Abstract
The intricate interplay between the gut microbiome and colorectal cancer (CRC) presents novel avenues for early diagnosis and prognosis, crucial for improving patient outcomes. This comprehensive review synthesizes current findings on the gut microbiome’s contribution to CRC pathogenesis, highlighting its potential as a biomarker for non-invasive CRC screening strategies. We explore the mechanisms through which the microbiome influences CRC, including its roles in inflammation, metabolism, and immune response modulation. Furthermore, we assess the viability of microbial signatures as predictive tools for CRC prognosis, offering insights into personalized treatment approaches. Our analysis underscores the necessity for advanced metagenomic studies to elucidate the complex microbiome-CRC nexus, aiming to refine diagnostic accuracy and prognostic assessment in clinical settings. This review propels forward the understanding of the microbiome’s diagnostic and prognostic capabilities, paving the way for microbiome-based interventions in CRC management.
Keywords: colorectal cancer, gut microbiome, disease detection, disease progression, health research
1. Introduction
Colorectal cancer (CRC) is a significant health challenge worldwide and is a leading cause of cancer-related deaths globally (1, 2). According to a study, the link between gut microbiota and CRC seems to be primarily present in the white population, and this connection varies among different races. Certain bacteria are significantly associated with CRC in white women, while the link between Bacillus and CRC is most prominent in black women (3). The development of the CRC condition is influenced by genetic predisposition, lifestyle factors like physical inactivity and smoking, dietary habits such as the consumption of red and processed meats and alcohol, and exposure to various environmental substances (4). Research has shown that chronic psychosocial stress may increase inflammation, disrupt the gut microbiome, and increase the risk of early-onset colorectal cancer (5). All of these factors contribute to the complex nature of the condition. Despite advances in screening and treatment, early detection and accurate prognosis are crucial for improving survival rates. Recent studies have shown that the gut microbiome plays a pivotal role in CRC pathogenesis, indicating its potential for developing non-invasive diagnostic and prognostic tool (6).
The gut microbiome is a complex group of microorganisms that reside in the human gastrointestinal tract. It has been increasingly recognized for its impact on host health and disease development, including colorectal cancer. Studies have shown that an imbalance of gut microbiota, known as microbial dysbiosis, is linked to the initiation, progression, and treatment response of CRC (7). This review aims to explore the current understanding of the gut microbiome’s role in CRC, with a focus on how it can improve diagnostic accuracy and prognostic assessment.
There is mounting evidence to suggest that the gut microbiota plays a crucial role in the initiation, progression, and metastasis of CRC (8–10). In terms of gene count, the human gut microbiota surpasses the human genome, despite the vast array of microorganisms present (11, 12). A healthy gut microbiota is essential for various critical roles, such as energy acquisition, defending against pathogens, shaping the intestinal epithelium, and maintaining immune function (13–16). Dysbiosis of the gut microbiota can disrupt host physiological functions, leading to various diseases (17, 18). The gut microbiota is also influential in several crucial physiological processes, including metabolism, immunity, and the production of nutritional substances (19).
Kostic et al. proposed a mechanism in which Fusobacterium nucleatum increases the concentration of immune cells that infiltrate tumors, leading to the establishment of an inflammatory environment that promotes the progression of CRC (20, 21). Wu et al. reported that enterotoxigenic Bacteroides fragilis (ETBF) secretes toxin BFT, which stimulates the translation and transcription of the proto-oncogene c-Myc, resulting in sustained cell proliferation (22). ETBF induces colitis, activates signaling transducer and activator of transcription 3 (STAT3), and T-helper 17 cell responses in multiple intestinal neoplasia (Min) mice with colon tumors (23). Researchers have identified cyclopropane rings in colibactin secreted by Escherichia coli, which may induce DNA alkylation in vivo, potentially contributing to the development of CRC (24). Rohani et al. demonstrated at the genetic level that E. coli K-12 can be considered an important probiotic that may reduce the risk of CRC development (25). There is limited research on the role of bacterial depletion in the development of intestinal tumors due to a lack of appropriate technologies. The absence of certain bacterial strains that are either protective or beneficial may be as crucial as the overgrowth of bacteria associated with tumors (26). The gut microbiota’s diversity, relative abundance, and distribution differ significantly between adenoma patients and healthy individuals (27). CRC patients have alterations in the abundance of bacteria such as Fusobacterium, Enterococcus, and Prevotella, which are associated with mucosal gene expression patterns and may contribute to CRC development. Studies have also shown an increased incidence of CRC in individuals with Salmonella enterica infection. The overall abundance of Enterobacteriaceae in CRC tissues exceeds that in normal tissues by more than 400 times (28).
There are different theories about the relationship between CRC and the gut microbiota. One of these theories is the alpha-bug hypothesis, which suggests that certain bacterial groups can cause cancer and change the bacterial community in a way that supports cancer growth (23, 29–32). Another theory is the CRC gene driver-passenger model, which divides microbiota into driver and passenger bacteria and explains differences in research findings (33, 34).
We analyze how gut microbiota contribute to CRC by examining the findings from recent metagenomic studies. We explore their effects on inflammation, immune modulation, and metabolic processes. Furthermore, we evaluate the potential of emerging microbiome-based biomarkers for CRC screening and prognosis. These biomarkers could complement existing diagnostic methods and facilitate personalized treatment strategies. By summarizing these advancements, we underscore the importance of microbiome research in the quest for better cancer management strategies. This introduction sets the stage for a detailed examination of the gut microbiome’s diagnostic and prognostic potential in CRC.
2. Deciphering the gut microbiome: toward early detection and precise prognosis in CRC
2.1. Underlying mechanisms: how the gut microbiome influences CRC development
2.1.1. Inflammation and immune
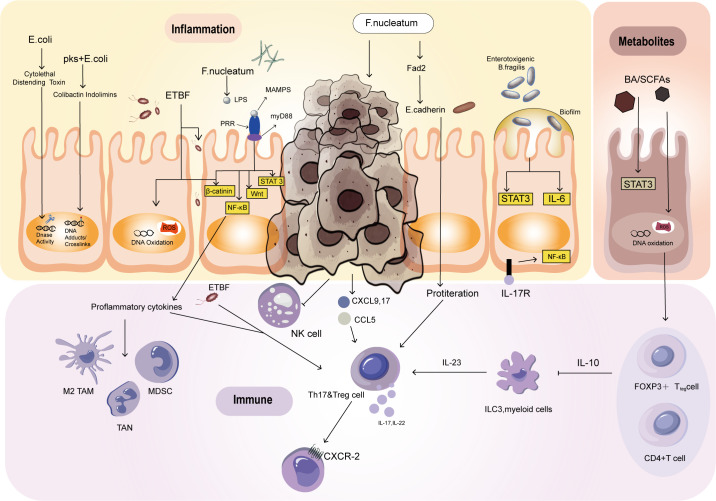
Colorectal cancer (CRC) and inflammatory bowel disease (IBD) are both characterized by ecological imbalances that can disrupt the mucosal barrier, leading to inflammation and the development of cancer. This ecological imbalance can create a positive feedback loop, which forms the foundation of the inflammation dysplasia cancer sequence (35). It is worth noting that the presence of IBD is often linked to an increased risk of CRC in younger people, but it is not a prerequisite for developing CRC (36–38). Inflammatory reactions can break down the barrier and perpetuate the process of inflammation by causing bacteria to migrate to the intestinal cavity and initiate immunity and inflammation. Inflammatory cells can promote the growth of CRC and induce maldevelopment. Under normal circumstances, epithelial cells reduce oxygen utilization to create an anaerobic environment. However, chronic inflammation leads to an increase in oxygen availability, creating an ecological imbalance that allows pathogenic bacteria such as E. coli to proliferate. These bacteria produce colibactin, a toxin that can destroy DNA and stimulate the growth of tumors. Colibactin induces DNA double-strand breaks by alkylating DNA bases, which triggers replication stress and promotes mutagenesis (39). This direct DNA damage can accumulate over time, ultimately driving tumorigenesis (40). In addition to being a direct DNA-damaging agent, colibactin is linked to inflammation: colibactin-induced DNA damage can activate inflammatory pathways, such as NF-κB, triggering the release of inflammatory cytokines (41). This sets off a feedback loop in which DNA damage triggers inflammation (42), and inflammation further promotes bacterial colonization and DNA damage (43). Over time, chronic inflammation and DNA damage synergistically drive CRC by enhancing cell proliferation and mutagenesis (44), and genomic instability (45). As a result, the inflammatory environment in the gut not only facilitates the colonization of colibactin-producing bacteria but also amplifies DNA damage, further driving cancer development. Moreover, ETBF produces a toxin that triggers inflammatory signaling, promoting the growth of colorectal cancer ( Figure 1 ) (35). The toxin known as Bacteroides fragilis toxin (BFT), also referred to as fragilysin (46). BFT is a metalloprotease that specifically targets the E-cadherin protein, which plays a critical role in maintaining tight junctions between epithelial cells (47). The toxin cleaves E-cadherin, thereby disrupting the epithelial barrier and resulting in increased intestinal permeability (48). This disruption triggers an inflammatory response by activating the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway (49). BFT’s activity can lead to diarrhea and inflammation, and it has been linked to the promotion of colorectal cancer due to its role in chronic inflammation and cellular alterations (50).
Figure 1.
Mechanisms by which the intestinal flora affects the development of CRC. Escherichia coli and ETBF can cause DNA damage. The development of colon tumors is closely associated with the activation of STAT3 and the Th17 response. BA, SCFAs, ETBF, and F. nucleatum can activate STAT3, promoting tumor initiation and growth. However, STAT3 activation alone is not sufficient to trigger colon tumors. Escherichia coli participates in tumor development through Th1 and Th17 immune responses, which induce inflammation. F. nucleatum inhibits NK cells by interacting with T-cell immune receptors. After activating STAT3, some bacteria lead to IL-17-dependent NF-kB activation, resulting in CXCR2 expression and promoting the recruitment of bone marrow cells. SCFAs produced through metabolism promote the differentiation of naive T cells into Th1 cells. BA induces oxidative DNA damage and activates NF-κB, affecting overall immune function.
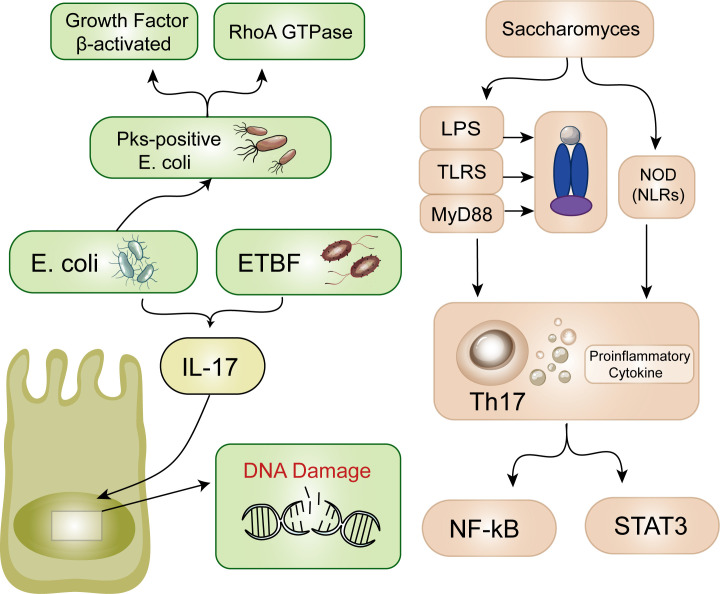
In patients with IBD, damage to the mucus layer allows bacteria to penetrate the mucosa, resulting in hyperplasia and inflammation (51). Inflammatory damage-induced mucosal disruption allows more bacteria to enter, forming a malignant positive feedback loop of antigen exposure and mucosal injury (52). ETBF is the most common anaerobic bacterium, and its presence is positively correlated with IBD and CRC (53). According to the research of Appunni et al., the cocolonization of toxigenic E. coli and ETBF in mice leads to increased generation of proinflammatory IL-17 followed by DNA damage, potentially accelerating CRC development ( Figure 2 ) (54). Currently, E. coli strains containing pks islands express colibactin genes, which have certain effects on the host’s DNA, genes, and chromosomes ( Figure 1 ) (55). This may be due to the association of E. coli effector proteins with the DNA mismatch repair system (56). Recent findings have also identified this pathogenic island in E. coli (57). Pks-positive E. coli promotes the development of cancer by activating transforming growth factor β-activated kinase 1 and RhoA GTPase (58). Minute amounts of Saccharomyces cerevisiae, a yeast, induce a Th-17 cell response and proinflammatory cytokine secretion via the activation of signaling pathways mediated by Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors. These receptors, which act through NF-κB or STAT3, serve as primary sensors for bacterial products and play crucial roles in the prevention of CRC and the inhibition of inflammatory processes ( Figure 2 ) (59). According to previous studies, Streptococcus bovis induces the release of inflammatory cytokines (TNF-α、IL-6、IL-1β、IL-8) in CRC cells, creating a pro-inflammatory environment that promotes tumor progression (60). Preliminary clinical trials have demonstrated that ginger consumption leads to a notable decrease in inflammatory markers among participants. While the impact on the overall microbiome appears to be modest, there is a significant reduction in certain microbial taxa, which correlates with the observed anti-inflammatory effects (61). These findings bring hope to those affected by this disease and emphasize the significance of ongoing research on the interplay between microorganisms and cancer cells. Partial inflammation-induced disturbances in intestinal homeostasis directly affect the development of colorectal cancer.
Figure 2.
Inflammation. Escherichia coli and ETBF lead to increased generation of IL-17 followed by DNA damage. Additionally, pks-positive Escherichia coli promotes the development of cancer through two pathways. S. cerevisiae induces TLRs and NOD. Thus, TH-17 cells and proinflammatory cytokines are secreted. These receptors act through NF-κB or barrier STAT3.
Immune system dysfunction in intestinal epithelial cells plays a crucial role in maintaining the stability of the intestinal microenvironment. Research indicates that infection-induced carcinogenesis is directly associated with endogenous T-cell immune responses.
In a study conducted by Wu et al., it was revealed that the Th17 response plays a significant role in the early stages of carcinogenesis (23). This response is driven by STAT3 activation, which is closely linked to the Th17 response. In mice, ETBF infection induces colitis, strongly promoting colon tumor development. However, the IL-17-mediated signaling pathway and the IL-23 receptor can be blocked using antibodies to inhibit ETBF-induced colitis, colonic hyperplasia, and tumor formation (26). The presence of ETBF can stimulate the recruitment and growth of specific immune cells such as CD4+, CCR6+, IL-17A+, and Th17 cells. This happens through the IL-17 signaling pathway, which can contribute to the initiation and growth of tumors. However, while STAT3 activation is a necessary condition for colon tumor development, it is not enough to trigger colon tumors solely through ETBF (47, 62). IL-17-dependent NF-kB activation leads to CXCR2 expression, promoting the recruitment of myeloid cells and thus facilitating ETBF-mediated colon tumor development ( Figure 1 ) (63).
The reduction of ETBF toxin in T lymphocyte utilization of IL-2 promotes the polarization of Th17 lymphocytes and leads to colon tumor development (64, 65). Research on other bacteria, such as Citrobacter rodentium and E. coli, has also revealed their involvement in tumor development through Th1/Th17 immune responses, which induce inflammation ( Figure 1 ) (66).
Fusobacterium nucleatum, through interaction with T-cell immune receptors, inhibits the cytotoxicity of NK cells, contributing to the regulation of tumor immunity ( Figure 1 ) (67). These studies provide a new perspective for in-depth exploration of the regulatory network between the intestinal microbiome and the immune system.
2.1.2. Metabolism
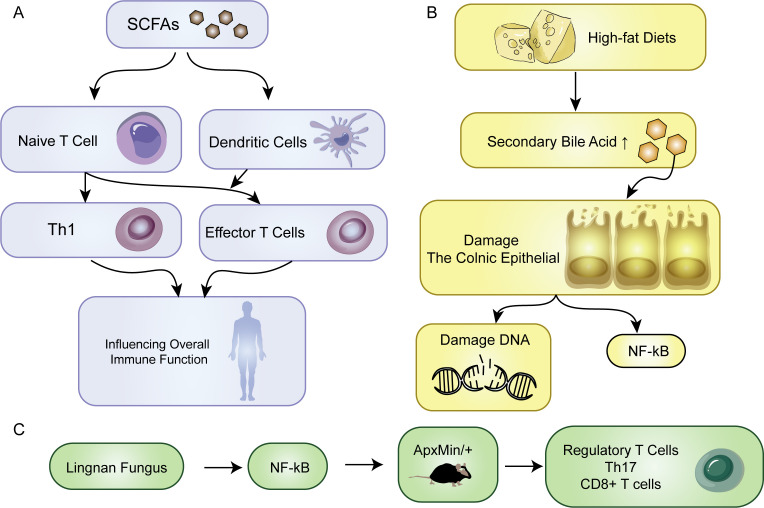
The development of tumors is accompanied by changes in metabolic status that affect the microenvironment of the tumor tissue and its surroundings. Both host and dietary components are metabolized together, and the gut microbiota produces various compounds that fulfill their survival requirements while metabolizing different dietary and parasitic components. Diet, in particular, has a significant impact on the metabolic output of the gut microbiota. Bile acids (BAs) and short-chain fatty acids (SCFAs) are important components that play a vital role in the interaction between microorganisms and the host. They are the products of metabolism and can act as protective or harmful agents in the development of CRC ( Figure 1 ) (68). The impact of metformin on the gut microbiota has been reported, with some results indicating that metformin increases the abundance of short-chain fatty acid-producing bacteria and mucin-degrading bacteria, thereby promoting the enrichment of beneficial bacteria through metabolic processes (69). The immune system is triggered by the interaction of immune cells with the microbiota in the intestine. This interaction is facilitated by SCFAs produced by various types of bacteria including Firmicutes, Bacteroides, Actinobacteria, Proteobacteria, and Verrucomicrobia (70). SCFAs provide energy to intestinal cells in the host. The activation of immune cells by SCFAs helps in the differentiation of naive T cells into Th1 cells thereby enhancing immune defense. Additionally, dendritic cells that are activated by SCFAs help guide the differentiation of naive T cells into effector T cells thus affecting overall immune function ( Figure 3A ) (71). There are certain bacteria, such as Fusobacterium nucleatum and E. coli, which are related to colorectal cancer. These bacteria impact tumor development and immune responses through different mechanisms. Additionally, the Lingnan fungus activates NF-kB, which in turn induces the proliferation of tumor bone marrow cells in ApcMin/+ mice. Simultaneously, it modulates the frequencies of regulatory T cells, Th-17 cells, and CD8+ T cells ( Figure 3C ) (72). E. coli microbiota stimulation prompts colorectal cancer cells to release chemokines, facilitating the recruitment of T cells into the tumor tissue (73).
Figure 3.
Metabolism. (A) SCFAs promote naive T cells to transform into Th1 cells. SCFA-activated dendritic cells direct the maturation of naive T cells into effector T cells, influencing overall immune function. (B) The impact of a high-fat diet on the body. (C) Effect of Lingnan fungi on the proliferation of tumor bone marrow cells.
In the onset of tumors, metabolites from microbial metabolism play a crucial role. Bacterial bile salt hydrolases catalyze the conversion of primary bile acids into secondary bile acids, including deoxycholic acid and lithocholic acid (74). Individuals consuming high-fat diets commonly exhibit an increase in secondary bile acid in the colon, which is correlated with an increased risk of colon cancer. It contributes to oncogenesis through distinct mechanisms. They directly damage the colonic epithelial barrier, induce oxidative DNA damage, cause genomic instability, and activate NF-κB, collectively facilitating the initiation of tumors ( Figure 3B ). Specific secondary bile acids, such as deoxycholic acid and lithocholic acid, accelerate carcinogen-induced colon cancer, particularly in the presence of a functional mutation in the Apc gene. However, ursodeoxycholic acid, produced by Lactobacillus, plays a protective role in inhibiting CRC development (59). Through comprehensive analyses of the gut microbiota and metabolome, ongoing research continues to unravel the intricate interplay between microbial communities and dietary factors in the CRC environment (75, 76). Polyamines are organic polycations involved in cell proliferation, differentiation, tissue repair, apoptosis, angiogenesis, immune response, signal transduction, and gene expression (77). Elevated levels of polyamine metabolites in biofilms are linked with cell proliferation and CRC. One particular polyamine metabolite, which has been proposed as an early CRC marker, increases in tumors regardless of the presence of biofilm. Compared to healthy tissues, CRC tissues display increased polyamine concentrations, and polyamines promote carcinogenic signaling (78). Current evidence suggests that any polyamine from various sources, such as diet, microbiota, or tissues, can drive tumorigenesis (79). Lysophosphatidic acid (LPA), a promoter of cell proliferation and cell cycle acceleration in CRC cell lines and organoids derived from CRC patients, has emerged as a significant player in CRC pathogenesis. Relevant analysis revealed a close association between infective species and LPA, the metabolic product known to stimulate proliferation (80). Distinct changes are observed in the serum metabolome of CRC patients, particularly in amino acid profiles. The levels of metabolites related to branched-chain amino acids (BCAAs), aromatic amino acids, and phenylalanine typically increase (81). However, evidence indicates that a higher intake of dietary BCAAs may not be associated with an increased risk of CRC but could be related to a lower risk of colorectal cancer (82). Alanine, which was identified as relevant to CRC in one study, has been reported to be a crucial survival signal in certain gastrointestinal tumors (83). Lactobacillus subtilis has the ability to break down tryptophan, thereby producing indole-3 -lactic acid (ILA) that targets ROR γ t. Through this action, it can effectively inhibit Th17 differentiation, reduce IL-17 expression, and potentially prevent the occurrence of colorectal cancer (84).
Metabolic changes in tumor cells can provide valuable information for detecting biomarkers of tumor onset. These changes can more directly reflect the state of tumor cells compared to changes in the genome and proteome. We propose that exploring the relationship between amino acid metabolites and the gut microbiota can reveal distinctive characteristics across various stages of CRC. Studies have suggested that the metabolites produced by the gut microbiota can enter the circulation and regulate distant organs. Therefore, investigating serum metabolites that are closely associated with colorectal cancer holds promise for developing novel diagnostic biomarkers.
Compared to changes in the genome and proteome, metabolic changes can more directly reflect the state of tumor cells. This makes them a promising source for detecting biomarkers of tumor onset. We believe that by studying the relationship between amino acid metabolites and the gut microbiota, we can identify distinct characteristics at different stages of colorectal cancer. Several studies suggest that metabolites produced by the gut microbiota can enter circulation and regulate distant organs. Recent evidence indicates that a relationship exists between sex hormones and the gut microbiome. The gut microbiome’s composition and function, as well as its metabolites, are regulated by sex hormones, while the gut microbiome significantly impacts sex hormone levels. A study has revealed that estrogen may have a protective effect on female CRC (85). Therefore, exploring serum metabolites closely associated with colorectal cancer can hold promising potential for developing novel diagnostic biomarkers. Potential biomarkers for early detection of CRC based on gut microbiome are summarized in Table 1 .
Table 1.
Potential biomarkers for early detection of CRC based on gut microbiome.
| Marker | Mechanism/Role in CRC | Potential Application | References |
|---|---|---|---|
| Fusobacterium nucleatum | Promotes inflammation, immune evasion, and tumor growth via adhesion to E-cadherin and modulation of the immune system | Early detection, prognosis | (20, 86) |
| Enterotoxigenic Bacteroides fragilis (ETBF) | Produces Bacteroides fragilis toxin (BFT), disrupting epithelial integrity and inducing chronic inflammation | Biomarker for CRC risk in inflammatory conditions | (87, 88) |
| Escherichia coli (pks+) | Induces DNA double-strand breaks through colibactin, promoting genomic instability and mutagenesis | Early detection in high-risk individuals | (89, 90) |
| Bacteroides spp. | Dysbiosis in gut microbiome leads to imbalance in short-chain fatty acids and promotes carcinogenesis | Detection of early dysbiosis linked to CRC | (91) |
| Short-chain fatty acids (SCFAs) | Anti-inflammatory properties; SCFA dysregulation associated with CRC | Biomarker for microbial dysbiosis and CRC risk | (92, 93) |
| Lipopolysaccharide (LPS) | Pro-inflammatory component that stimulates immune response; elevated in CRC patients | Biomarker for gut inflammation and CRC development | (94) |
| Akkermansia muciniphila | Modulates mucin production and maintains gut barrier function; low levels linked to inflammation and CRC development | Prognostic marker for disease progression | (95, 96) |
| Streptococcus gallolyticus | Associated with CRC through bacterial adhesion, triggering inflammation and promoting carcinogenesis | Marker for CRC risk, especially in CRC-associated bacteremia | (97, 98) |
| Faecalibacterium prausnitzii | Anti-inflammatory properties through butyrate production, maintaining epithelial barrier integrity | Lower abundance associated with CRC; marker for gut health | (99, 100) |
3. Microbiome-based diagnostics: revolutionizing CRC detection
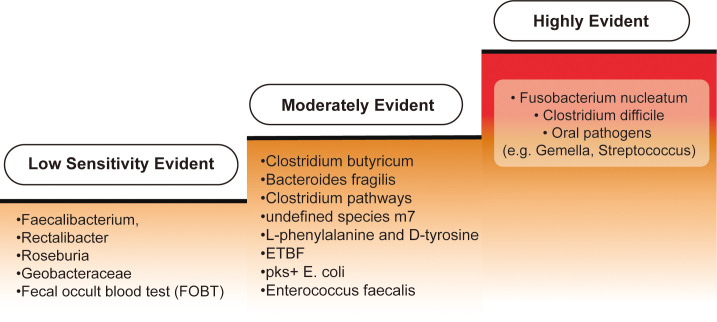
According to current research, biomarkers are classified into three categories: highly evident, moderately evident, and biomarkers with low sensitivity for CRC diagnosis, reflecting varying levels of validation. An overview is provided in Figure 4 .
Figure 4.
Categorization of biomarkers for colorectal cancer diagnosis. Biomarkers for colorectal cancer (CRC) diagnosis can be categorized into three levels of evidence based on their sensitivity and specificity. The "Highly Evident Biomarkers" group includes Fusobacterium nucleatum, frequently enriched in CRC patients and shown to enhance the sensitivity of the fecal immunochemical test (FIT) when used in conjunction with other diagnostic tools. Additionally, Clostridium difficile has been found to increase in both early- and late-onset CRC cases, suggesting a strong correlation with CRC development. Oral pathogens such as Fusobacterium, Gemella, and Streptococcus are also strongly associated with CRC. In the "Moderately Evident Biomarkers" category, Clostridium butyricum, Bacteroides fragilis, and Clostridium pathways—along with an undefined species, m7—provide moderate detection capabilities, albeit less prominent than the highly evident group. Elevated levels of L-phenylalanine and D-tyrosine are observed in amino acid metabolism specific to early-onset CRC samples. Furthermore, enriched bacteria such as enterotoxigenic Bacteroides fragilis (ETBF), pks+ Escherichia coli, and Enterococcus faecalis are found in CRC patients, though their independent or synergistic roles in CRC development are not fully understood. Lastly, the "Low Sensitivity Biomarkers" category includes reduced levels of Faecalibacterium, Rectalibacter, and Roseburia in late-onset CRC cases, though their absence alone is insufficient for a definitive diagnosis. Geobacteraceae, which exhibits a mutual exclusion relationship with other bacteria, shows weaker evidence for CRC detection. Finally, the fecal occult blood test (FOBT) has limited sensitivity and specificity, often yielding false positives, making it a less reliable biomarker.
3.1. Microbial biomarkers: pioneering noninvasive screening and diagnosis in CRC
3.1.1. Fecal metagenomic analysis
Studies of gut bacteria associated with adenoma and/or CRC development are summarized in Table 2 . CRC can be divided into two types: early-onset (EO-CRC) and late-onset (LO-CRC) colorectal cancer. Fecal metagenomic analysis showed that perfluorobutane sulfonic acid (PFOS) continuously accumulated in LO-CRC and BO-CRC samples, indicating potential ecological toxicity (101). In both types of colorectal cancer, L-phenylalanine and D-tyrosine levels increase significantly in amino acid metabolism. However, only in EO-CRC samples, certain amino acids and their microbial derivatives were enriched. In fecal samples, a decrease in butyrate-producing bacteria (Faecalibacterium, Rectalibacter, and Roseburia), a reduction in short-chain fatty acid (SCFA) acetate, and downregulation of GABA biosynthetic genes were observed in LO-CRC (102). Both CRC types showed an increase in Clostridium difficile and Helicobacter pylori, which had a negative correlation with reduced SCFA acetate in LO-CRC (103, 104).
Table 2.
Studies of gut bacteria associated with adenoma and/or CRC development.
| Indicators/Discoveries | Colorectal Cancer Types | Relevant Information/Trends |
|---|---|---|
| Perfluorooctane Sulfonic Acid (PFOSA) | LO-CRC | Accumulates continuously in LO-CRC and BO-CRC samples, with potential ecological toxicity (101). |
| Amino Acid Metabolites | EO-C O-CRC | Levels of L-phenylalanine and D-tyrosine significantly increased in both CRC types, while specific amino acids and their microbial derivatives were enriched only in EO-CRC samples (102). |
| Butyrate-Producing Bacteria | EO-CRC | Decreased in fecal samples, including fecal Enterobacter, rectal Clostridium, and Roseburia (102). |
| Short-Chain Fatty Acids (SCFAs) Acetate | LO-CRC | Decreased in LO-CRC, negatively correlated with increased frail spore rods and Helicobacter pylori (103, 104). |
| GABA Biosynthetic Genes | LO-CRC | Downregulated in LO-CRC (102). |
| Oral Pathogens | CRC | Elevated levels in the gut microbiota of colorectal adenoma patients, including Clostridium difficile, Moryella, and Lachnoclostridium species (105). |
| Enriched Oral Pathogens | CRC | Enriched in colorectal cancer patients, including Clostridium difficile, Micrococcus, Streptococcus, etc (106, 107). |
| Colorectal Resident Bacteria | CRC | Specific colon-resident bacteria enriched in colorectal cancer patients, such as enterotoxigenic frail rod-like bacteria, pks+ Escherichia coli, lithocholic acid chain cocci, etc (108, 109). |
| Bacterial Combination Testing | CRC | Slightly improved sensitivity in detecting CRC, including Fusobacterium, Bacteroides, Haemophilus, and an undefined species. |
| Depleted Probiotics | CRC | Includes thermophilic Streptococcus, Streptococcus salivarius, Lactobacillus gallinarum, butyric acid bacillus, and malt-flavored meat rod bacillus, These probiotics engage in competitive or antagonistic interactions with pathogenic microorganisms (110–114). |
| Fungal Colonization | CRC | Continuously enriched fungi include Rhizopus oryzae, and Cordyceps, while continuously decreased fungi include Aspergillus. Coexistence and mutual exclusion patterns exist between fungi and bacteria (84, 116). |
| Archaea | CRC | Patient's fecal samples have more halophilic archaea and fewer methane-producing archaea, halophilic bacteria, etc (122). |
Apart from colorectal cancer, elevated levels of oral pathogens, including Fusobacterium, Moryella, and Lachnoclostridium, were found in the intestinal microbiota affected by colorectal adenomas, indicating early events of intestinal dysbiosis in colorectal tumor development (105). The enriched oral pathogens in CRC patients include Fusobacterium, Gemella, and Streptococcus (106, 107). Certain colon-resident bacteria, such as ETBF, pks+ E. coli, and Enterococcus faecalis, are enriched in colorectal cancer patients (108, 109). It has been established that certain bacteria are associated with the development of colorectal cancer, but it’s not clear whether they act alone or in combination with other microorganisms to accelerate the disease. A combination of four bacteria found in feces, namely Clostridium butyricum, Bacteroides fragilis, Clostridium pathways, and an undefined species m7, only slightly increased the ability to detect colorectal cancer.
There is a hypothesis that certain beneficial probiotics could be absent in the development of CRC. These probiotics could have mutually exclusive relationships with pathogenic microbes and could also engage in competitive or antagonistic interactions (110–114). Research has aimed to identify factors influencing pathogenic probiotics in the human body, to normalize gut microbiota, improve gastrointestinal barrier function, reduce tumor formation, and inhibit CRC cell proliferation, growth, and metastasis (115). Studies indicate that alterations in the composition of intestinal fungi are associated with colorectal cancer development. Complex relationships between fungi and bacteria, including antagonistic interactions, may contribute to the onset of colorectal cancer. Bacterial differential abundance analysis revealed a significant mutual exclusion relationship only between Geobacteraceae and bacteria. Changes in CRC-specific intra- and interdomain ecological networks suggest coexistence and antagonistic interactions within the bacteria-fungi network, with potential contributions from fungal synergies to CRC development (116). It’s worth noting that the gut microbiome of CRC patients exhibits varying enrichments of specific bacteria based on their body weight. In particular, Actinomyces, Desulfovibrio, and Bacteroides are more prevalent in the gut microbiota of obese CRC patients, while Bacteroides and Prevotella are more abundant in the microbiota of CRC patients with normal body weight (117). Research on archaea indicates an association between the enrichment of halophilic archaea and the reduction of methanogenic archaea with CRC. Studies further revealed mutualistic and antagonistic relationships between archaea enriched in CRC and bacteria enriched or depleted in CRC, collectively promoting CRC (84, 118). Research indicates that bacteria linked to gum disease may spread to the colon, causing microbial imbalance, compromising colonic defense mechanisms, and elevating the levels of harmful metabolites and proteolytic activity, resulting in inflammation and potentially cancerous growth (119). Analysis of mouthwash samples through 16S rRNA gene sequencing indicated that oral pathogenic taxa positively and negatively impact CRC development (120). Several studies, including the one conducted by Flemer et al., have identified particular oral bacteria linked to CRC. They analyzed microbial communities in oral, colonic mucosal tissues, and fecal samples from CRC patients to make these findings. Additionally, after radiotherapy, patients with rectal cancer may experience changes in their oral microbiota due to damage to the intestinal mucosal barrier (121).
3.1.2. Fecal immunochemical test
In CRC screening, the performance of the fecal immunochemical test (FIT) is somewhat limited due to the influence of the critical value of hemoglobin, with a sensitivity ranging from 91% to 71% (123).
3.1.3. Nanogold particles
GNPs have attracted significant interest in disease detection because of their distinctive properties. Colloidal gold immunological analysis technique is a crucial technology in FIT. It serves as a linking scaffold to improve the collective detection sensitivity of DNA and antibodies (124). The colloidal gold approach primarily involves utilizing primary and related secondary antibodies to achieve sensitivities and specificities exceeding 80% for detecting colorectal cancer. Therefore, the colloidal gold method has become one primary approach for colorectal cancer diagnosis (125). Comparing several bacterial species using FIT and fecal occult blood tests revealed that incorporating F. nucleatum screening can enhance the accuracy and sensitivity of FIT. When combined with other bacterial strains, it showed increased sensitivity and specificity. SEPTIN9 combined with FIT improved the sensitivity of CRC detection to 94.4-98%, while maintaining a specificity of 69%. Fecal microbial markers have demonstrated potential in detecting colorectal adenomas, with a combination of several bacteria obtaining an area under the curve (AUC) of 0.90 (126, 127). The determination of differences in BAs and SCFAs in CRC patients provides a direction for further large-scale clinical studies (68).
3.1.4. Fecal occult blood test based on guaia wood resin
There are two methods: the FIT and the fecal occult blood test (FOBT). It can be utilized to detect the presence of blood in the feces. FOBT has benefits including speed, convenience, and noninvasiveness, however its sensitivity and specificity are rather limited. FOBT utilizes tetramethylbenzidine (TMB) as a chromogen, enabling quick findings for at-home testing. However, its drawbacks consist of dietary constraints and a potential for false positive results (128, 129).
In addition to the conventional detection methods mentioned above, we have also summarized some new biomarker methods in Table 3 .
Table 3.
Other biomarkers.
| Biomarker | Detection Method | CRC diagnosis AUC |
Research Findings |
|---|---|---|---|
| GMSM Model | LASSO algorithm selects metabolite features | 0.93 (early-stage CRC) | The GMSM model, correlating serum metabolites with colorectal cancer and adenoma, accurately distinguishes patients from healthy individuals. Its performance significantly exceeds that of CEA and FOBT, holding promise as a noninvasive method for colorectal cancer and adenoma detection (130, 131). |
| Targeted Metabolite Analysis | Orthogonal partial least squares discriminant analysis and ROC analysis | 0.969 | Metabolomic analysis reveals different enrichment pathways of intestinal metabolites in colorectal cancer and adenoma. Combined analysis effectively distinguishes patient populations. Certain metabolites, such as 9,10-dihydroxy-12-octadecenoic acid and cholesterol esters, can be used to differentiate colorectal cancer from healthy controls. Relevant metabolites correlate with patient survival (132). |
| Blood cfDNA Detection | Detects tumor cells, organotypic DNA, and donor DNA in tumors, using CRISPR technology | 0.96 | Engineered bacteria are employed to detect colorectal cancer cells, organotypic DNA, and donor DNA. This approach holds promise in the diagnosis and treatment of colorectal cancer. Clinically occult and radiologically undetectable minimal residual disease (MRD) during surgery is considered a major source of disease recurrence. Compared to the traditional blood marker CEA, preoperative ctDNA exhibits higher sensitivity, allowing more accurate prediction of recurrence risk (133). |
| Methylation Biomarker MYO1-G | ddPCR and ROC analysis | 0.94 | The MYO1-G methylation biomarker, detected using ddPCR technology, accurately distinguishes colorectal cancer patients from normal controls. It is also correlated with disease progression and treatment response. Postoperative monitoring reveals a correlation between methylation levels and patient treatment response and survival, providing a new biomarker for colorectal cancer diagnosis and monitoring (134–136). |
4. Prognostic power of the microbiome: shaping CRC outcomes
The most effective diagnostic techniques for CRC are intrusive and expensive. Developing sensitive, noninvasive, and cost-effective approaches for detecting and predicting the outcome of colorectal cancer is crucial to improve the likelihood of a cure (137).
4.1. Survival insights: the microbiome’s prognostic significance in CRC
Mima et al.’s research found that elevated nuclear F. nucleatum levels in colorectal cancer tissues were linked to negative clinical outcomes, such as reduced survival periods and unfavorable prognoses (138). Recent research has sought to validate the hypothesis that greater amounts of F. nucleatum nuclear DNA in tissues may be correlated with adverse clinical outcomes in patients with colorectal cancer. By examining databases of 1,069 CRC patients from two nationwide prospective cohort studies in the United States, it was found that increased amounts of nuclear F. nucleatum nucleoplasmic DNA were associated with increased colorectal cancer-specific mortality rates. Recent research suggests that F. nucleatum may promote the development of colorectal cancer through the expression of the virulence factor FadA. Moreover, this study indicated that tissue-resident F. nucleatum may inhibit T-cell-mediated immune responses, which is correlated with unfavorable clinical outcomes in patients with colorectal cancer. These findings support the notion that high F. nucleatum expression may indicate a more invasive subtype of colorectal cancer. Future research may need to further explore the impact of tissue-resident F. nucleatum on T-cell-based immunotherapy. Overall, nuclear DNA may serve as a biomarker for CRC prognosis, but further validation in other populations is needed. These findings provide insights for developing colorectal cancer prevention and treatment strategies targeting the microbiota (138).
Wang et al.’s study showed that combining regorafenib with toripalimab is safe, effective, and improves survival in colorectal cancer patients. The study indicated that the results of the gut microbiota, particularly the negative correlation between Bacteroides and response and survival rates, provided a combined treatment option for refractory metastatic colorectal cancer patients (139).
This prospective study aimed to explore the role of the gut microbiota in predicting the response to neoadjuvant chemoradiotherapy (nCRT) in locally advanced rectal cancer (LARC) patients. Yuxi Yi et al. constructed a random forest classifier that successfully predicted the nCRT response using 10 microbial biomarkers. These findings suggest that the gut microbiota may serve as a potential biomarker for predicting nCRT response in LARC patients, with clinical significance for their management (140).
While re-establishing the microbiota following intensive antibiotic treatment is a critical step, limited attention has been directed toward understanding the state of health after microbiota cultivation. In the context of clinical trials evaluating microbiota-based therapies, whether employing fecal microbiota transplantation (FMT) or engineered microbial consortia, it is imperative to incorporate comprehensive, long-term studies of the microbiota. Appropriate metrics for such studies may involve monitoring microbial diversity, examining the existence of multidrug-resistant microorganisms, and determining the prevalence of antibiotic-resistant genes. Assessing the carcinogenic potential of the microbiota may be possible one day, and once validated, these indicators should be incorporated (141).
María Antonia Martínez-Sánchez et al. provided the initial study assessing the effect of preoperative nutritional intervention based on high-fiber intake and high levels of polyunsaturated fatty acids (PUFAs) on changes in the intestinal microbiota and their link with postoperative complications, particularly anastomotic leakage, and site infection. The regulation of the microbiota through dietary intervention has been proven in recent explorations of human testees (142–144). In this way, the consumption of a diet that contains a large amount of PUFAs regulates the composition of the gut microbiota (145). It also favors the development of protective bacteria such as Bifidobacterium and Lactobacillus while reducing the presence of pathogenic bacteria such as Pseudomonas (146–148). In summary, they hypothesize that consuming a diet that has a large content of fiber and PUFA before CRC surgery may result in changes in the intestinal microbiota, making it more balanced and potentially reducing postoperative complications.
Lelde Lauka et al.’s systematic review comprehensively analyzed the relationship between gut microbiota and short-term and long-term outcomes after colorectal cancer surgery. This study revealed that specific bacteria, such as F. nucleatum and Co-abundance Groups, may be independent predictors of prognosis and postoperative complications in colorectal cancer patients. However, due to limited data and the influence of confounding factors, more research is needed to further explain these findings. Overall, this research emphasizes the importance of comparing the microbiota composition before and after surgery, providing insights into how the gut microbiota is linked to the underlying mechanisms of colorectal cancer. When interpreting the results, heterogeneity in studies, sample sources, and analysis methods should be considered. Although evidence is limited, this field holds potential clinical importance for improving colorectal cancer surgery outcomes (149).
Although the value of bacterial markers in colorectal cancer diagnosis still requires further research, this direction provides a promising area due to the wide range of microbes and fewer interfering factors. Future study can go deeper into the utilization of microbiota in diagnosing and predicting outcomes of CRC, particularly in the realms of personalized medicine and microbiome intervention treatment. The efforts will reveal the intricate connection between the microbiota and survival rates in colorectal cancer patients, offering more accurate targets for future treatment options.
4.2. Association of the gut microbiota with colorectal cancer disease progression
The gut microbiota has a substantial impact on the initiation, growth, and treatment outcomes of CRC (150). Research in this field involves exploring the potential applications of microbial markers in CRC screening and prognosis. Disease recurrence and metastasis are also being explored.
In addition to their potential applications in CRC screening, microbial markers can also serve as prognostic biomarkers for CRC. In colorectal tissue samples, Fusobacterium nucleatum (Fn) is one of the most common microbes, and it is associated with resistance in colorectal cancer (151, 152). Studies suggest that the enrichment of Fn in colon tissues is correlated with poor prognosis in right-sided colon cancer patients, while Fn-negative right-sided colon cancer patients have a prognosis comparable to that of left-sided colon cancer patients (153). This indicates that Fn may undergo stage-specific changes during colorectal cancer recurrence and metastasis, impacting the response to systemic chemotherapy in palliative treatment settings.
4.3. Dysbiosis of the gut microbiota and CRC development
CRC is associated with an imbalance in the gut microbiota, with proximal feces reflecting changes in structure and metabolism (154). Analyzing fecal metabolites in CRC patients and comparing them with healthy subjects may identify potential biomarkers related to CRC and explore the impact of metabolites on CRC progression (65). The progression of colorectal tumors along the adenoma-carcinoma sequence disrupts the homeostasis of intestinal metabolites, leading to significant changes in key metabolic pathways, such as the upregulation of cholesterol metabolites and sphingolipids, which are associated with increased fat intake and cholesterol during CRC development (155, 156).
4.4. Reprogramming of amino acid and lipid metabolism in CRC development
In the tumor microenvironment, the reprogramming of amino acid and lipid metabolism contributes to the growth of cancer cells and tumor formation. Metabolomic analysis revealed the enrichment of various metabolites, such as 2-aminoethylphosphonic acid salts and amino acid derivatives, in the CRC group, which is crucial for promoting cancer cell proliferation (157, 158). The presence of these metabolites may serve as potential markers for CRC development.
4.5. Detection of microbial translocation in blood and its association with CRC prognosis
The detection of microbial translocation in the blood of CRC patients has prognostic significance. Studies have identified key biological associations with CRC through the grouping of OCS, inferring the metabolic potential of OCS, and emphasizing the association between grouping and disease prognosis (159). Changes in the gut microbiota associated with colorectal cancer, demonstrated through microbial markers, show excellent performance in the early detection of CRC in the precancerous stage with high accuracy (160). This study, employing rigorous validation methods, identified biomarkers derived from the microbiota. This discovery instills optimism for the prospect of noninvasive diagnosis of colorectal adenomas and, in the future, may emerge as a promising target for the treatment of colorectal cancer (161).
5. Conclusion and future perspectives
In-depth research has been conducted on the gut microbiota of CRC patients. The key role of the gut microbiota in the three carcinogenic processes of CRC has been revealed. First, the continuous proliferation of the gut microbiota during inflammation disrupts the intestinal ecological balance and damages the mucosal barrier, allowing more bacteria to enter and form a vicious positive feedback loop that accelerates the progression of colorectal cancer. Second, certain metabolites of the gut microbiota, such as polyamines and secondary bile acids, participate in carcinogenic signal transduction, leading to tumor formation. Third, the gut microbiota induces the recruitment and proliferation of immune cells by mediating cytokines, which contribute to tumor initiation and growth. Research in this area reveals that there are distinct variations in the prevalence of particular bacterial species, a reduction in microbial diversity, and changes in metabolites in the gut microbiome of patients with colorectal cancer. They also identified biomarker associations between the gut microbiome and CRC. The tight interaction between these bacteria and host epithelial cells impacts the development of CRC.
The gut microbiota once referred to as the forgotten organ, has gradually unveiled its mysterious nature. Studies indicate that the gut microbiome is crucially involved in the pathogenesis of CRC. The occurrence of CRC has been consistently associated with the gut microbiota in numerous clinical studies and experimental models. Robust functional data have reliably demonstrated microbial involvement in the pathways and molecular mechanisms that lead to CRC. Despite achieving preliminary success, a deeper exploration is still needed to determine whether the gut microbiota is a predictor and/or prognostic biomarker for CRC. Conducting thorough longitudinal studies to monitor the alterations in the gut microbiota of colorectal cancer patients over a prolonged period and analyzing these changes in relation to patient outcomes can aid in pinpointing particular compositional aspects of the gut microbiota that may be linked to survival rates, recurrence rates, and other prognostic indicators in colorectal cancer patients. Metabolomics has become an effective method for detecting tumors. Subsequent research can establish a CRC detection model through metabolomic analysis, increasing the probability of diagnosing CRC and predicting favorable outcomes (162). Interventional studies, such as those involving probiotics, prebiotics, and fecal microbiota transplantation, can be conducted to observe their impact on the prognosis of colorectal cancer patients, thereby validating whether the gut microbiota could serve as a potential therapeutic target. Mutual recognition plays a role in identifying potential agonistic and antagonistic interactions, and if a breakthrough is made in this area, it can facilitate the detection of pathogens and subsequent drug research (59).
The utilization of noninvasive biomarkers in current practice, coupled with the repeated improvement in the AUC, highlights ongoing progress in understanding the correlation between the gut microbiota and CRC incidence. Experimental findings employing predictive random forest classifiers underscore a close association between alterations in the gut microbiota and the initiation of carcinogenesis (140).
Microbiome research, confronted by the challenge of limited samples and a multitude of features, innovates logistic regression techniques with penalty methods such as least absolute shrinkage and selection operator (LASSO), ridge, and elastic net methods. In studying tumor microbiota communities, the integration of artificial intelligence (AI) and high-throughput sequencing aims to identify at-risk individuals early, emphasizing significant features such as mutations, methylation, and structural variations.
Unsupervised learning methods, encompassing clustering and dimensionality reduction, aid in feature selection and visualization. Techniques such as random forest (RF), support vector machine (SVM), and penalty regression explore the relationships between microbiota species and CRC features. Time-event machine learning algorithms, including random survival forest (RSF), gradient boosting machine (GBM), Cox-time, and neural multitask logistic regression (N-MTLR), predict CRC-specific survival potential, providing transparent and interpretable models. These models assist clinicians in accurately predicting patient survival rates, formulating personalized treatment plans, and ultimately enhancing patient outcomes (162, 163).
Although the carcinogenic potential of specific bacterial genera, such as Fusobacterium nucleatum, pks-positive E. coli, and Bacteroides fragilis, has been established, the oncogenic properties of other related genera remain unclear. Research confirms the close relationship between CRC and the gut microbiota, emphasizing the need for further exploration of microbial communities in the tumor microenvironment and their impact on CRC. The dynamic interaction between these microbes and host epithelial cells significantly influences CRC development.
During the literature search, it was noted that relevant clinical data are limited. Future studies should focus on multidisciplinary approaches and extensive clinical data analysis to explore the complex link between the gut microbiota and CRC, leading to improved treatment strategies. Challenges in clinical practice, such as individual variations, tumor staging, and cross-species translation, need to be overcome when exploring more accurate diagnostic and prognostic biomarkers.
Therefore, despite promising initial findings, continuous efforts are needed in future preclinical and clinical research to better comprehend the connection between the gut microbiota and CRC. Overall, the study of the gut microbiota provides unprecedented opportunities to explore new diagnostic, therapeutic, and prognostic strategies for colorectal cancer.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by (1) the Research Council of the University of Hong Kong (104004092 and 104004460); (2) Wong’s donation (200006276); (3) a donation from the Gaia Family Trust of New Zealand (200007008); (4) the Research Grants Committee (RGC) of Hong Kong, HKSAR (740608, 766211, 17152116 and 17121419); (5) Health and Medical Research Fund (15162961,18192141 and 16172751); (6) Enhanced new staff start-up fund (204610519); and (7) Pre-emptive retention fund (202007002).
Author contributions
GC: Writing – original draft, Writing – review & editing. QR: Writing – original draft, Writing – review & editing. ZZ: Writing – original draft, Writing – review & editing. QL: Writing – original draft, Writing – review & editing. ZH: Visualization, Writing – original draft. CZ: Writing – original draft. HY: Resources, Visualization, Writing – review & editing. ZF: Visualization, Writing – review & editing. BC: Visualization, Writing – original draft. NW: Writing – original draft. YF: Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Cheng Y, Ling Z, Li L. The intestinal microbiota and colorectal cancer. Front Immunol. (2020) 11:615056. doi: 10.3389/fimmu.2020.615056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3. Carson TL, Byrd DA, Smith KS, Carter D, Abaskaron M, Little RB, et al. A case-control study of the association between the gut microbiota and colorectal cancer: exploring the roles of diet, stress, and race. Gut Pathog. (2024) 16:13. doi: 10.21203/rs.3.rs-2475944/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oliveira ML, Biggers A, Oddo VM, Yanez B, Booms E, Sharp L, et al. A perspective review on diet quality, excess adiposity, and chronic psychosocial stress and implications for early-onset colorectal cancer. J Nutr. (2024) 154:1069–79. doi: 10.1016/j.tjnut.2024.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lima OM, Biggers A, Oddo VM, Naylor KB, Chen Z, Hamm A, et al. Design of a remote time-restricted eating and mindfulness intervention to reduce risk factors associated with early-onset colorectal cancer development among young adults. Nutrients. (2024) 16:504. doi: 10.3390/nu16040504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khan M, Shah S, Shah W, Khan I, Ali H, Ali I, et al. Gut microbiome as a treatment in colorectal cancer. Int Rev Immunol. (2024) 43:229–47. doi: 10.1080/08830185.2024.2312294 [DOI] [PubMed] [Google Scholar]
- 7. Vogelstein B, Kinzler KW. The multistep nature of cancer. Trends Genet. (1993) 9:138–41. doi: 10.1016/0168-9525(93)90209-z [DOI] [PubMed] [Google Scholar]
- 8. Garrett WS. Cancer and the microbiota. Science. (2015) 348:80–6. doi: 10.1126/science.aaa4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jobin C. Colorectal cancer: looking for answers in the microbiota. Cancer Discovery. (2013) 3:384–87. doi: 10.1158/2159-8290.CD-13-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tlaskalova-Hogenova H, Vannucci L, Klimesova K, Stepankova R, Krizan J, Kverka M. Microbiome and colorectal carcinoma: insights from germ-free and conventional animal models. Cancer J. (2014) 20:217–24. doi: 10.1097/PPO.0000000000000052 [DOI] [PubMed] [Google Scholar]
- 11. Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. (2006) 312:1355–59. doi: 10.1126/science.1124234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsai YL, Lin TL, Chang CJ, Wu TR, Lai WF, Lu CC, et al. Probiotics, prebiotics and amelioration of diseases. J BioMed Sci. (2019) 26:3. doi: 10.1186/s12929-018-0493-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. (2013) 54:2325–40. doi: 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Natividad JM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res. (2013) 69:42–51. doi: 10.1016/j.phrs.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 15. Bäumler AJ, Sperandio V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. (2016) 535:85–93. doi: 10.1038/nature18849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. (2016) 352:539–44. doi: 10.1126/science.aad9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Helmink BA, Khan M, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. (2019) 25:377–88. doi: 10.1038/s41591-019-0377-7 [DOI] [PubMed] [Google Scholar]
- 18. Oliva M, Mulet-Margalef N, Ochoa-De-Olza M, Napoli S, Mas J, Laquente B, et al. Tumor-associated microbiome: where do we stand? Int J Mol Sci. (2021) 22:1446. doi: 10.3390/ijms22031446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wong-Rolle A, Wei HK, Zhao C, Jin C. Unexpected guests in the tumor microenvironment: microbiome in cancer. Protein Cell. (2021) 12:426–35. doi: 10.1007/s13238-020-00813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. (2013) 14:207–15. doi: 10.1016/j.chom.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. (2013) 14:195–206. doi: 10.1016/j.chom.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu S, Morin PJ, Maouyo D, Sears CL. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology. (2003) 124:392–400. doi: 10.1053/gast.2003.50047 [DOI] [PubMed] [Google Scholar]
- 23. Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. (2009) 15:1016–22. doi: 10.1038/nm.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson MR, Jiang Y, Villalta PW, Stornetta A, Boudreau PD, Carrá A, et al. The human gut bacterial genotoxin colibactin alkylates DNA. Science. (2019) 363:eaar7785. doi: 10.1126/science.aar7785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rohani Z, Sazegar H, Rahimi E. Unlocking the potential of Escherichia coli K-12: A novel approach for Malignancy reduction in colorectal cancer through gene expression modulation. Gene. (2024) 906:148266. doi: 10.1016/j.gene.2024.148266 [DOI] [PubMed] [Google Scholar]
- 26. Collins D, Hogan AM, Winter DC. Microbial and viral pathogens in colorectal cancer. Lancet Oncol. (2011) 12:504–12. doi: 10.1016/S1470-2045(10)70186-8 [DOI] [PubMed] [Google Scholar]
- 27. Peters BA, Dominianni C, Shapiro JA, Church TR, Wu J, Miller G, et al. The gut microbiota in conventional and serrated precursors of colorectal cancer. Microbiome. (2016) 4:69. doi: 10.1186/s40168-016-0218-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Repass J, Maherali N, Owen K. Registered report: Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Elife. (2016) 5:e10012. doi: 10.7554/eLife.10012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huycke MM, Abrams V, Moore DR. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis. (2002) 23:529–36. doi: 10.1093/carcin/23.3.529 [DOI] [PubMed] [Google Scholar]
- 30. Herrera P, Kwon YM, Ricke SC. Ecology and pathogenicity of gastrointestinal Streptococcus bovis. Anaerobe. (2009) 15:44–54. doi: 10.1016/j.anaerobe.2008.11.003 [DOI] [PubMed] [Google Scholar]
- 31. Martin HM, Campbell BJ, Hart CA, Mpofu C, Nayar M, Singh R, et al. Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology. (2004) 127:80–93. doi: 10.1053/j.gastro.2004.03.054 [DOI] [PubMed] [Google Scholar]
- 32. Maddocks OD, Short AJ, Donnenberg MS, Bader S, Harrison DJ. Attaching and effacing Escherichia coli downregulate DNA mismatch repair protein in vitro and are associated with colorectal adenocarcinomas in humans. PloS One. (2009) 4:e5517. doi: 10.1371/journal.pone.0005517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tjalsma H, Boleij A, Marchesi JR, Dutilh BE. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol. (2012) 10:575–82. doi: 10.1038/nrmicro2819 [DOI] [PubMed] [Google Scholar]
- 34. Yu YN, Fang JY. Gut microbiota and colorectal cancer. Gastrointest Tumors. (2015) 2:26–32. doi: 10.1159/000380892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Quaglio A, Grillo TG, De Oliveira E, Di Stasi LC, Sassaki LY. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol. (2022) 28:4053–60. doi: 10.3748/wjg.v28.i30.4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. (2013) 62:933–47. doi: 10.1136/gutjnl-2013-304701 [DOI] [PubMed] [Google Scholar]
- 37. Shao T, Yang YX. Cholecystectomy and the risk of colorectal cancer. Am J Gastroenterol. (2005) 100:1813–20. doi: 10.1111/j.1572-0241.2005.41610.x [DOI] [PubMed] [Google Scholar]
- 38. Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. (2005) 97:1679–87. doi: 10.1093/jnci/dji375 [DOI] [PubMed] [Google Scholar]
- 39. Altshuller M, He X, MacKrell EJ, Wernke KM, Wong J, Sellés-Baiget S, et al. The Fanconi anemia pathway repairs colibactin-induced DNA interstrand cross-links. Biorxiv. (2024), 2024.01.30.576698. doi: 10.1101/2024.01.30.576698 [DOI] [Google Scholar]
- 40. Klapp V, Álvarez-Abril B, Leuzzi G, Kroemer G, Ciccia A, Galluzzi L. The DNA damage response and inflammation in cancer. Cancer Discovery. (2023) 13:1521–45. doi: 10.1158/2159-8290.CD-22-1220 [DOI] [PubMed] [Google Scholar]
- 41. Li S, Liu J, Zheng X, Ren L, Yang Y, Li W, et al. Tumorigenic bacteria in colorectal cancer: mechanisms and treatments. Cancer Biol Med. (2021) 19:147–62. doi: 10.20892/j.issn.2095-3941.2020.0651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kay J, Thadhani E, Samson L, Engelward B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair (Amst). (2019) 83:102673. doi: 10.1016/j.dnarep.2019.102673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rivas-Domínguez A, Pastor N, Martínez-López L, Colón-Pérez J, Bermúdez B, Orta ML. The role of DNA damage response in dysbiosis-induced colorectal cancer. Cells. (2021) 10:1934. doi: 10.3390/cells10081934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muthusami S, Ramachandran IK, Babu KN, Krishnamoorthy S, Guruswamy A, Queimado L, et al. Role of inflammation in the development of colorectal cancer. Endocr Metab Immune Disord Drug Targets. (2021) 21:77–90. doi: 10.2174/1871530320666200909092908 [DOI] [PubMed] [Google Scholar]
- 45. Kawanishi S, Ohnishi S, Ma N, Hiraku Y, Murata M. Crosstalk between DNA damage and inflammation in the multiple steps of carcinogenesis. Int J Mol Sci. (2017) 18:1808. doi: 10.3390/ijms18081808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parida S, Wu S, Siddharth S, Wang G, Muniraj N, Nagalingam A, et al. A procarcinogenic colon microbe promotes breast tumorigenesis and metastatic progression and concomitantly activates notch and β-catenin axes. Cancer Discovery. (2021) 11:1138–57. doi: 10.1158/2159-8290.CD-20-0537 [DOI] [PubMed] [Google Scholar]
- 47. Yang J, Wang X, Hu T, Huang H, Chen G, Jin B, et al. Entero-toxigenic Bacteroides fragilis contributes to intestinal barrier injury and colorectal cancer progression by mediating the BFT/STAT3/ZEB2 pathway. Cell Cycle. (2024) 23:70–82. doi: 10.1080/15384101.2024.2309005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee C, Hwang S, Gwon S, Park C, Jo M, Hong J, et al. Bacteroides fragilis toxin induces intestinal epithelial cell secretion of interleukin-8 by the E-cadherin/β-catenin/NF-κB dependent pathway. Biomedicines. (2022) 10:827. doi: 10.3390/biomedicines10040827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roh HC, Yoo DY, Ko SH, Kim YJ, Kim JM. Bacteroides fragilis enterotoxin upregulates intercellular adhesion molecule-1 in endothelial cells via an aldose reductase-, MAPK-, and NF-κB-dependent pathway, leading to monocyte adhesion to endothelial cells. J Immunol. (2011) 187:1931–41. doi: 10.4049/jimmunol.1101226 [DOI] [PubMed] [Google Scholar]
- 50. Jeon JI, Ko SH, Kim JM. Intestinal Epithelial Cells Exposed to Bacteroides fragilis Enterotoxin Regulate NF-kappaB Activation and Inflammatory Responses through beta-Catenin Expression. Infect Immun. (2019) 87. doi: 10.1128/IAI.00312-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ghouri YA, Tahan V, Shen B. Secondary causes of inflammatory bowel diseases. World J Gastroenterol. (2020) 26:3998–4017. doi: 10.3748/wjg.v26.i28.3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. (2020) 113:2019–40. doi: 10.1007/s10482-020-01474-7 [DOI] [PubMed] [Google Scholar]
- 53. Haghi F, Goli E, Mirzaei B, Zeighami H. The association between fecal enterotoxigenic B. fragilis colorectal cancer. BMC Cancer. (2019) 19:879. doi: 10.1186/s12885-019-6115-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Appunni S, Rubens M, Ramamoorthy V, Tonse R, Saxena A, McGranaghan P, et al. Emerging evidence on the effects of dietary factors on the gut microbiome in colorectal cancer. Front Nutr. (2021) 8:718389. doi: 10.3389/fnut.2021.718389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cuevas-Ramos G, Petit CR, Marcq I, Boury M, Oswald E, Nougayrède JP. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci U.S.A. (2010) 107:11537–42. doi: 10.1073/pnas.1001261107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maddocks OD, Scanlon KM, Donnenberg MS. An Escherichia coli effector protein promotes host mutation via depletion of DNA mismatch repair proteins. Mbio. (2013) 4:e113–52. doi: 10.1128/mBio.00152-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bronowski C, Smith SL, Yokota K, Corkill JE, Martin HM, Campbell BJ, et al. A subset of mucosa-associated Escherichia coli isolates from patients with colon cancer, but not Crohn’s disease, share pathogenicity islands with urinary pathogenic E. coli. Microbiol (Reading). (2008) 154:571–83. doi: 10.1099/mic.0.2007/013086-0 [DOI] [PubMed] [Google Scholar]
- 58. Choi HJ, Kim J, Do KH, Park SH, Moon Y. Enteropathogenic Escherichia coli-induced macrophage inhibitory cytokine 1 mediates cancer cell survival: an in vitro implication of infection-linked tumor dissemination. Oncogene. (2013) 32:4960–69. doi: 10.1038/onc.2012.508 [DOI] [PubMed] [Google Scholar]
- 59. Wong CC, Yu J. Gut microbiota in colorectal cancer development and therapy. Nat Rev Clin Oncol. (2023) 20:429–52. doi: 10.1038/s41571-023-00766-x [DOI] [PubMed] [Google Scholar]
- 60. Mignini I, Piccirilli G, Galasso L, Termite F, Esposto G, Ainora ME, et al. From the colon to the liver: how gut microbiota may influence colorectal cancer metastatic potential. J Clin Med. (2024) 13:420. doi: 10.3390/jcm13020420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Prakash A, Rubin N, Staley C, Onyeaghala G, Wen YF, Shaukat A, et al. Effect of ginger supplementation on the fecal microbiome in subjects with prior colorectal adenoma. Sci Rep. (2024) 14:2988. doi: 10.1038/s41598-024-52658-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Levy DE, Darnell JJ. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. (2002) 3:651–62. doi: 10.1038/nrm909 [DOI] [PubMed] [Google Scholar]
- 63. Chung L, Thiele OE, Geis AL, Chan JL, Fu K, DeStefano SC, et al. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe. (2018) 23:203–14. doi: 10.1016/j.chom.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Geis AL, Fan H, Wu X, Wu S, Huso DL, Wolfe JL, et al. Regulatory T-cell response to enterotoxigenic bacteroides fragilis colonization triggers IL17-dependent colon carcinogenesis. Cancer Discovery. (2015) 5:1098–109. doi: 10.1158/2159-8290.CD-15-0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oke S, Martin A. Insights into the role of the intestinal microbiota in colon cancer. Therap Adv Gastroenterol. (2017) 10:417–28. doi: 10.1177/1756283X17694832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zou S, Fang L, Lee MH. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol Rep (Oxf). (2018) 6:1–12. doi: 10.1093/gastro/gox031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. (2015) 42:344–55. doi: 10.1016/j.immuni.2015.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Singhal S, Bhadana R, Jain BP, Gautam A, Pandey S, Rani V. Role of gut microbiota in tumorigenesis and antitumoral therapies: an updated review. Biotechnol Genet Eng Rev. (2023) 40:1–27. doi: 10.1080/02648725.2023.2166268 [DOI] [PubMed] [Google Scholar]
- 69. Zhou L, Zheng L, Xu B, Ye Z, Li D, Wang W. Clinical efficacy of metformin in familial adenomatous polyposis and the effect of intestinal flora. Orphanet J Rare Dis. (2024) 19:88. doi: 10.1186/s13023-024-03064-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xie W, Zhong YS, Li XJ, Kang YK, Peng QY, Ying HZ. Postbiotics in colorectal cancer: intervention mechanisms and perspectives. Front Microbiol. (2024) 15:1360225. doi: 10.3389/fmicb.2024.1360225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sun J, Chen F, Wu G. Potential effects of gut microbiota on host cancers: focus on immunity DNA damage, cellular pathways, and anticancer therapy. Isme J. (2023) 17:1535–51. doi: 10.1038/s41396-023-01483-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Long X, Wong CC, Tong L, Chu E, Ho SC, Go M, et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat Microbiol. (2019) 4:2319–30. doi: 10.1038/s41564-019-0541-3 [DOI] [PubMed] [Google Scholar]
- 73. Cremonesi E, Governa V, Garzon J, Mele V, Amicarella F, Muraro MG, et al. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut. (2018) 67:1984–94. doi: 10.1136/gutjnl-2016-313498 [DOI] [PubMed] [Google Scholar]
- 74. Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci U.S.A. (2008) 105:13580–85. doi: 10.1073/pnas.0804437105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Krautkramer KA, Kreznar JH, Romano KA, Vivas EI, Barrett-Wilt GA, Rabaglia ME, et al. Diet-microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol Cell. (2016) 64:982–92. doi: 10.1016/j.molcel.2016.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell. (2012) 48:612–26. doi: 10.1016/j.molcel.2012.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gerner EW, Meyskens FJ. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. (2004) 4:781–92. doi: 10.1038/nrc1454 [DOI] [PubMed] [Google Scholar]
- 78. DeDecker L, Coppedge B, Avelar-Barragan J, Karnes W, Whiteson K. Microbiome distinctions between the CRC carcinogenic pathways. Gut Microbes. (2021) 13:1854641. doi: 10.1080/19490976.2020.1854641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Niekamp P, Kim CH. Microbial metabolite dysbiosis and colorectal cancer. Gut Liver. (2023) 17:190–203. doi: 10.5009/gnl220260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yang J, Wei H, Zhou Y, Szeto CH, Li C, Lin Y, et al. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology. (2022) 162:135–49. doi: 10.1053/j.gastro.2021.08.041 [DOI] [PubMed] [Google Scholar]
- 81. Long L, Yang W, Liu L, Tobias DK, Katagiri R, Wu K, et al. Dietary intake of branched-chain amino acids and survival after colorectal cancer diagnosis. Int J Cancer. (2020) 148:2471–80. doi: 10.1002/ijc.33449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rossi M, Mascaretti F, Parpinel M, Serraino D, Crispo A, Celentano E, et al. Dietary intake of branched-chain amino acids and colorectal cancer risk. Br J Nutr. (2021) 126:22–7. doi: 10.1017/S0007114520003724 [DOI] [PubMed] [Google Scholar]
- 83. Coker OO, Liu C, Wu W, Wong SH, Jia W, Sung J, et al. Altered gut metabolites and microbiota interactions are implicated in colorectal carcinogenesis and can be non-invasive diagnostic biomarkers. Microbiome. (2022) 10:35. doi: 10.1186/s40168-021-01208-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Liu NN, Jiao N, Tan JC, Wang Z, Wu D, Wang AJ, et al. Multi-kingdom microbiota analyses identify bacterial-fungal interactions and biomarkers of colorectal cancer across cohorts. Nat Microbiol. (2022) 7:238–50. doi: 10.1038/s41564-021-01030-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wu Z, Huang Y, Zhang R, Zheng C, You F, Wang M, et al. Sex differences in colorectal cancer: with a focus on sex hormone-gut microbiome axis. Cell Commun Signal. (2024) 22:167. doi: 10.1186/s12964-024-01549-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shang FM, Liu HL. Fusobacterium nucleatum and colorectal cancer: A review. World J Gastrointest Oncol. (2018) 10:71–81. doi: 10.4251/wjgo.v10.i3.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cheng WT, Kantilal HK, Davamani F. The mechanism of bacteroides fragilis toxin contributes to colon cancer formation. Malays J Med Sci. (2020) 27:9–21. doi: 10.21315/mjms2020.27.4.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Valguarnera E, Wardenburg JB. Good gone bad: one toxin away from disease for bacteroides fragilis. J Mol Biol. (2020) 432:765–85. doi: 10.1016/j.jmb.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 89. Pleguezuelos-Manzano C, Puschhof J, Rosendahl HA, van Hoeck A, Wood HM, Nomburg J, et al. Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nat. (2020) 580:269–73. doi: 10.1038/s41586-020-2080-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nougayrède JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science. (2006) 313:848–51. doi: 10.1126/science.1127059 [DOI] [PubMed] [Google Scholar]
- 91. Jaye K, Li CG, Bhuyan DJ. The complex interplay of gut microbiota with the five most common cancer types: From carcinogenesis to therapeutics to prognoses. Crit Rev Oncol Hematol. (2021) 165:103429. doi: 10.1016/j.critrevonc.2021.103429 [DOI] [PubMed] [Google Scholar]
- 92. Wang LY, He LH, Xu LJ, Li SB. Short-chain fatty acids: bridges between diet, gut microbiota, and health. J Gastroenterol Hepatol. (2024) 39:1728–36. doi: 10.1111/jgh.16619 [DOI] [PubMed] [Google Scholar]
- 93. Archana, Gupta AK, Noumani A, Panday DK, Zaidi F, Sahu GK, et al. Gut microbiota derived short-chain fatty acids in physiology and pathology: An update. Cell Biochem Funct. (2024) 42:e4108. doi: 10.1002/cbf.4108 [DOI] [PubMed] [Google Scholar]
- 94. Sulit AK, Daigneault M, Allen-Vercoe E, Silander OK, Hock B, McKenzie J, et al. Bacterial lipopolysaccharide modulates immune response in the colorectal tumor microenvironment. NPJ Biofilms Microbiomes. (2023) 9:59. doi: 10.1038/s41522-023-00429-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhang T, Li Q, Cheng L, Buch H, Zhang F. Akkermansia muciniphila is a promising probiotic. Microb Biotechnol. (2019) 12:1109–25. doi: 10.1111/1751-7915.13410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ouyang J, Lin J, Isnard S, Fombuena B, Peng X, Marette A, et al. The bacterium akkermansia muciniphila: A sentinel for gut permeability and its relevance to HIV-related inflammation. Front Immunol. (2020) 11:645. doi: 10.3389/fimmu.2020.00645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Taylor JC, Kumar R, Xu J, Xu Y. A pathogenicity locus of Streptococcus gallolyticus subspecies gallolyticus. Sci Rep. (2023) 13:6291. doi: 10.1038/s41598-023-33178-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Périchon B, Lichtl-Häfele J, Bergsten E, Delage V, Trieu-Cuot P, Sansonetti P, et al. Detection of Streptococcus gallolyticus and Four Other CRC-Associated Bacteria in Patient Stools Reveals a Potential “Driver” Role for Enterotoxigenic Bacteroides fragilis. Front Cell Infect Microbiol. (2022) 12:794391. doi: 10.3389/fcimb.2022.794391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hodgkinson K, El AF, Dobranowski P, Manoogian J, Butcher J, Figeys D, et al. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin Nutr. (2023) 42:61–75. doi: 10.1016/j.clnu.2022.10.024 [DOI] [PubMed] [Google Scholar]
- 100. Leonel AJ, Alvarez-Leite JI. Butyrate: implications for intestinal function. Curr Opin Clin Nutr Metab Care. (2012) 15:474–79. doi: 10.1097/MCO.0b013e32835665fa [DOI] [PubMed] [Google Scholar]
- 101. Zhu Y, Yang D, Duan X, Zhang Y, Chen D, Gong Z, et al. Perfluorooctane sulfonate promotes doxycycline-induced liver tumor progression in male Kras(v12) transgenic zebrafish. Environ Res. (2021) 196:110962. doi: 10.1016/j.envres.2021.110962 [DOI] [PubMed] [Google Scholar]
- 102. Kong C, Liang L, Liu G, Du L, Yang Y, Liu J, et al. Integrated metagenomic and metabolomic analysis reveals distinct gut-microbiome-derived phenotypes in early-onset colorectal cancer. Gut. (2023) 72:1129–42. doi: 10.1136/gutjnl-2022-327156 [DOI] [PubMed] [Google Scholar]
- 103. Wang G, Yu Y, Wang YZ, Wang JJ, Guan R, Sun Y, et al. Role of SCFAs in gut microbiome and glycolysis for colorectal cancer therapy. J Cell Physiol. (2019) 234:17023–49. doi: 10.1002/jcp.28436 [DOI] [PubMed] [Google Scholar]
- 104. O’Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. (2016) 13:691–706. doi: 10.1038/nrgastro.2016.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liang JQ, Li T, Nakatsu G, Chen YX, Yau TO, Chu E, et al. A novel faecal Lachnoclostridium marker for the non-invasive diagnosis of colorectal adenoma and cancer. Gut. (2020) 69:1248–57. doi: 10.1136/gutjnl-2019-318532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yachida S, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat Med. (2019) 25:968–76. doi: 10.1038/s41591-019-0458-7 [DOI] [PubMed] [Google Scholar]
- 107. McCoy AN, Araújo-Pérez F, Azcárate-Peril A, Yeh JJ, Sandler RS, Keku TO. Fusobacterium is associated with colorectal adenomas. PloS One. (2013) 8:e53653. doi: 10.1371/journal.pone.0053653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. (2012) 338:120–23. doi: 10.1126/science.1224820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Boleij A, Tjalsma H. The itinerary of Streptococcus gallolyticus infection in patients with colonic Malignant disease. Lancet Infect Dis. (2013) 13:719–24. doi: 10.1016/S1473-3099(13)70107-5 [DOI] [PubMed] [Google Scholar]
- 110. Corrêa NB, Péret FL, Penna FJ, Lima FM, Nicoli JR. A randomized formula controlled trial of Bifidobacterium lactis and Streptococcus thermophilus for prevention of antibiotic-associated diarrhea in infants. J Clin Gastroenterol. (2005) 39:385–89. doi: 10.1097/01.mcg.0000159217.47419.5b [DOI] [PubMed] [Google Scholar]
- 111. Horz HP, Meinelt A, Houben B, Conrads G. Distribution and persistence of probiotic Streptococcus salivarius K12 in the human oral cavity as determined by real-time quantitative polymerase chain reaction. Oral Microbiol Immunol. (2007) 22:126–30. doi: 10.1111/j.1399-302X.2007.00334.x [DOI] [PubMed] [Google Scholar]
- 112. Roy D, Ward P, Vincent D, Mondou F. Molecular identification of potentially probiotic lactobacilli. Curr Microbiol. (2000) 40:40–6. doi: 10.1007/s002849910008 [DOI] [PubMed] [Google Scholar]
- 113. Szajewska H, Ruszczyński M, Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. (2006) 149:367–72. doi: 10.1016/j.jpeds.2006.04.053 [DOI] [PubMed] [Google Scholar]
- 114. Pilchová T, Pilet MF, Cappelier JM, Pazlarová J, Tresse O. Protective Effect of Carnobacterium spp. against Listeria monocytogenes during host cell invasion using in vitro HT29 Model. Front Cell Infect Microbiol. (2016) 6:88. doi: 10.3389/fcimb.2016.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Eslami M, Yousefi B, Kokhaei P, Hemati M, Nejad ZR, Arabkari V, et al. Importance of probiotics in the prevention and treatment of colorectal cancer. J Cell Physiol. (2019) 234:17127–43. doi: 10.1002/jcp.28473 [DOI] [PubMed] [Google Scholar]
- 116. Coker OO, Nakatsu G, Dai RZ, Wu W, Wong SH, Ng SC, et al. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut. (2019) 68:654–62. doi: 10.1136/gutjnl-2018-317178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Huang Y, Huang X, Wang Z, He F, Huang Z, Chen C, et al. Analysis of differences in intestinal flora associated with different BMI status in colorectal cancer patients. J Transl Med. (2024) 22:142. doi: 10.1186/s12967-024-04903-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Coker OO, Wu W, Wong SH, Sung J, Yu J. Altered gut archaea composition and interaction with bacteria are associated with colorectal cancer. Gastroenterology. (2020) 159:1459–70. doi: 10.1053/j.gastro.2020.06.042 [DOI] [PubMed] [Google Scholar]
- 119. Lin Y, Lau HC, Liu Y, Kang X, Wang Y, Ting NL, et al. Altered mycobiota signatures and enriched pathogenic aspergillus rambellii are associated with colorectal cancer based on multicohort fecal metagenomic analyses. Gastroenterology. (2022) 163:908–21. doi: 10.1053/j.gastro.2022.06.038 [DOI] [PubMed] [Google Scholar]
- 120. Koliarakis I, Messaritakis I, Nikolouzakis TK, Hamilos G, Souglakos J, Tsiaoussis J. Oral bacteria and intestinal dysbiosis in colorectal cancer. Int J Mol Sci. (2019) 20:4146. doi: 10.3390/ijms20174146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wang K, Zhang J, Zhang Y, Xue J, Wang H, Tan X, et al. The recovery of intestinal barrier function and changes in oral microbiota after radiation therapy injury. Front Cell Infect Microbiol. (2023) 13:1288666. doi: 10.3389/fcimb.2023.1288666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gao R, Kong C, Li H, Huang L, Qu X, Qin N, et al. Dysbiosis signature of mycobiota in colon polyp and colorectal cancer. Eur J Clin Microbiol Infect Dis. (2017) 36:2457–68. doi: 10.1007/s10096-017-3085-6 [DOI] [PubMed] [Google Scholar]
- 123. Ferlizza E, Solmi R, Sgarzi M, Ricciardiello L, Lauriola M. The roadmap of colorectal cancer screening. Cancers (Basel). (2021) 13:1101. doi: 10.3390/cancers13051101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tabatabaei MS, Islam R, Ahmed M. Size and macromolecule stabilizer-dependent performance of gold colloids in immuno-PCR. Anal Bioanal Chem. (2022) 414:2205–17. doi: 10.1007/s00216-021-03857-9 [DOI] [PubMed] [Google Scholar]
- 125. Xu H, Chen H, Hu J, Xiong Z, Li D, Wang S, et al. Feasibility of quantification based on novel evaluation with stool DNA and fecal immunochemical test for colorectal cancer detection. BMC Gastroenterol. (2022) 22:384. doi: 10.1186/s12876-022-02470-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wu D, Zhou G, Jin P, Zhu J, Li S, Wu Q, et al. Detection of colorectal cancer using a simplified SEPT9 gene methylation assay is a reliable method for opportunistic screening. J Mol Diagn. (2016) 18:535–45. doi: 10.1016/j.jmoldx.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 127. Lu DC, Zhang QF, Li L, Luo XK, Liang B, Lu YH, et al. Methylated Septin9 has moderate diagnostic value in colorectal cancer detection in Chinese population: a multicenter study. BMC Gastroenterol. (2022) 22:232. doi: 10.1186/s12876-022-02313-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Dybdahl JH, Haug K, Bakkevold K, Olsen KO, Vetvik K. Screening for occult faecal blood loss in a community by means of Hemoccult II slides and a tetramethylbenzidine test. Scand J Gastroenterol. (1984) 19:343–49. [PubMed] [Google Scholar]
- 129. Zhang Y, Wang Y, Zhang B, Li P, Zhao Y. Methods and biomarkers for early detection, prediction, and diagnosis of colorectal cancer. BioMed Pharmacother. (2023) 163:114786. doi: 10.1016/j.biopha.2023.114786 [DOI] [PubMed] [Google Scholar]
- 130. Wilmanski T, Rappaport N, Earls JC, Magis AT, Manor O, Lovejoy J, et al. Blood metabolome predicts gut microbiome α-diversity in humans. Nat Biotechnol. (2019) 37:1217–28. doi: 10.1038/s41587-019-0233-9 [DOI] [PubMed] [Google Scholar]
- 131. Chen F, Dai X, Zhou CC, Li KX, Zhang YJ, Lou XY, et al. Integrated analysis of the faecal metagenome and serum metabolome reveals the role of gut microbiome-associated metabolites in the detection of colorectal cancer and adenoma. Gut. (2022) 71:1315–25. doi: 10.1136/gutjnl-2020-323476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xie Z, Zhu R, Huang X, Yao F, Jin S, Huang Q, et al. Metabolomic analysis of gut metabolites in patients with colorectal cancer: Association with disease development and outcome. Oncol Lett. (2023) 26:358. doi: 10.3892/ol.2023.13944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cooper RM, Wright JA, Ng JQ, Goyne JM, Suzuki N, Lee YK, et al. Engineered bacteria detect tumor DNA. Science. (2023) 381:682–86. doi: 10.1126/science.adf3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lin WH, Xiao J, Ye ZY, Wei DL, Zhai XH, Xu RH, et al. Circulating tumor DNA methylation marker MYO1-G for diagnosis and monitoring of colorectal cancer. Clin Epigenet. (2021) 13:232. doi: 10.1186/s13148-021-01216-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Esteller M. Epigenetics in cancer. N Engl J Med. (2008) 358:1148–59. doi: 10.1056/NEJMra072067 [DOI] [PubMed] [Google Scholar]
- 136. Karpf AR, Peterson PW, Rawlins JT, Dalley BK, Yang Q, Albertsen H, et al. Inhibition of DNA methyltransferase stimulates the expression of signal transducer and activator of transcription 1, 2, and 3 genes in colon tumor cells. Proc Natl Acad Sci U.S.A. (1999) 96:14007–12. doi: 10.1073/pnas.96.24.14007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Villéger R, Lopès A, Veziant J, Gagnière J, Barnich N, Billard E, et al. Microbial markers in colorectal cancer detection and/or prognosis. World J Gastroenterol. (2018) 24:2327–47. doi: 10.3748/wjg.v24.i22.2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. (2016) 65:1973–80. doi: 10.1136/gutjnl-2015-310101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wang F, He MM, Yao YC, Zhao X, Wang ZQ, Jin Y, et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med. (2021) 2:100383. doi: 10.1016/j.xcrm.2021.100383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yi Y, Shen L, Shi W, Xia F, Zhang H, Wang Y, et al. Gut microbiome components predict response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: A prospective, longitudinal study. Clin Cancer Res. (2021) 27:1329–40. doi: 10.1158/1078-0432.CCR-20-3445 [DOI] [PubMed] [Google Scholar]
- 141. Khoruts A. Can FMT cause or prevent CRC? Maybe, but there is more to consider. Gastroenterology. (2021) 161:1103–05. doi: 10.1053/j.gastro.2021.06.074 [DOI] [PubMed] [Google Scholar]
- 142. Fritsch J, Garces L, Quintero MA, Pignac-Kobinger J, Santander AM, Fernández I, et al. Low-fat, high-fiber diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with ulcerative colitis. Clin Gastroenterol Hepatol. (2021) 19:1189–99. doi: 10.1016/j.cgh.2020.05.026 [DOI] [PubMed] [Google Scholar]
- 143. Biruete A, Cross TL, Allen JM, Kistler BM, de Loor H, Evenepoel P, et al. Effect of dietary inulin supplementation on the gut microbiota composition and derived metabolites of individuals undergoing hemodialysis: A pilot study. J Ren Nutr. (2021) 31:512–22. doi: 10.1053/j.jrn.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Guthrie L, Spencer SP, Perelman D, Van Treuren W, Han S, Yu FB, et al. Impact of a 7-day homogeneous diet on interpersonal variation in human gut microbiomes and metabolomes. Cell Host Microbe. (2022) 30:863–74. doi: 10.1016/j.chom.2022.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Peng D, Wang Y, Yao Y, Yang Z, Wu S, Zeng K, et al. Long-chain polyunsaturated fatty acids influence colorectal cancer progression via the interactions between the intestinal microflora and the macrophages. Mol Cell Biochem. (2024). doi: 10.1007/s11010-023-04904-y [DOI] [PubMed] [Google Scholar]
- 146. Watson H, Mitra S, Croden FC, Taylor M, Wood HM, Perry SL, et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut. (2018) 67:1974–83. doi: 10.1136/gutjnl-2017-314968 [DOI] [PubMed] [Google Scholar]
- 147. Vetrani C, Maukonen J, Bozzetto L, Della PG, Vitale M, Costabile G, et al. Diets naturally rich in polyphenols and/or long-chain n-3 polyunsaturated fatty acids differently affect microbiota composition in high-cardiometabolic-risk individuals. Acta Diabetol. (2020) 57:853–60. doi: 10.1007/s00592-020-01494-9 [DOI] [PubMed] [Google Scholar]
- 148. Kjølbæk L, Benítez-Páez A, Gómez DPE, Brahe LK, Liebisch G, Matysik S, et al. Arabinoxylan oligosaccharides and polyunsaturated fatty acid effects on gut microbiota and metabolic markers in overweight individuals with signs of metabolic syndrome: A randomized cross-over trial. Clin Nutr. (2020) 39:67–79. doi: 10.1016/j.clnu.2019.01.012 [DOI] [PubMed] [Google Scholar]
- 149. Lauka L, Reitano E, Carra MC, Gaiani F, Gavriilidis P, Brunetti F, et al. Role of the intestinal microbiome in colorectal cancer surgery outcomes. World J Surg Oncol. (2019) 17:204. doi: 10.1186/s12957-019-1754-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. (2019) 16:690–704. doi: 10.1038/s41575-019-0209-8 [DOI] [PubMed] [Google Scholar]
- 151. Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. (2017) 170:548–63. doi: 10.1016/j.cell.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhang S, Yang Y, Weng W, Guo B, Cai G, Ma Y, et al. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J Exp Clin Cancer Res. (2019) 38:14. doi: 10.1186/s13046-018-0985-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lee JB, Kim KA, Cho HY, Kim D, Kim WK, Yong D, et al. Association between Fusobacterium nucleatum and patient prognosis in metastatic colon cancer. Sci Rep. (2021) 11:20263. doi: 10.1038/s41598-021-98941-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Vymetalkova V, Vodicka P, Vodenkova S, Alonso S, Schneider-Stock R. DNA methylation and chromatin modifiers in colorectal cancer. Mol Aspects Med. (2019) 69:73–92. doi: 10.1016/j.mam.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 155. Zhang C, Wang XY, Zhang P, He TC, Han JH, Zhang R, et al. Cancer-derived exosomal HSPC111 promotes colorectal cancer liver metastasis by reprogramming lipid metabolism in cancer-associated fibroblasts. Cell Death Dis. (2022) 13:57. doi: 10.1038/s41419-022-04506-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Vernia F, Longo S, Stefanelli G, Viscido A, Latella G. Dietary factors modulating colorectal carcinogenesis. Nutrients. (2021) 13:143. doi: 10.3390/nu13010143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Tomonaga N, Manabe Y, Aida K, Sugawara T. Dietary ceramide 2-aminoethylphosphonate, a marine sphingophosphonolipid, improves skin barrier function in hairless mice. Sci Rep. (2020) 10:13891. doi: 10.1038/s41598-020-70888-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Vučetić M, Cormerais Y, Parks SK, Pouysségur J. The central role of amino acids in cancer redox homeostasis: vulnerability points of the cancer redox code. Front Oncol. (2017) 7:319. doi: 10.3389/fonc.2017.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Mouradov D, Greenfield P, Li S, In EJ, Storey C, Sakthianandeswaren A, et al. Oncomicrobial community profiling identifies clinicomolecular and prognostic subtypes of colorectal cancer. Gastroenterology. (2023) 165:104–20. doi: 10.1053/j.gastro.2023.03.205 [DOI] [PubMed] [Google Scholar]
- 160. Wu Y, Jiao N, Zhu R, Zhang Y, Wu D, Wang AJ, et al. Identification of microbial markers across populations in early detection of colorectal cancer. Nat Commun. (2021) 12:3063. doi: 10.1038/s41467-021-23265-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Qu R, Zhang Y, Ma Y, Zhou X, Sun L, Jiang C, et al. Role of the gut microbiota and its metabolites in tumorigenesis or development of colorectal cancer. Adv Sci (Weinh). (2023) 10:e2205563. doi: 10.1002/advs.202205563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Yang X, Qiu H, Wang L, Wang X. Predicting colorectal cancer survival using time-to-event machine learning: retrospective cohort study. J Med Internet Res. (2023) 25:e44417. doi: 10.2196/44417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Zhang W, Chen X, Wong KC. Noninvasive early diagnosis of intestinal diseases based on artificial intelligence in genomics and microbiome. J Gastroenterol Hepatol. (2021) 36:823–31. doi: 10.1111/jgh.15500 [DOI] [PubMed] [Google Scholar]