Abstract
BACKGROUND:
Female infants with congenital heart disease (CHD) face significantly higher postoperative mortality rates after adjusting for cardiac complexity. Sex differences in metabolic adaptation to cardiac stressors may be an early contributor to cardiac dysfunction. In adult diseases, hypoxic/ischemic cardiomyocytes undergo a cardioprotective metabolic shift from oxidative phosphorylation to glycolysis which appears to be regulated in a sexually dimorphic manner. We hypothesize sex differences in cardiac metabolism are present in cyanotic CHD and detectable as early as the infant period.
METHODS:
RNA sequencing was performed on blood samples (cyanotic CHD cases, n = 11; controls, n = 11) and analyzed using gene set enrichment analysis (GSEA). Global plasma metabolite profiling (UPLC-MS/MS) was performed using a larger representative cohort (cyanotic CHD, n = 27; non-cyanotic CHD, n = 11; unaffected controls, n = 12).
RESULTS:
Hallmark gene sets in glycolysis, fatty acid metabolism, and oxidative phosphorylation were significantly enriched in cyanotic CHD females compared to male counterparts, which was consistent with metabolomic differences between sexes. Minimal sex differences in metabolic pathways were observed in normoxic patients (both controls and non-cyanotic CHD cases).
CONCLUSION:
These observations suggest underlying differences in metabolic adaptation to chronic hypoxia between males and females with cyanotic CHD.
INTRODUCTION
Congenital heart diseases (CHD) are the most common type of birth defect worldwide, with the highest rates of birth defect-related morbidity and mortality.1 Over the past forty years, medical and surgical advancements have resulted in steady improvements in survival rates and long-term outcomes.2 And yet, postoperative morbidity and mortality for the most severe forms of CHD remains high with a one-year survival rate of ~75%.3 Overall, infant deaths make up half of all CHD mortality.4 In this high-risk age group, females with cyanotic CHDs have a 30–50% higher odds of mortality following cardiac surgery compared to their male counterparts after risk adjustment for CHD complexity and other attributable factors.5–7 Considering the high energy demands of the heart, metabolic response to chronic hypoxia may be different between males and females contributing to sex differences in mortality rates.
During normal fetal development, the placental blood supply to the fetus is relatively hypoxic. Hypoxia upregulates transcription factor hypoxia-inducible factor 1 subunit alpha (HIF-1α), driving anaerobic glycolysis to provide the main source of energy for the heart and promoting cardiomyocyte proliferation.8–11 At the birth of unaffected newborns, delivery into an oxygen-rich environment inactivates HIF-1α and activates transcription factor peroxisome proliferator activated receptor alpha (PPARα), triggering a gradual metabolic shift from glycolysis to oxygen-dependent oxidative phosphorylation.8,12–14 In contrast, neonates with cyanotic CHD remain chronically hypoxic after birth, but the newborn heart is tolerant to oxygen deprivation likely due to an increased capacity for glycolysis.14,15 Adult studies have shown the counterbalancing roles of HIF-1α and PPARα also regulate metabolic shifts triggered by cardiac stressors such as hypoxia/ischemic injury, ventricular hypertrophy, and heart failure, reverting back to glycolysis as a form of cardioprotection.12,16–19 Impaired or inappropriate metabolic regulation results in cardiac dysfunction and appears to be a key driver in sex-dependent heart failure.18,20–24
Compared to acquired adult diseases, less is known on the early metabolic impact of hypoxia exposure in CHD. Prior studies involving infants with CHD have sought to detect global differences in metabolite levels related to cardiopulmonary bypass during surgery, identifying biomarkers associated with clinical outcomes.25–29 However, dedicated pediatric studies remain limited and sex differences remain relatively unexplored. Our study objective is to investigate sex differences in gene expression and metabolic profiles of infants with cyanotic CHD along key metabolic pathways utilized by cardiomyocytes. We hypothesize that there is a sexually dimorphic metabolic adaptation to chronic hypoxia in cyanotic CHD cases, providing insight into the biological mechanisms that may contribute to early infant mortality in females.
METHODS
Patient sample collection
Patients were enrolled into biobank programs at two separate institutions. In Houston, Texas, newborns admitted to the neonatal intensive care unit (NICU) were enrolled into the UTHealth Houston BioRepository for NICU Diseases with parental consent from January 2020 to December 2021. A small volume of blood (1 mL) was collected in EDTA-coated tubes and tubes containing RNA stabilizer in the first month of the NICU hospitalization and prior to any cardiac surgery for CHD cases. EDTA tubes were centrifuged within six hours of collection at 1850 g for 10 min at 4 °C. Approximately 100 to 200 μL of plasma was extracted without disturbing the buffy coat and stored at −80 °C. Blood samples in tubes containing RNA stabilizer were stored unprocessed at −80 °C for subsequent use. In Jackson, Mississippi, pediatric patients undergoing cardiac surgery at the University of Mississippi Medical Center (UMMC) were enrolled into the UMMC Biobank with parental consent from January 2013 to December 2017. Blood samples were collected in the operating room prior to surgical incision; no blood samples were stored in tubes containing RNA stabilizer. Similar processing and storage procedures for plasma were used at UMMC. The age of the patients at the time of collection was influenced by the biobank protocol at each participating institution. The timing and frequency of blood collection was limited to minimize extraneous needle sticks and iatrogenic blood loss. Samples at UMMC were collected prior to cardiac surgery and so were influenced by the timing of surgery. See other patient characteristics and cardiac phenotypes in Supplementary Table 1. Informed patient consent was obtained for study participants, and the study received approval from the Institutional Review Board at the University of Texas Health Science Center at Houston.
Patients from both institutions were selected and divided into three groups based on cardiac phenotype: cyanotic CHD, non-cyanotic (normoxic) CHD, and normoxic controls without CHD (Fig. 1). Inclusion criteria for control patients were the absence of cardiopulmonary anomalies, gestational age greater than 32 weeks, and absent or minimal respiratory support, as defined as non-invasive respiratory support with fractional inspired oxygen (FiO2) of 21% (room air) with normal oxygen saturations. Exclusion criteria were mechanical ventilation or requirement of supplementary oxygen (FiO2 > 21%).
Fig. 1. Comparison of differential expression of genes between cyanotic CHD females vs. males.
Cohort groups for (a) blood transcriptomics and (b) plasma metabolomics studies. Created with BioRender.com.
Bulk RNA sequencing and data analysis
Cyanotic CHD cases (n = 11) and controls without CHD (n = 11) were chosen as comparative cohorts for the blood transcriptomics study as they were the most phenotypically distinct and similar in age. RNA-stabilized samples were thawed and incubated for a minimum of 2 h at room temperature to allow for complete lysis of blood cells. Total RNA extraction was performed using RNeasy® blood kits in accordance with the manufacturer’s protocol (Qiagen, Germantown, Maryland). RNA samples were submitted to the Cancer Genomics Center at The University of Texas Health Science Center at Houston. Total RNA was quality-checked using Agilent RNA 6000 Pico kit (#5067–1513) by Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, California). RNA with an integrity number of greater than 7 was used for library preparation. Libraries were prepared with New England Biolabs’s NEBNext Ploy(A) mRNA Magnetic Isolation Module (E7490L), NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (E7760L), and NEBNext Multiplex Oligos for Illumina (E6609S) by following the manufacturer’s instructions. The quality of the final libraries was examined using Agilent High Sensitive DNA Kit (#5067–4626) by Agilent Bioanalyzer 2100, and the library concentrations were determined by qPCR using Collibri Library Quantification kit (#A38524500, Thermo Fisher Scientific, Waltham, Massachusetts). The libraries were pooled evenly and underwent paired-end 75-cycle sequencing on an Illumina NextSeq 550 System using High Output Kit v2.5 (#20024907, Illumina, San Diego, California).
Raw mRNA sequence reads were processed using Cutadatp and bases with quality scores less than 20 were removed.30 Filtered RNA-sequence reads were aligned to the human reference genome (GRCh38.102) and gene read counts were generated by STAR.31 Genes with less than 5 reads among the samples were removed. The differential gene expression analysis was conducted by DESeq2, which models the read counts to follow the negative binomial distribution.32 Differentially expressed genes (DEG) were defined as genes with a fold change greater than 1.2 (log2 fold change > 0.26) and p < 0.05 after adjusting for multiple comparisons. Functional enrichment was analyzed by gene set enrichment analysis (GSEA) to compare gene expression levels to an a priori established gene set.33,34 GSEA was utilized to compare gene expression between females and males (cyanotic CHD patients analyzed separately from controls). The magnitude of enrichment was quantified by normalized enrichment score (NES) with significance determined by |NES | > 1, nominal p < 0.05, and q < 0.25. The comparison was performed using the Molecular Signatures Database (MSigDB) Hallmark gene set35 as a reference, which highlights well-characterized biological states and processes by summarizing multiple gene sets and eliminating overlapping genes.
Global plasma metabolite profiling and data analysis
Cyanotic CHD patients (n = 27), non-cyanotic CHD (n = 11), and normoxic controls without CHD (n = 12) were included in the plasma metabolomics study. Plasma samples were thawed on ice, aliquoted, and shipped on dry ice to Metabolon (Metabolon, Morrisville, North Carolina). Following receipt, all samples were maintained at −80 °C until processed per standardized protocols and quality control measures using the automated MicroLab STAR® system (Hamilton Company, Reno, Nevada). The extract was divided into five fractions: two for analysis by two separate reverse phrase (RP)/ultra-performance liquid chromatography (UPLC)- mass spectrometry (MS)/MS methods with positive ion mode electrospray ionization (ESI), one for analysis by RP/UPLC-MS/MS with negative ion mode ESI, one for analysis by hydrophilic interaction chromatography (HILIC)/UPLC-MS/MS with negative ion mode ESI, and one sample was reserved for backup. Instrument variability was determined by calculating the median relative standard deviation for the standards added to each sample. Process variability was determined by calculating the median relative standard deviation for all endogenous metabolites present in 100% of the pooled matrix samples.
Raw data was extracted, peak-identified, and processed for quality control. Compounds were identified by comparison to library entries of purified standards or recurrent unknown entities. A total of 1012 compounds of known identity were included in the analysis. Biochemical identifications were based on three criteria: retention index within a narrow window of the proposed biochemical identification, accurate mass match to the library +/− 10 ppm, and the MS/MS forward and reverse scores between the experimental data and standards. Peaks were quantified using area-under-the-curve.
In the statistical analysis, Welch’s two-sample t-test was used to compare metabolite levels between the study groups. Threshold of statistical significance was p < 0.05. An estimate of the false discovery rate (q value) for multiple comparisons was set at q < 0.10 for statistical significance. Sparse partial least squares discriminant analysis (sPLS-DA) was performed to provide a comparative overview of the metabolomics data by study groups.
RESULTS
Gene expression in metabolic pathways
Gene expression was compared between sexes within each study cohort of cyanotic CHD cases and controls without CHD. This study design allowed for more precise examination of sex differences in hypoxic and normoxic conditions. Among cyanotic CHD cases, there were 655 differentially expressed genes between females and males (see volcano plot, Fig. 2a). As expected, the expression of genes located on sex chromosomes (e.g., RPS4Y1, EIF1AY, KDM5D, UTY, XIST) were differentially expressed between sexes in both study groups, confirming that expression of those genes is sex-determined in neonates.36 The top differentially-expressed genes in both study groups were present on sex chromosomes (Table 1). Eight other genes were differentially expressed only in cyanotic CHD patients, and one gene was differentially expressed exclusively in normoxic controls. Differential expression of target genes of HIF-1α were explored but none were statistically significant after adjusting for multiple comparisons (data not shown).
Fig. 2. Comparison of differential expression of genes between sexes.
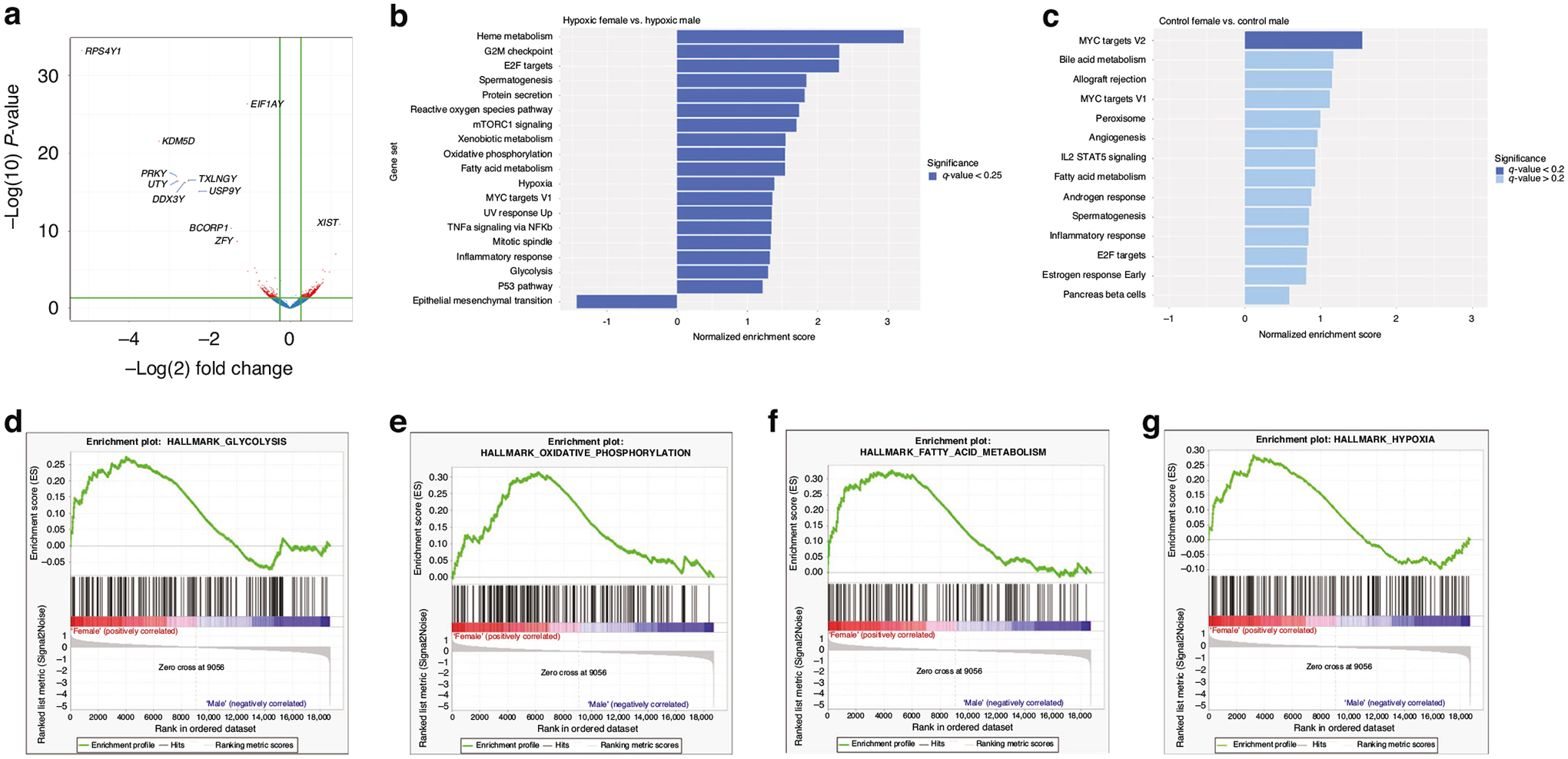
a Volcano plot comparing expression of genes between cyanotic CHD females vs. males. Threshold for significance (in red) of fold change >1.2, p < 0.05. b GSEA between cyanotic females vs. cyanotic males and (c) normoxic control females vs. normoxic control males using MSigDB Hallmark gene set ranked by normalized enrichment score (NES) with significance threshold of |NES | > 1, nominal p < 0.05. Enrichment plots between cyanotic females vs. cyanotic males of (d) Hallmark_glycolysis, (e) Hallmark_oxidative_phosphorylation, (f) Hallmark_fatty_acid_metabolism, and (g) Hallmark_hypoxia.
Table 1.
Differential expression of genes reported in log2 fold change (LFC) between sexes (F, females; M, males) within study groups of cyanotic congenital heart defects (CHD) and normoxic control patients.
| Symbol | Chromosome | Gene name | Cyanotic F:M | Control F:M | ||
|---|---|---|---|---|---|---|
| LFC | Padj value | LFC | Padj value | |||
| RPS4Y1 | chrY | ribosomal protein S4, Y-linked 1 | −5.15 | 1.14E-29 | −12.51 | 5.81E-44 |
| EIF1AY | chrY | eukaryotic translation initiation factor 1A, Y-linked | −1.06 | 4.03E-23 | −12.19 | 2.65E-44 |
| KDM5D | chrY | lysine demethylase 5D | −3.25 | 1.75E-18 | −11.58 | 6.68E-23 |
| PRKY | chrY | protein kinase, Y-linked, pseudogene | −2.81 | 4.64E-14 | −11.00 | 5.46E-19 |
| UTY | chrY | ubiquitously transcribed tetratricopeptide repeat containing, Y-linked | −2.78 | 1.15E-13 | −10.98 | 1.33E-19 |
| TXLNGY | chrY | taxilin gamma pseudogene, Y-linked | −2.52 | 1.15E-13 | −11.07 | 5.46E-19 |
| DDX3Y | chrY | DEAD-box helicase 3, Y-linked | −2.60 | 1.48E-13 | −9.60 | 2.08E-22 |
| USP9Y | chrY | ubiquitin specific peptidase 9, Y-linked | −2.26 | 1.95E-12 | −8.14 | 3.60E-16 |
| XIST | chrX | X inactive specific transcript (non-protein coding) | 1.23 | 2.72E-08 | 8.31 | 3.52E-09 |
| ZFY | chrY | zinc finger protein, Y-linked | −1.31 | 3.59E-06 | −9.64 | 1.24E-12 |
| BCORP1 | chrY | BCL6 corepressor pseudogene 1 | −1.46 | 7.94E-08 | −8.28E-06 | 3.14E-07 |
| AC100801.1 | chr8 | long non-protein coding RNA | 1.14 | 0.0001 | −3.83E-07 | 0.9999 |
| TTTY14 | chrY | long non-protein coding RNA | −0.87 | 0.0092 | −2.37E-06 | 0.5363 |
| AC021713.1 | chr11 | long non-protein coding RNA | 0.81 | 0.0146 | 9.26E-08 | 0.9999 |
| IGHV4–34 | chr14 | immunoglobulin variable gene | −1.05 | 0.0188 | −1.20E-06 | 0.9999 |
| CA2 | chr8 | carbonic anhydrase 2 | 0.86 | 0.0198 | 7.91E-07 | 0.9999 |
| MICALL2 | chr7 | MICAL like 2 | 0.98 | 0.0213 | 1.02E-06 | 0.9999 |
| LINC00278 | chrY | long non-protein coding RNA | −0.83 | 0.0246 | −5.08E-06 | 1.28E-04 |
| OLR1 | chr12 | oxidized low density lipoprotein receptor 1 | 0.29 | 0.9998 | 2.56 | 0.0447 |
Threshold of statistical significance of fold change > 1.2 (or |LFC | > 0.26) and p < 0.05 after adjusting for multiple comparisons.
Bold indicates statistical significant of p < 0.05 adjusted for multiple comparisons.
Utilizing GSEA allows incorporation of enriched transcriptional events along signaling pathways as opposed to single gene events.33 Critical hypoxia-regulated transcriptional events involving HIF-1α and PPAR regulate glycolysis and fatty acid oxidation.37 In the comparison of cyanotic CHD females and cyanotic CHD males, GSEA identified 19 Hallmark gene sets that were significantly enriched (Fig. 2b). Key metabolic pathways were significantly overrepresented in cyanotic females including Hallmark_glycolysis (NES = 1.298, p = 0.031, q = 0.109); Hallmark_oxidative phosphorylation (NES = 1.543, p < 0.002, q = 0.024); Hallmark_fatty acid metabolism (NES = 1.543, p = 0.002, q = 0.022) (see enrichment plots in Fig. 2d–g). Additionally, expression of hypoxic-related genes was sexually dimorphic with Hallmark_hypoxia gene set overrepresented in cyanotic females (NES = 1.387, p = 0.007, q value 0.076). No metabolism gene sets were enriched by sex among normoxic controls without CHD (Fig. 2c).
Metabolic adaptation to hypoxia
A larger representative cohort was used for global plasma metabolomic profiling including cyanotic CHD, non-cyanotic CHD, and controls without CHD. To illustrate overall group effects, comprehensive sPLS-DA was performed involving the three study groups. The results of sPLS-DA revealed that non-cyanotic CHD patients exhibited the highest degree of clustering for normoxic groups and therefore served as the reference group for subsequent analysis (Fig. 3a). When cyanotic CHD cases were analyzed separately, there was distinct clustering of males and females (Fig. 3b). Random forest analysis was performed to identify top-ranking discriminating metabolites between study groups. A predictive accuracy of 75% was obtained compared to 33% expected by random chance alone. The top discriminating metabolites included markers of amino acid, carbohydrate, lipid, and nucleic acid metabolism (Fig. 3c). Among these, 5-oxoproline (or pyroglutamic acid), the end-product of glutathione metabolism,38 was the most discriminating metabolite between study groups. Aggregated cyanotic CHD cases had a 1.44-fold increase in 5-oxoproline when compared to non-cyanotic CHD cases (p = 0.0011, q = 0.0010). When disaggregated by sex, heightened levels of 5-oxoproline were predominantly observed in female cyanotic CHD cases by a 1.55-fold increase compared to female non-cyanotic CHD cases (p = 0.051, q = 0.0157). Conversely, no significant elevation in 5-oxoproline levels was noted in the male comparison groups (p = 0.1111, q = 0.0480).
Fig. 3. Overall group effects in plasma metabolic profiling between study groups.
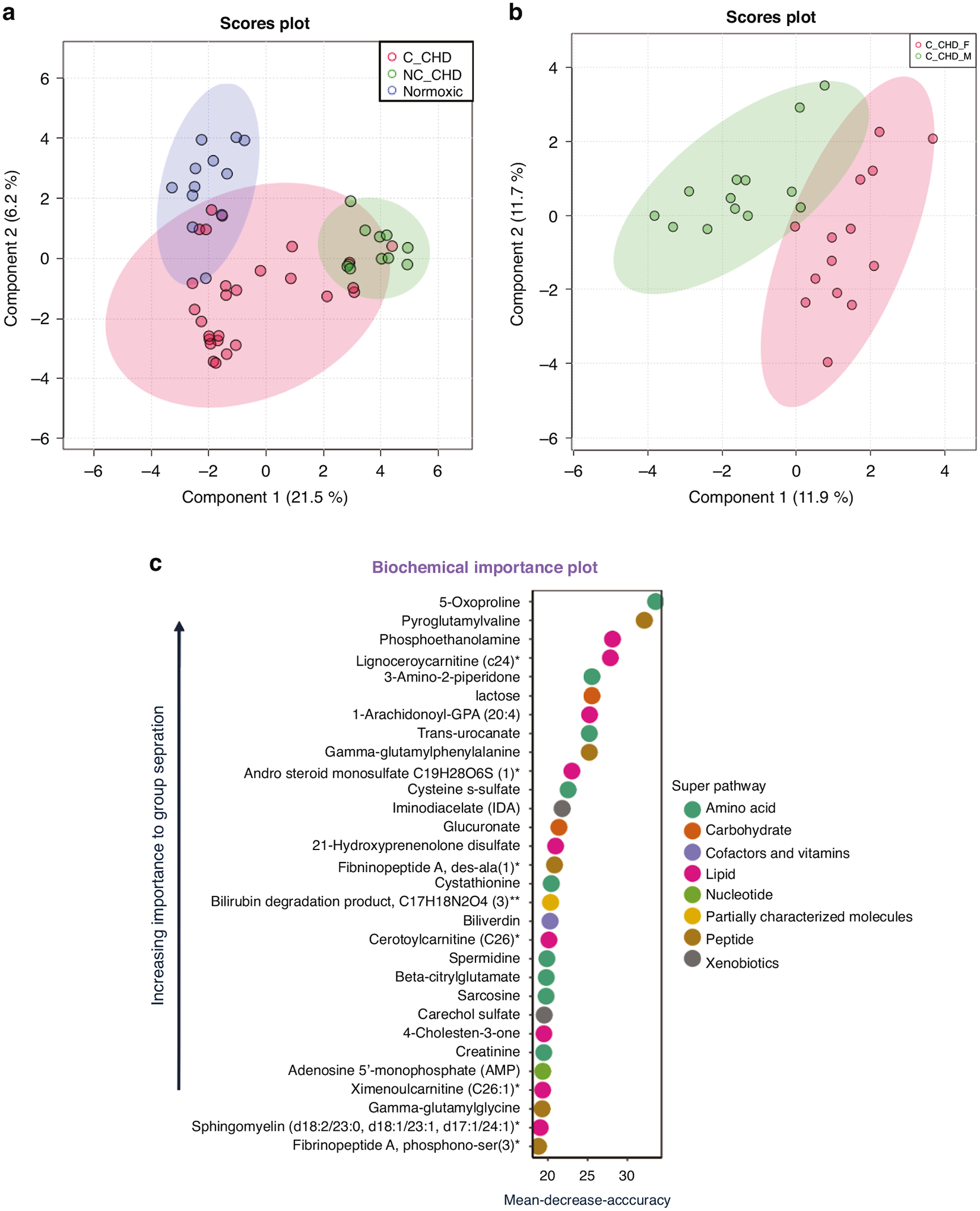
a Sparse partial least squares discriminant analysis plot of plasma metabolites grouped by study groups (normoxic control, non-cyanotic congenital heart defect (NC_CHD), and cyanotic CHD (C_CHD) patients). b Sparse partial least squares discriminant analysis plot of plasma metabolites grouped by cyanotic congenital heart defect (CDH) females and cyanotic CDH males. c Random forest results generated with normoxic control, non-cyanotic congenital heart defect (NC_CHD), and cyanotic CHD (C_CHD) patients.
Results of GSEA were used to inform the approach for comparative metabolic analysis between cyanotic CHD cases, non-cyanotic CHD cases, and controls without CHD. Each study group underwent separate analysis to pinpoint sex differences while addressing potential confounding variables such as differences in age and the presence/absence of CHD across groups. In normoxic patients (non-cyanotic CHD cases and controls without CHD), the metabolic profiles were similar between females and males. However, in cyanotic CHD cases, hypoxic females demonstrated nominally significant elevations in glycolytic intermediates (e.g., 2,3-diphosphoglycerate and 3-phophoglycerate) and long-chain acylcarnitines compared to hypoxic males (Supplementary Table 2A). These differences suggested increased activity in glycolysis and fatty acid oxidation in female cyanotic CHD cases compared to males.
To further test our hypothesis, hypoxic patients were directly compared against normoxic patients with non-cyanotic CHD cases serving as the reference group. In the aggregated data, cyanotic CHD cases exhibited increased levels of lactate (fold change 1.32, p = 0.0113, q = 0.0073), the final by-product of glycolysis,39 suggesting increased glycolytic activity in the presence of hypoxia which was an expected result. Along the citric acid cycle, early intermediates (e.g., citrate, isocitrate) were decreased but downstream intermediates were significantly increased, likely through a compensatory pathway.40 Levels of long-chain acylcarnitines, markers of fatty acid oxidation activity,41,42 were significantly reduced in cyanotic CHD cases (e.g., stearoylcarnitine, fold change 0.77, p = 0.0045, q = 0.0034). Overall, a metabolic shift away from fatty acid oxidation towards glycolysis was observed in cyanotic CHD cases compared to the reference group but the data was confounded by age differences limiting our interpretation (Supplementary Table 2B). However, these findings are consistent with the anticipated metabolic responses attributed to varying levels of oxygen. As a result, they provide a solid basis for deeper exploration of sex differences between these two groups.
In the sex disaggregated comparative analysis, male cyanotic CHD cases were compared with male non-cyanotic CHD cases as the reference group. Similarly, female cyanotic CHD cases were compared with female non-cyanotic CHD cases. If findings in the sex disaggregated data were similar to the aggregated data, this would suggest our observations were due to age differences alone and sex was unlikely a contributing factor. However, intermediates in glycolysis, citric acid cycle, and fatty acid oxidation were more disparate when analyzed by sex. In the aggregated dataset, glycolytic intermediates were mostly similar between cyanotic CHD cases and the reference group. Upon closer examination by sex, heightened levels of glycolytic intermediates were predominantly observed in female cyanotic CHD cases compared to female non-cyanotic CHD cases, including a significant 1.5-fold increase in lactate compared to female non-cyanotic CHD cases (p = 0.0005, q = 0.0075). In contrast, no significant elevation in lactate was seen in the male comparison groups (Fig. 4c). Evaluation of mitochondrial oxidation revealed that suppression of early intermediates in the citric acid cycle was only observed in male cyanotic CHD cases (p = 0.0188, q = 0.0128); there was no difference in the female comparison groups (p = 0.5077, q = 0.3074; Fig. 4d). Downstream citric cycle intermediates were significantly increased in both sexes, likely from a downstream compensatory pathway. Similarly for fatty acid oxidation, significant reductions in long-chain acylcarnitines were observed in the male comparison groups with no difference in the female comparison groups (Fig. 4h). Overall, these findings further confirm a sexually dimorphic response in glycolysis and mitochondrial oxidation in neonates exposed to chronic hypoxia (Fig. 5).
Fig. 4. Normalized metabolite peak area levels among study groups of cyanotic congenital heart defect (C-CHD) and non-cyanotic CHD (NC-CHD) by sex (F, female; M, male).
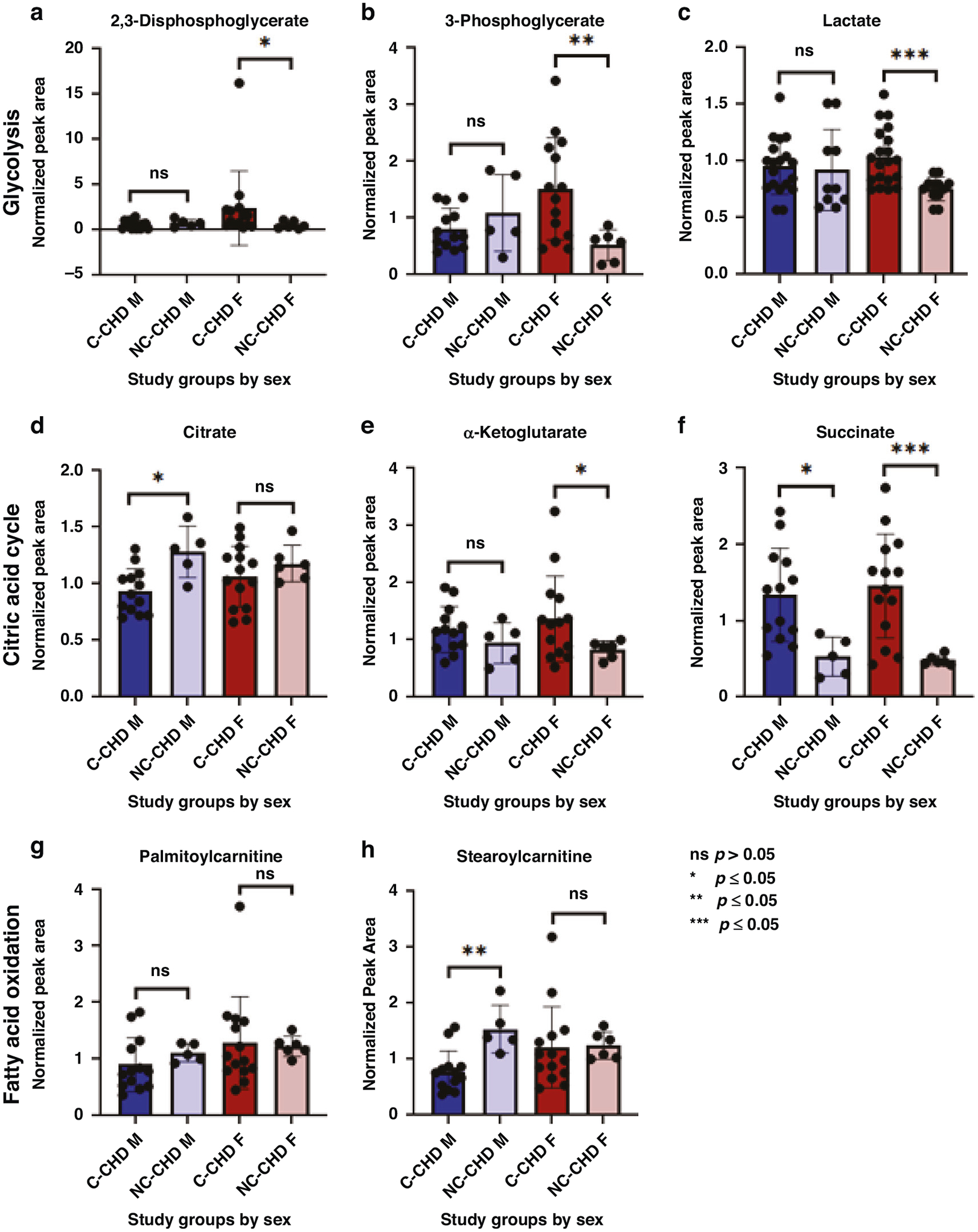
Intermediates in glycolysis are represented by (a) 2,3-diphosphoglycerate, (b) 3-phosphoglycerate, and (c) lactate; citric acid cycle are represented by (d) citrate, (e) α-ketoglutarate, and (f) succinate; fatty acid oxidation are represented by (g) palmitoylcarnitine (C16) and (h) stearoylcarnitine (C18).
Fig. 5. Comparison of metabolic levels in fold of change (FC) between cyanotic and non-cyanotic congenital heart defect patients by sex (F, female; M, male) in interrelated metabolic pathways (glycolysis, citric acid or TCA cycle, fatty acid oxidation, and glutamine metabolism).
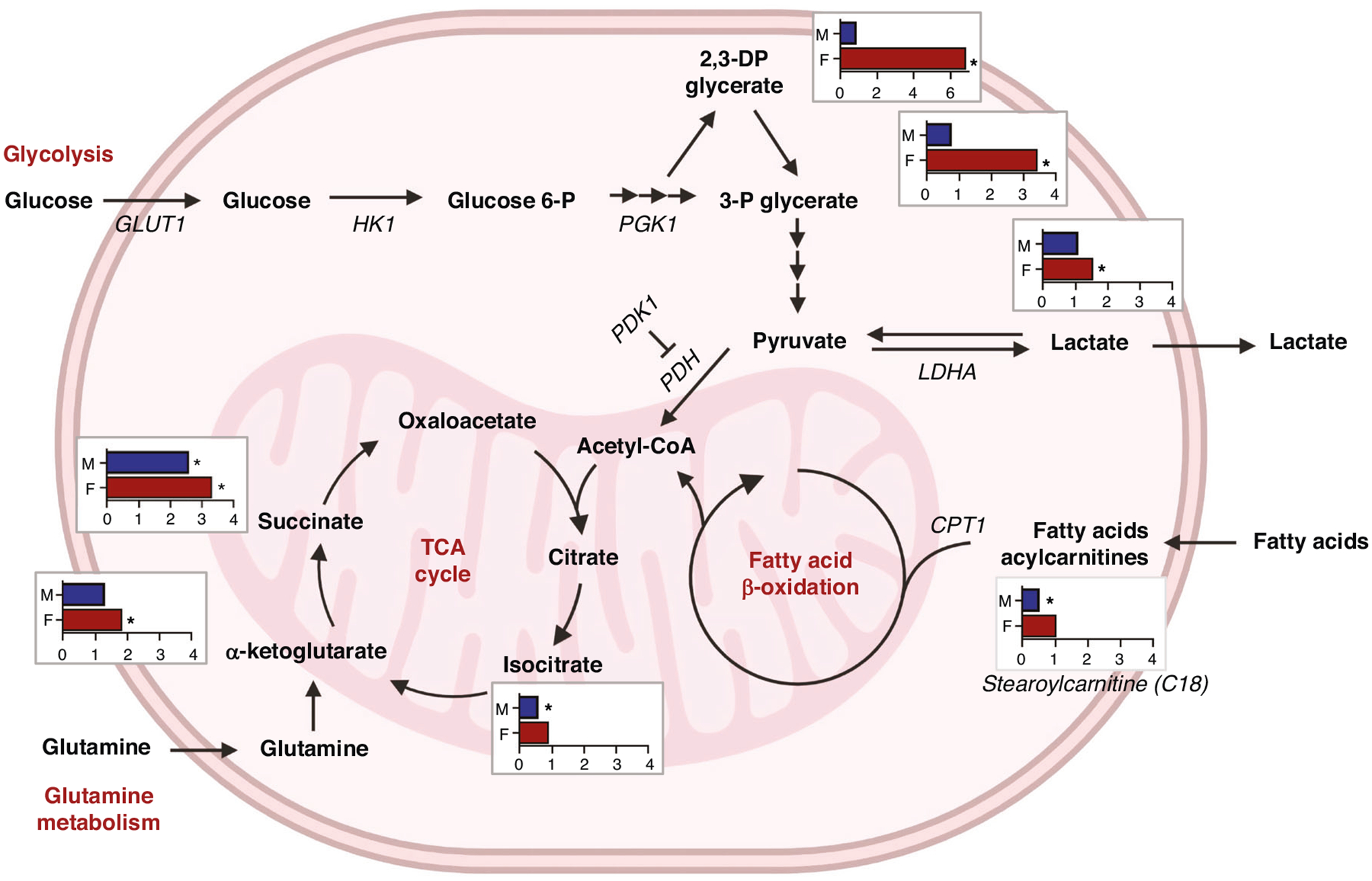
FC of 1 indicates no difference; * indicates p < 0.05. Metabolites: glucose 6-P, glucose 6-phosphate; 2,3-DP glycerate, 2,3-diphosphoglycerate; 3-P glycerate, 3-phosphoglycerate. Gene names: GLUT1, glucose transporter 1; HK1, hexokinase 1; PGK1, phosphoglycerate kinase; LDHA, lactate dehydrogenase A; PDK1, pyruvate dehydrogenase kinase 1; PDH, pyruvate dehydrogenase complex; CPT1, carnitine palmitoyltransferase. Created with BioRender.com.
DISCUSSION
Chronic hypoxia triggers a cascade of cellular mechanisms leading to a metabolic shift towards alternative sources of energy for cardioprotection.12,43,44 Sex differences in metabolic responses to both acute and chronic hypoxia have been observed in animal models and adult diseases.18,20,45–48 However, this remains understudied in children with CHD even though female sex is an independent risk factor for mortality after adjusting for age and surgical complexity.5 Further investigation is warranted in this fragile patient population. Our study demonstrates that a sexually dimorphic metabolic change occurs in neonates with cyanotic CHD, likely attributable to chronic hypoxia. Females with cyanotic CHD exhibit augmented transcriptomic and metabolomic fatty acid and oxidative phosphorylation activity compared to their male counterparts. Suppression of mitochondrial oxidative activity is a known cardioprotective response in the setting of oxygen deprivation.12,17,18 Somewhat unexpectedly, hypoxic females also showed increased expression of hypoxia-related genes and glycolytic activity.45 Although differential expression of individual target genes of HIF-1α were similar between sexes, expression of the Hallmark_hypoxia gene set representing 200 genes targeted by HIF-1α was upregulated in females with cyanotic CHD.35 Analyzing the collective expression pattern of gene sets can amplify coordinated gene changes compared to more subtle changes that may be missed when analyzing genes separately. While HIF and PPAR may appear to be in opposition, in postnatal cardiac mitochondria, PPAR gamma co-activator 1 (PGC1α) expression does not depend on downregulation of HIF, but instead on oxygen levels.49 We speculate that females with cyanotic CHD may have inappropriate responses to hypoxia that underlie these observed differences. Differences in energy capacity and utilization in the neonatal myocardium continues to be explored, such as whether increased glycolytic activity in neonatal females indicates early exhaustion of glycogen stores.45,50,12,17,18 Overall, these findings are consistent with animal studies indicating females may be at a metabolic disadvantage to ischemic/hypoxic injury with higher rates of cardiac dysfunction and ultimately, ventricular failure.22,45,51 Possible mechanisms include the PPARα/estrogen signaling pathway which has been shown to be differentially regulated by sex and implicated in the pathophysiology of heart failure.20,23,24,52–54
Pediatric studies on metabolic effects of CHD have been more limited and will be reviewed here. Mitochondrial dysfunction and oxidative stress were found to be important contributors to severe heart failure in iPSC-derived cardiomyocytes in hypoplastic left heart syndrome, a severe form of cyanotic CHD.55 In this study by Xu et al., groups were defined by severity of heart failure. However, females were only represented in the group of the most severe phenotype and excluded in the single-cell transcriptome profiling of iPSC-derived cardiomyocytes, limiting analysis of sex-related factors. Other metabolic effects of hypoxia on pediatric cardiomyocytes have been examined from discarded tissue excised during cardiac repair.56–58 Of note, these studies lack unaffected controls as tissue from normal, healthy hearts is extremely difficult to obtain due to ethical and practical constraints. One study involving patients with cyanotic CHD (n = 25) demonstrated that at the onset of puberty, suppression of HIF-1α in cardiac tissue co-occurred with metabolic maladaptation and impaired cardiac function.57 Others studies involving infants with CHD have utilized serum or plasma metabolites as a more feasible, non-invasive alternative.25,28,29,59 Differentially expressed genes in oxidative phosphorylation, citric acid cycle, and fatty acid metabolism, in blood obtained from HLHS neonates were associated with post-operative low cardiac output syndrome (n = 13), but the effect of sex was not explored.60 Differences in metabolite panels (e.g., targeted vs. untargeted LC-MS panels) hinders grouped interpretation of multiple studies, nonetheless, glutathione metabolism was identified as a top discriminating pathways in another study comparing cyanotic and non-cyanotic CHD infants, consistent with our findings.28 In these studies including ours, total metabolite concentrations do not accurately represent metabolite flux, limiting the interpretation of metabolic activity along interrelated pathways, including intracellular redox homeostasis. However, our findings indicate oxidative stress is handled differently between cyanotic males and females, potentially bearing implications for cell survival mechanisms.
Our study is limited to the use of blood samples which may not directly reflect the metabolic activity of the heart. However, plasma shares a similar profile with cardiac biochemistry while being minimally invasive,42,61 permitting the inclusion of unaffected controls in our study. Another study limitation was that age differences were not balanced across the study groups introducing a potential confounder as the duration of hypoxia could influence results. This is a common issue in studies involving blood and tissue collected at the time of cardiac surgery since children with non-cyanotic CHD often have cardiac surgery performed at an older age.28 Our approach to first compare transcriptomic data between sexes confined to each study group was performed to abate potential age-related confounders. As a result, non-cyanotic CHD patients were not included in the transcriptomic analysis. While this is a notable study limitation, our primary objective was to elucidate potential pathways of interest specific to hypoxia and cyanotic CHD. Focusing the scope to two relatively homogeneous groups (cyanotic CHD and unaffected controls) avoided potential confounders related to the wider phenotypic variability in non-cyanotic CHD. Lastly, the small sample size posed a limitation to the robustness and generalizability of our findings. To mitigate the risk of spurious results from multiple comparisons, stringent correction methods using FDR adjustment was used, ultimately influencing the significance of the results.
This study presents evidence that genetic expression and metabolic activity in circulating blood undergo alterations in the setting of chronic hypoxia from cyanotic CHD. Furthermore, these alterations occur in a sexually dimorphic manner. Based on the known cardioprotective and diverse cellular functions driven by hypoxia, glycolysis and mitochondrial oxidation appear to be key distinguishing metabolic pathways between males and females with cyanotic CHD. Larger-scale metabolomic and transcriptomic sex-disaggregated studies involving pediatric patients with CHD are needed to confirm findings. Importantly, our findings suggest alternative metabolic pathways are utilized in neonates with chronically hypoxic conditions.
Supplementary Material
IMPACT:
Children with cyanotic CHD exhibit sex differences in utilization of glycolysis vs. fatty acid oxidation pathways to meet the high-energy demands of the heart in the neonatal period.
Transcriptomic and metabolomic results suggest that under hypoxic conditions, males and females undergo metabolic shifts that are sexually dimorphic. These sex differences were not observed in neonates in normoxic conditions (i.e., non-cyanotic CHD and unaffected controls).
The involved metabolic pathways are similar to those observed in advanced heart failure, suggesting metabolic adaptations beginning in the neonatal period may contribute to sex differences in infant survival.
ACKNOWLEDGEMENTS
We thank the technical support from the Cancer Prevention and Research Institute of Texas (CPRIT RP180734). This work was supported by NIH/NCATS grants UL1 TR000445 and UL1 TR001105. Study data were collected and managed using REDCap electronic data capture tools hosted at The University of Texas School of Biomedical Informatics (SBMI) at Houston.62 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources. T.O.F. was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through UTHealth-CCTS Grant Number UL1TR003167 and by 5KL2TR003168-02. A.C.P. was supported by NIH K01HL159032, R01HL148191, and U54GM115428. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
COMPETING INTERESTS
The authors declare no competing interests
INFORMED CONSENT
Parental permission was obtained for patient participation for each respective institutional biorepository.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41390-024-03291-4.
DATA AVAILABILITY
The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE269353 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE269353).
REFERENCES
- 1.Liu Y et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int. J. Epidemiol 48, 455–463, 10.1093/ije/dyz009 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohye RG, Schranz D & D’Udekem Y Current therapy for hypoplastic left heart syndrome and related single ventricle lesions. Circulation 134, 1265–1279, 10.1161/CIRCULATIONAHA.116.022816 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oster ME et al. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics 131, e1502–e1508, 10.1542/peds.2012-3435 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilboa SM, Salemi JL, Nembhard WN, Fixler DE & Correa A Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation 122, 2254–2263, 10.1161/CIRCULATIONAHA.110.947002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochilas LK, Vinocur JM & Menk JS Age-dependent sex effects on outcomes after pediatric cardiac surgery. J. Am. Heart Assoc 3, e000608, 10.1161/JAHA.113.000608 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang RKR, Chen AY & Klitzner TS Female sex as a risk factor for in-hospital mortality among children undergoing cardiac surgery. Circulation 106, 1514–1522, 10.1161/01.CIR.0000029104.94858.6F (2002). [DOI] [PubMed] [Google Scholar]
- 7.Seifert HA, Howard DL, Silber JH & Jobes DR Female gender increases the risk of death during hospitalization for pediatric cardiac surgery. J. Thorac. Cardiovasc. Surg 133, 668–675, 10.1016/j.jtcvs.2006.11.014 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Puente BN et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157, 565–579, 10.1016/j.cell.2014.03.032 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza GL, Roth PH, Fang HM & Wang GL Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem 269, 23757–23763, 10.1016/S0021-9258(17)31580-6 (1994). [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase a gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem 271, 32529–32537, 10.1074/JBC.271.51.32529 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Wang GL, Jiang BH, Rue EA & Semenza GL Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl Acad. Sci. USA 92, 5510–5514, 10.1073/pnas.92.12.5510(1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karwi QG, Uddin GM, Ho KL & Lopaschuk GD Loss of metabolic flexibility in the failing heart. Front. Cardiovasc. Med 5, 68, 10.3389/fcvm.2018.00068 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JW, Ko J, Ju C & Eltzschig HK Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med 51, 1–13, 10.1038/s12276-019-0235-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopaschuk GD, Collins-Nakai RL & Itoi T Developmental changes in energy substrate use by the heart. Cardiovasc. Res 26, 1172–1180, 10.1093/cvr/26.12.1172 (1992). [DOI] [PubMed] [Google Scholar]
- 15.Eckersley LG et al. The perinatal transition and early neonatal period in hypoplastic left heart syndrome is associated with reduced systemic and cerebral perfusion. Can. J. Cardiol 37, 1923–1933, 10.1016/j.cjca.2021.07.002 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Neely JR & Morgan HE Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu. Rev. Physiol 36, 413–459, 10.1146/annurev.ph.36.030174.002213 (1974). [DOI] [PubMed] [Google Scholar]
- 17.Cole MA et al. On the pivotal role of PPARa in adaptation of the heart to hypoxia and why fat in the diet increases hypoxic injury. FASEB J 30, 2684–2697, 10.1096/fj.201500094R (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy E, Amanakis G, Fillmore N, Parks RJ & Sun J Sex differences in metabolic cardiomyopathy. Cardiovasc. Res 113, 370–377, 10.1093/cvr/cvx008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JW, Tchernyshyov I, Semenza GL & Dang CV HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3, 177–185, 10.1016/J.CMET.2006.02.002 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Naumenko N et al. PGC-1α deficiency reveals sex-specific links between cardiac energy metabolism and EC-coupling during development of heart failure in mice. Cardiovasc. Res 118, 1520–1534, 10.1093/cvr/cvab188(2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy E & Steenbergen C Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc. Res 75, 478–486, 10.1016/j.cardiores.2007.03.025 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Broderick TL & Glick B Effect of gender and fatty acids on ischemic recovery of contractile and pump function in the rat heart. Gend. Med 1, 86–99, 10.1016/s1550-8579(04)80014-7 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Wang T et al. Estrogen-related receptor α (ERRα) and ERRγ are essential coordinators of cardiac metabolism and function. Mol. Cell Biol 35, 1281–1298, 10.1128/MCB.01156-14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson PA et al. Cardiac-specific overexpression of dominant-negative CREB leads to increased mortality and mitochondrial dysfunction in female mice. Am. J. Physiol. Heart Circ. Physiol 299, 2056–2068, 10.1152/ajpheart.00394.2010.-Cardiac (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagano E et al. Alterations in metabolites associated with hypoxemia in neonates and infants with congenital heart disease. Congenit. Heart Dis 15, 251–265, 10.32604/CHD.2020.012219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Correia GDS et al. Metabolic profiling of children undergoing surgery for congenital heart disease. Crit. Care Med 43, 1467–1476, 10.1097/CCM.0000000000000982 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heibel J et al. Perioperative metabolites are associated with adverse neonatal congenital heart disease surgical outcomes. J. Am. Heart Assoc. J. Am. Heart Assoc 11, 24996, 10.1161/JAHA.121.024996 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davidson JA et al. Metabolomic fingerprinting of infants undergoing cardiopulmonary bypass: changes in metabolic pathways and association with mortality and cardiac intensive care unit length of stay. J. Am. Heart Assoc 7, e010711, 10.1161/JAHA.118.010711 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidson JA et al. Serum metabolic profile of postoperative acute kidney injury following infant cardiac surgery with cardiopulmonary bypass. Pediatr. Nephrol 36, 3259–3269, 10.1007/s00467-021-05095-8/Published (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin M Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17, 10–12, http://www-huber.embl.de/users/an-(2011). [Google Scholar]
- 31.Dobin A et al. Sequence analysis STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21, 10.1093/bioinformatics/bts635 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anders S & Huber W Differential expression analysis for sequence count data. Genome Biol 11, R106, 10.1186/gb-2010-11-10-r106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian A et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550, 10.1073/pnas.0506580102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mootha VK et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet 34, 267–273, 10.1038/ng1180 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Liberzon A et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 1, 417–425, 10.1016/j.cels.2015.12.004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi MW et al. SAGD: a comprehensive sex-associated gene database from transcriptomes. Nucleic Acids Res 47, D835–D840, 10.1093/nar/gky1040 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montaigne D, Butruille L & Staels B PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol 18, 809–823, 10.1038/s41569-021-00569-6 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Palekar AG, Tate SS, Meister A Formation of 5-Oxoproline from Glutathione in Erythrocytes by the 7-Glutamyltranspeptidase-Cyclotransferase Pathway (Pyroglutamate/Pyrrolidone Carboxylate/7y-Glutamyl Cycle/-y-Glutamyl Cyclotransferase) Vol 71.; 1974. https://www.pnas.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogatzki MJ, Ferguson BS, Goodwin ML & Gladden LB Lactate is always the end product of glycolysis. Front. Neurosci 9, 22, 10.3389/fnins.2015.00022 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe K et al. Critical role of glutamine metabolism in cardiomyocytes under oxidative stress. Biochem. Biophys. Res. Commun 534, 687–693, 10.1016/j.bbrc.2020.11.018 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Angelini A et al. PHDs/CPT1B/VDAC1 axis regulates long-chain fatty acid oxidation in cardiomyocytes. Cell Rep 37, 109767, 10.1016/j.celrep.2021.109767 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makrecka-Kuka M et al. Plasma acylcarnitine concentrations reflect the acylcarnitine profile in cardiac tissues. Sci. Rep 7, 17528, 10.1038/s41598-017-17797-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnan J et al. Activation of a HIF1α-PPARγ axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab 9, 512–524, 10.1016/j.cmet.2009.05.005 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Fuhrmann DC et al. Chronic hypoxia enhances β-oxidation-dependent electron transport via electron transferring flavoproteins. Cells 8, 1–18, 10.3390/cells8020172 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wittnich C, Quaglietta D, Tan L & Belanger MP Sex differences in newborn myocardial metabolism and response to ischemia. Pediatr. Res 70, 148–152, 10.1203/PDR.0b013e3182218c6c (2011). [DOI] [PubMed] [Google Scholar]
- 46.Trexler CL, Odell AT, Jeong MY, Dowell RD & Leinwand LA Transcriptome and functional profile of cardiac myocytes is influenced by biological sex. Circ. Cardiovasc. Genet 10, e001770, 10.1161/CIRCGENETICS.117.001770 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ritterhoff J et al. Increasing fatty acid oxidation elicits a sex-dependent response in failing mouse hearts. J. Mol. Cell Cardiol 158, 1–10, 10.1016/j.yjmcc.2021.05.004 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith KLM et al. Chronic developmental hypoxia alters mitochondrial oxidative capacity and reactive oxygen species production in the fetal rat heart in a sex-dependent manner. J. Pineal Res 10.1111/jpi.12821 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neary MT et al. Hypoxia signaling controls postnatal changes in cardiac mitochondrial morphology and function. J. Mol. Cell Cardiol 74, 340–352, 10.1016/j.yjmcc.2014.06.013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan M et al. Glutathione system enhancement for cardiac protection: pharmacological options against oxidative stress and ferroptosis. Cell Death Dis 14, 131, 10.1038/s41419-023-05645-y (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ip WTK et al. Dietary omega-6 fatty acid replacement selectively impairs cardiac functional recovery after ischemia in female (but not male) rats. Am. J. Physiol. Heart Circ. Physiol 311, H768–H780, 10.1152/ajpheart.00690.2015 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Harrington J et al. A systems biology approach to investigating sex differences in cardiac hypertrophy. J. Am. Heart Assoc 6, e005838, 10.1161/JAHA.117.005838 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Djouadi F et al. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor α-deficient mice. J. Clin. Investig 102, 1083–1091, 10.1172/JCI3949 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sihag S, Cresci S, Li AY, Sucharov CC & Lehman JJ PGC-1α and ERRα target gene downregulation is a signature of the failing human heart. J. Mol. Cell Cardiol 46, 201–212, 10.1016/j.yjmcc.2008.10.025 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu X et al. Uncompensated mitochondrial oxidative stress underlies heart failure in an iPSC-derived model of congenital heart disease. Cell Stem Cell 29, 840–855.e7, 10.1016/j.stem.2022.03.003 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong S et al. Metabolic profile of heart tissue in cyanotic congenital heart disease. Am. J. Transl. Res 13, 4224–4232 (2021). [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y et al. Suppression of myocardial hypoxia-inducible factor-1α compromises metabolic adaptation and impairs cardiac function in patients with cyanotic congenital heart disease during puberty. Circulation 143, 2254–2272, 10.1161/CIRCULATIONAHA.120.051937 (2021). [DOI] [PubMed] [Google Scholar]
- 58.Liu J et al. Metabolic variation dictates cardiac pathogenesis in patients with tetralogy of fallot. Front. Pediatr 9, 819195, 10.3389/fped.2021.819195 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Najm HK et al. Does the degree of cyanosis affect myocardial adenosine triphosphate levels and function in children undergoing surgical procedures for congenital heart disease? J. Thorac. Cardiovasc. Surg 119, 515–524, 10.1067/mtc.2000.104339 (2000). [DOI] [PubMed] [Google Scholar]
- 60.Jain PN et al. Altered metabolic and inflammatory transcriptomics after cardiac surgery in neonates with congenital heart disease. Sci. Rep 11, 4965, 10.1038/s41598-021-83882-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruder ED & Raff H Cardiac and plasma lipid profiles in response to acute hypoxia in neonatal and young adult rats. Lipids Health Dis 9, 3, 10.1186/1476-511X-9-3 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harris PA et al. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatic support. J. Biomed. Inf 42, 377–381, 10.1016/j.jbi.2008.08.010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE269353 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE269353).


