148:213356. [DOI] [PubMed] [Google Scholar]
- 123.Jiang S, Lyu C, Zhao P, Li W, Kong W, Huang C, et al. Cryoprotectant enables structural control of porous scaffolds for exploration of cellular mechano-responsiveness in 3D. Nat Commun. 2019;10:3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu Z, Liu J, Li J, Li Y, Sun J, Deng Y, et al. Substrate stiffness can affect the crosstalk between adipose derived mesenchymal stem cells and macrophages in bone tissue engineering. Front Bioeng Biotechnol. 2023;11:1133547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xing XWY, Zhang X, Gao X, Li M, Wu S, Zhao Y, et al. Matrix stiffness-mediated effects on macrophages polarization and their LOXL2 expression. FEBS J. 2021;288:3465–3477. [DOI] [PubMed] [Google Scholar]
- 126.Konar S, Bolam SM, Coleman B, Dalbeth N, McGlashan SR, Leung S, et al. Changes in physiological tendon substrate stiffness have moderate effects on tendon-derived cell growth and immune cell activation. Front Bioeng Biotechnol. 2022;10:800748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sridharan R, Cavanagh B, Cameron AR, Kelly DJ, O’Brien FJ. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019;89:47–59. [DOI] [PubMed] [Google Scholar]
- 128.You YZQ, Dong Y, Xie X, Wang Y, Wu S, Zhang L, et al. Matrix stiffness-mediated effects on stemness characteristics occurring in HCC cells. Oncotarget. 2016;7:32221–32231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kieswetter KSZ, Dean DD, Boyan BD. The role of implant surface characteristics in the healing of bone. Crit Rev Oral Biol Med. 1996;7:329–345. [DOI] [PubMed] [Google Scholar]
- 130.Wang Y, Li Y, Zhong J, Li M, Zhou Y, Lin Q, et al. Tumor-derived Cav-1 promotes pre-metastatic niche formation and lung metastasis in breast cancer. Theranostics. 2023;13:1684–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Taufalele PV, Wang W, Simmons AJ, Southard-Smith AN, Chen B, Greenlee JD, et al. Matrix stiffness enhances cancer-macrophage interactions and M2-like macrophage accumulation in the breast tumor microenvironment. Acta Biomater. 2023;163:365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Larsen AMH, Kuczek DE, Kalvisa A, Siersbaek MS, Thorseth ML, Johansen AZ, et al. Collagen density modulates the immunosuppressive functions of macrophages. J Immunol. 2020;205:1461–1472. [DOI] [PubMed] [Google Scholar]
- 133.Lu Y, Jin Z, Hou J, Wu X, Yu Z, Yao L, et al. Calponin 1 increases cancer-associated fibroblasts-mediated matrix stiffness to promote chemoresistance in gastric cancer. Matrix Biol. 2023;115:1–15. [DOI] [PubMed] [Google Scholar]
- 134.Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol Cancer. 2021;20:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mayer S, Milo T, Isaacson A, Halperin C, Miyara S, Stein Y, et al. The tumor microenvironment shows a hierarchy of cell-cell interactions dominated by fibroblasts. Nat Commun. 2023;14:5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Afik RZE, Vugman M, Klepfish M, Shimshoni E, Pasmanik-Chor M, Shenoy A, et al. Tumor macrophages are pivotal constructors of tumor collagenous matrix. J Exp Med. 2016;213:2315–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.LaRue MM, Parker S, Puccini J, Cammer M, Kimmelman AC, Bar-Sagi D. Metabolic reprogramming of tumor-associated macrophages by collagen turnover promotes fibrosis in pancreatic cancer. Proc Natl Acad Sci USA. 2022;119:e2119168119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tariq M, Zhang J, Liang G, Ding L, He Q, Yang B. Macrophage polarization: Anti-cancer strategies to target tumor-associated macrophage in breast cancer. J Cell Biochem. 2017;118:2484–2501. [DOI] [PubMed] [Google Scholar]
- 140.Albrektsson TBP, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52:155–170. [DOI] [PubMed] [Google Scholar]
- 141.Zhang YCX, Jansen JA, Yang F, van den Beucken JJJP. Titanium surfaces characteristics modulate macrophage polarization. Mater Sci Eng C Mater Biol Appl. 2019;95:143–151. [DOI] [PubMed] [Google Scholar]
- 142.Luu TUGS, Woo BW, Rao MP, Liu WF. Micro- and nanopatterned topographical cues for regulating macrophage cell shape and phenotype. ACS Appl Mater Interfaces. 2015;7:28665–28672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.He YGY, Ma Q, Zhang X, Zhang Y, Song W. Nanotopographical cues for regulation of macrophages and osteoclasts: Emerging opportunities for osseointegration. J Nanobiotechnology. 2022;20:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vassey MJFG, Scurr DJ, Vasilevich AS, Vermeulen S, Carlier A, Luckett J, et al. Immune modulation by design: Using topography to control human monocyte attachment and macrophage differentiation. Adv Sci (Weinh). 2020;7:1903392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhu YLH, Liu X, Wu J, Yang C, Wong TM, Kwan KYH, et al. Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration. Sci Adv. 2021;7:eabf6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Refai AKTM, Brunette DM, Waterfield JD. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J Biomed Mater Res A. 2004;70:194–205. [DOI] [PubMed] [Google Scholar]
- 147.Hamlet SMLR, Moon HJ, Alfarsi MA, Ivanovski S. Hydrophilic titanium surface-induced macrophage modulation promotes pro-osteogenic signalling. Clin Oral Implants Res. 2019;30:1085–1096. [DOI] [PubMed] [Google Scholar]
- 148.Hotchkiss KMSK, Olivares-Navarrete R. Novel in vitro comparative model of osteogenic and inflammatory cell response to dental implants. Dent Mater. 2019;35:176–184. [DOI] [PubMed] [Google Scholar]
- 149.Park JWHS, Hanawa T. Effects of surface nanotopography and calcium chemistry of titanium bone implants on early blood platelet and macrophage cell function. Biomed Res Int. 2018;2018:1362958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lu JYC, Yang L, Webster TJ. Decreased platelet adhesion and enhanced endothelial cell functions on nano and submicron-rough titanium stents. Tissue Eng Part A. 2012;18:1389–1398. [DOI] [PubMed] [Google Scholar]
- 151.Linares JFA, Feito MJ, Matesanz MC, Sánchez-Salcedo S, Arcos D, Vallet-Regí M, et al. Effects of nanocrystalline hydroxyapatites on macrophage polarization. J Mater Chem B. 2016;4:1951–1959. [DOI] [PubMed] [Google Scholar]
- 152.Saino EFM, Gualandi C, Emanuele E, Cornaglia AI, Imbriani M, Visai L. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules. 2011;12:1900–1911. [DOI] [PubMed] [Google Scholar]
- 153.Jia YYW, Zhang K, Qiu S, Xu J, Wang C, Chai Y. Nanofiber arrangement regulates peripheral nerve regeneration through differential modulation of macrophage phenotypes. Acta Biomater. 2019;83:291–301. [DOI] [PubMed] [Google Scholar]
- 154.Provenzano PPEK, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cukierman EBD. Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. Semin Cancer Biol. 2010;20:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Conklin MWEJ, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178:1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.H RO. The extracellular matrix: Not just pretty fibrils. Science. 2009;326:1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Van Goethem EPR, Gauffre F, Maridonneau-Parini I, Le Cabec V. Matrix architecture dictates three-dimensional migration modes of human macrophages: Differential involvement of proteases and podosome-like structures. J Immunol. 2010;184:1049–1061. [DOI] [PubMed] [Google Scholar]
- 159.Naba ACK, Lamar JM, Carr SA, Hynes RO. Extracellular matrix signatures of human mammary carcinoma identify novel metastasis promoters. Elife. 2014;3:e01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Paszek MJZN, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. [DOI] [PubMed] [Google Scholar]
- 161.Levental KRYH, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Özdemir BCP-HT, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2015;28:831–833. [DOI] [PubMed] [Google Scholar]
- 163.Bota PCCA, Puolakkainen P, Vernon RB, Sage EH, Ratner BD, Stayton PS. Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. J Biomed Mater Res A. 2010;95:649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cho ESLK, Son YO, Jang YS, Lee SY, Kwak SY, Yang YM, et al. Compressive mechanical force augments osteoclastogenesis by bone marrow macrophages through activation of c-Fms-mediated signaling. J Cell Biochem. 2010;111:1260–1269. [DOI] [PubMed] [Google Scholar]
- 165.Evans CEMS, Andrew JG. Age of donor alters the effect of cyclic hydrostatic pressure on production by human macrophages and osteoblasts of sRANKL, OPG and RANK. BMC Musculoskelet Disord. 2006;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ferrier GMMA, Evans CE, Andrew JG. The effect of cyclic pressure on human monocyte-derived macrophages in vitro. J Bone Joint Surg Br. 2000;82:755–759. [DOI] [PubMed] [Google Scholar]
- 167.Facciabene APX, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. [DOI] [PubMed] [Google Scholar]
- 168.Ballotta VD-MA, Bouten CV, Baaijens FP. Strain-dependent modulation of macrophage polarization within scaffolds. Biomaterials. 2014;35:4919–4928. [DOI] [PubMed] [Google Scholar]
- 169.Fahy NMU, Alini M, Stoddart MJ. Shear and dynamic compression modulates the inflammatory phenotype of human monocytes in vitro. Front Immunol. 2019;10:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Purkayastha PJM, Lele TP. Molecular cancer cell responses to solid compressive stress and interstitial fluid pressure. Cytoskeleton (Hoboken). 2021;78:312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shah ADBM, Shieh AC. Interstitial fluid flow increases hepatocellular carcinoma cell invasion through CXCR4/CXCL12 and MEK/ERK signaling. PLoS One. 2015;10:e0142337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yu HSY, Jin J, Zhang Y, Feng T, Liu X. Fluid shear stress regulates HepG2 cell migration though time-dependent integrin signaling cascade. Cell Adh Migr. 2018;12:56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lien SC, Lee PL, Wei SY, Chang MD, Chang JY, Chiu JJ. Mechanical regulation of cancer cell apoptosis and autophagy: roles of bone morphogenetic protein receptor, Smad1/5, and p38 MAPK. Biochim Biophys Acta. 2013;1833:3124–3133. [DOI] [PubMed] [Google Scholar]
- 174.Wang XZY, Feng T, Su G, He J, Gao W, Shen Y, et al. Fluid shear stress promotes autophagy in hepatocellular carcinoma cells. Int J Biol Sci. 2018;14:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yan ZSG, Gao W, He J, Shen Y, Zeng Y, Liu X. Fluid shear stress induces cell migration and invasion via activating autophagy in HepG2 cells. Cell Adh Migr. 2019;13:152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yu HHJ, Su G, Wang Y, Fang F, Yang W, Gu K, et al. Fluid shear stress activates YAP to promote epithelial-mesenchymal transition in hepatocellular carcinoma. Mol Oncol. 2021;15:3164–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dai Z, Peng Y, Mansy HA, Sandler RH, Royston TJ. A model of lung parenchyma stress relaxation using fractional viscoelasticity. Med Eng Phys. 2015;37:752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mandal K, Gong Z, Rylander A, Shenoy VB, Janmey PA. Opposite responses of normal hepatocytes and hepatocellular carcinoma cells to substrate viscoelasticity. Biomater Sci. 2020;8:1316–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Adebowale K, Gong Z, Hou JC, Wisdom KM, Garbett D, Lee HP, et al. Enhanced substrate stress relaxation promotes filopodia-mediated cell migration. Nat Mater. 2021;20:1290–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Charrier EE, Pogoda K, Wells RG, Janmey PA. Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nat Commun. 2018;9:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nam S, Gupta VK, Lee HP, Lee JY, Wisdom KM, Varma S, et al. Cell cycle progression in confining microenvironments is regulated by a growth-responsive TRPV4-PI3K/Akt-p27(Kip1) signaling axis. Sci Adv. 2019;5:eaaw6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wisdom KM, Adebowale K, Chang J, Lee JY, Nam S, Desai R, et al. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat Commun. 2018;9:4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fan W, Adebowale K, Vancza L, Li Y, Rabbi MF, Kunimoto K, et al. Matrix viscoelasticity promotes liver cancer progression in the pre-cirrhotic liver. Nature. 2024;626:635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vining KH, Marneth AE, Adu-Berchie K, Grolman JM, Tringides CM, Liu Y, et al. Mechanical checkpoint regulates monocyte differentiation in fibrotic niches. Nat Mater. 2022;21:939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kalashnikov N, Moraes C. Substrate viscoelasticity affects human macrophage morphology and phagocytosis. Soft Matter. 2023;19:2438–2445. [DOI] [PubMed] [Google Scholar]
- 186.Zhang WZX, Sun HC, Xiong YQ, Zhuang PY, Xu HX, Kong LQ, et al. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420–3430. [DOI] [PubMed] [Google Scholar]
- 187.Zhuang ZZY, Sun S, Li Q, Chen K, An C, Wang L, et al. Control of matrix stiffness using methacrylate-gelatin hydrogels for a macrophage-mediated inflammatory response. ACS Biomater Sci Eng. 2020;6:3091–3102. [DOI] [PubMed] [Google Scholar]
- 188.Sun SCY, Ren K, Quan M, Song Z, Zou H, Li D, et al. 8-bromo-7-methoxychrysin reversed M2 polarization of tumor-associated macrophages induced by liver cancer stem-like cells. Anticancer Agents Med Chem. 2017;17:286–293. [DOI] [PubMed] [Google Scholar]
- 189.Dolor ASFJ. Digesting a path forward: The utility of collagenase tumor treatment for improved drug delivery. Mol Pharm. 2018;15:2069–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Song YKJ, Choi EK, Kim J, Kim KM, Seo HR. TGF-β-independent CTGF induction regulates cell adhesion mediated drug resistance by increasing collagen I in HCC. Oncotarget. 2017;8:21650–21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yang MLJ, Wang F, Tian Z, Ma B, Li Z, Wang B, et al. Lysyl oxidase assists tumor initiating cells to enhance angiogenesis in hepatocellular carcinoma. Int J Oncol. 2019;54:1398–1408. [DOI] [PubMed] [Google Scholar]
- 192.Ninomiya GYS, Hayashi M, Takeda S, Suenaga M, Takami H, Kanda M, et al. Significance of Lysyl oxidase like 2 gene expression on the epithelial mesenchymal status of hepatocellular carcinoma. Oncol Rep. 2018;39:2664–2672. [DOI] [PubMed] [Google Scholar]
- 193.Jiang YZH, Wang J, Liu Y, Luo T, Hua H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J Hematol Oncol. 2022;15:34. [DOI] [PMC free article] [PubMed] [Google Scholar]