Introduction
Silent atrial fibrillation (AF) represents 10% to 40% of all AF patients with morbidity and mortality rates similar to those of symptomatic AF.1, 2, 3 Stroke can be the first manifestation of silent AF with the lack of use of oral anticoagulation therapy.4 Patients with implantable cardioverter-defibrillators (ICDs) are at high risk (up to 50%) of developing AF.5
The availability of a single-lead ICD system with the ability to sense atrial rhythm from the floating electrode (a VDD-ICD) may permit accurate diagnosis of AF. Here, we report a randomized trial to compare a VDD-ICD system with a standard VVI-ICD for the ability to diagnose subclinical AF in patients without prior AF, receiving an ICD for standard indications.
Methods
Study design
The details of the design of the Dx-AF trial have been published previously.6 The Dx-AF was a prospective, multicenter, randomized controlled, open-label trial. Patients who were identified on a clinical ground for a single-chamber ICD were randomized to a LinoxSmart S DX VDD- or single-chamber ICD (Biotronik).
Inclusion criteria and exclusion criteria
The trial enrolled patients with cardiomyopathy and left ventricular ejection fraction of <50% who were scheduled for primary or secondary prevention ICD using standard guidelines. Patients were excluded if they (1) had a history of AF or flutter, (2) had active class I or III antiarrhythmic medications, or (3) were unwilling to attend study follow-up visits, considered unreliable for compliance, or with an anticipated life expectancy <3 years.
Study outcomes
The primary (efficacy) outcome of this study was the time to the first detected and confirmed episode of AF or atrial flutter lasting at least 6 minutes (detected by the ICD, electrocardiography, Holter monitor or telemetry).
The secondary (safety) outcome was a composite outcome of serious device-related complications including need for any ICD lead repositioning or replacement, pneumothorax, new pericardial effusion, cardiac tamponade, or procedure-related death or wound infection occurring in the 60 days from the time of ICD insertion.
The study was approved by the Institutional Review Board for each enrolling center as monitored by the steering committee.
Follow-up
The follow-up period in this trial was 36 months after randomization. Study follow-up occurred between 1 and 4 times per year and through remote monitoring continuously. At least 1 follow-up annually was by physical attendance.
Data analysis
Sample size calculations were conducted assuming an 80% power, 1-sided alpha of 5%, and a median follow-up of 2 years. For the main study, the rate of newly detected AF for each treatment arm was presented using the Kaplan-Meier method and comparison was made between the 2 treatment arms using a log-rank test; the treatment effect was expressed as a hazard ratio (HR) with 95% confidence interval (CI) computed from a Cox proportional hazards model. Death was a competing risk for AF; hence, cumulative incidence curves were also developed.
Results
Patients and follow-up
Between April 1, 2017, and February 28, 2021, 178 patients were enrolled in 8 Canadian centers and randomized to the treatment (VDD, n = 90) or control (VVI, n = 88) group. The average age was 65 ± 8 years (65 ± 8 years vs 66 ± 8 years), and 14% were female (10% vs 17%). The average left ventricular ejection fraction in the study population was 29 ± 6% (29 ± 7% vs 28 ± 6%). Ischemic cardiomyopathy was present in 72% (64% vs 81%), hypertension in 53%, and diabetes in 43%. Median follow-up was 2.4 years vs 2.2 years (Table 1).
Table 1.
Baseline characteristics of the patients
| Overall (N = 178) | VVI (n = 88) | VDD (n = 90) | Missing (%) | ||
|---|---|---|---|---|---|
| Age, y | — | 65.42 ± 8.01 | 65.57 ± 7.68 | 65.27 ± 8.36 | 0.6 |
| Sex | Male | 153 (86.4) | 72 (82.8) | 81 (90.0) | 0.6 |
| Female | 24 (13.6) | 15 (17.2) | 9 (10.0) | ||
| Hypertension | No | 83 (46.6) | 46 (52.3) | 37 (41.1) | 0.0 |
| Yes | 95 (53.4) | 42 (47.7) | 53 (58.9) | ||
| Diabetes | No | 101 (56.7) | 52 (59.1) | 49 (54.4) | 0.0 |
| Yes | 77 (43.3) | 36 (40.9) | 41 (45.6) | ||
| Cardiac history | Others | 49 (27.5) | 17 (19.3) | 32 (35.6) | 0.0 |
| Ischemic | 129 (72.5) | 71 (80.7) | 58 (64.4) | ||
| BMI, kg/m2 | — | 28.58 ± 5.39 | 28.16 ± 5.40 | 28.99 ± 5.38 | 1.7 |
| NYHA functional class | I | 42 (23.9) | 23 (26.4) | 19 (21.3) | 1.1 |
| II | 114 (64.8) | 57 (65.5) | 57 (64.0) | ||
| III | 20 (11.4) | 7 ( 8.0) | 13 (14.6) | ||
| IV | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| LVEF, % | — | 28.68 ± 6.65 | 28.31 ± 6.28 | 29.03 ± 7.00 | 1.7 |
Values are mean ± SD or n (%).
BMI = body mass index; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association.
Primary outcome
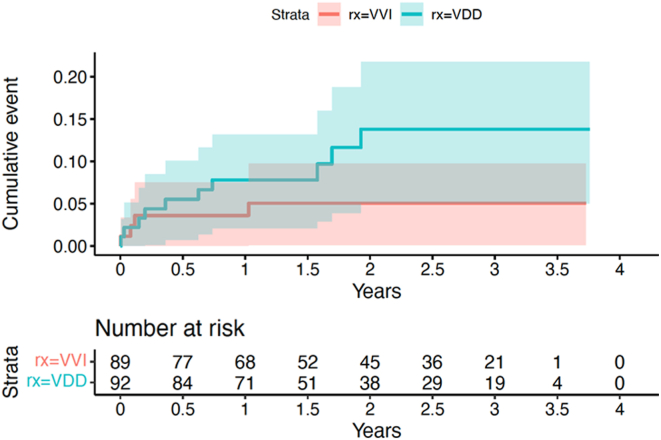
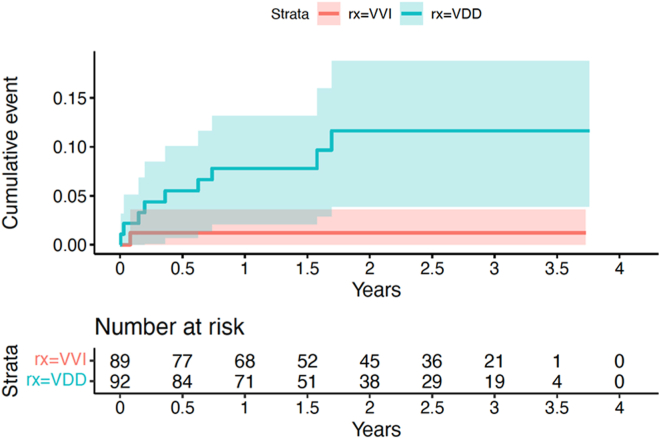
The primary outcome was diagnosed in 10 (11%) patients with VDD devices vs 4 (5%) patients with VVI devices (HR 2.36, 95% CI 0.73–7.58, P = .15). (Figure 1) The new-onset AF was detected by defibrillator in 9 patients in the VDD arm vs 1 patient in the VVI arm (HR 8.39, 95% CI 1.06–66.24, P = .04), (Figure 2) and by electrocardiography/electrocardiography monitoring in 1 vs 3 patients, respectively.
Figure 1.
Time to the first detected episode of new atrial fibrillation or flutter, with a duration of > 6 minutes, by the device, electrocardiography, or electrocardiography monitoring.
Figure 2.
Device detection of atrial fibrillation or flutter.
Secondary outcome
The secondary outcome of serious device-related complications (within 60 days of implant) was 1 (dislodgement) in the VDD group, and 2 (dislodgement, tamponade) in the VVI group.
Discussion
Our randomized controlled trial showed that the VDD-ICD increased the overall detection rate of atrial arrhythmias (HR 2.36), but due to the reduced sample size we failed to detect a statistically significant difference (P = .15). Furthermore, despite the reduced sample size, we demonstrated a difference in device detected atrial arrhythmias, which was of borderline statistical significance (HR 8.39, P = .04).
The randomized controlled trial by Sticherling and colleagues7 showed that a VDD-ICD system is equivalent to a standard DDD-ICD with regard to the detection of supraventricular tachyarrhythmias including AF. In the Management and Detection of Atrial Tachyarrhythmias in Patients Implanted with Biotronik DX Systems (MATRIX) registry, the capability of DX ICD system was assessed as 1841 DX ICD patients with daily remote monitoring were adjudicated with a 99.7% detection accuracy for device detected atrial high-rate episodes ≥1 hour.8
In the Non–Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial High Rate Episodes (NOAH-AFNET 6) trial, among patients with atrial high-rate episodes detected by implantable devices, anticoagulation with edoxaban did not significantly reduce the incidence of a composite of cardiovascular death, stroke, or systemic embolism as compared with placebo.9 However, in the Apixaban for the Reduction of Thrombo-Embolism in Patients with Device-Detected Subclinical Atrial Fibrillation (ARTESIA) trial, among patients with subclinical AF, apixaban resulted in a lower risk of stroke or systemic embolism than aspirin.10
In our study, serious device-related complications were similar between the VDD and the VVI groups. The VDD lead can be implanted safely without concerns related to an increased complications rate, as atrial lead dislodgement occurred in 4% of the patients in the DR-ICD group in the Belos A+ versus DR Clinical Investigation of Arrhythmia Discrimination (ADRIA) study.7 Our meta-analysis comparing VDD vs DDD pacemakers has revealed lower complication rates noted with VDD pacemakers.11 Our trial supports the benefit of VDD-ICD in detecting AF in comparison with VVI-ICD, without the need of implantation of additional lead and hence avoid the increased complication rate noted with DDD-ICD.
Limitations
Our trial had a small sample size and hence is underpowered to provide strong conclusions. The long-term performance of this VDD lead has not been evaluated in our study. The sample size was not large enough and hence is underpowered to assess the differences in stroke rate. Programming of ICDs in both groups was left to the treating physicians, and no data were collected regarding the pacing percentage in both groups. This study used a minimal duration of 6 minutes as an endpoint; AF of a longer duration may influence the outcomes and should be considered in future studies.
Conclusion
The VDD-ICD increased the overall detection rate of atrial arrhythmias, but due to the reduced sample size, the statistical evidence against no difference was weak and we failed to detect a statistically significant difference in our primary outcome. Furthermore, despite the reduced sample size, we demonstrated a difference in device-detected atrial arrhythmias, although this may be an overestimate due to small numbers.
Acknowledgments
Funding Sources
The study was sponsored by Biotronik.
Disclosures
The authors have no conflicts to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Written informed consent was provided by all participants in this study.
Ethics Statement
The study has been approved by the IRB for each enrolling center and was conducted according to the guidelines outlined in the Declaration of Helsinki.
Disclaimer
Given his role as Associate Editor, Jeff S. Healey had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Editor-in-Chief, Jeanne E. Poole.
References
- 1.Dobreanu D., Svendsen J.H., Lewalter T., et al. Current practice for diagnosis and management of silent atrial fibrillation: results of the European Heart Rhythm Association survey. Europace. 2013;15:1223–1225. doi: 10.1093/europace/eut227. [DOI] [PubMed] [Google Scholar]
- 2.Healey J.S., Connolly S.J., Gold M.R., et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 3.Savelieva I., Camm A.J. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J Interv Card Electrophysiol. 2000;4:369–382. doi: 10.1023/a:1009823001707. [DOI] [PubMed] [Google Scholar]
- 4.Benezet-Mazuecos J., Rubio J.M., Cortes M., et al. Silent ischaemic brain lesions related to atrial high rate episodes in patients with cardiac implantable electronic devices. Europace. 2015;17:364–369. doi: 10.1093/europace/euu267. [DOI] [PubMed] [Google Scholar]
- 5.Dries D.L., Exner D.V., Gersh B.J., Domanski M.J., Waclawiw M.A., Stevenson L.W. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32:695–703. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 6.Shurrab M., Janmohamed A., Sarrazin J.F., et al. The Dx-AF study: a prospective, multicenter, randomized controlled trial comparing VDD-ICD to VVI-ICD in detecting sub-clinical atrial fibrillation in defibrillator patients. J Interv Card Electrophysiol. 2017;50:57–63. doi: 10.1007/s10840-017-0276-0. [DOI] [PubMed] [Google Scholar]
- 7.Sticherling C., Zabel M., Spencker S., et al. Comparison of a novel, single-lead atrial sensing system with a dual-chamber implantable cardioverter-defibrillator system in patients without antibradycardia pacing indications: results of a randomized study. Circ Arrhythm Electrophysiol. 2011;4:56–63. doi: 10.1161/CIRCEP.110.958397. [DOI] [PubMed] [Google Scholar]
- 8.Hindricks G., Theuns D.A., Bar-Lev D., et al. Ability to remotely monitor atrial high-rate episodes using a single-chamber implantable cardioverter-defibrillator with a floating atrial sensing dipole. Europace. 2023;25 doi: 10.1093/europace/euad061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirchhof P., Toennis T., Goette A., et al. Anticoagulation with edoxaban in patients with atrial high-rate episodes. N Engl J Med. 2023;389:1167–1179. doi: 10.1056/NEJMoa2303062. [DOI] [PubMed] [Google Scholar]
- 10.Healey J.S., Lopes R.D., Granger C.B., et al. Apixaban for stroke prevention in subclinical atrial fibrillation. N Engl J Med. 2024;390:107–117. doi: 10.1056/NEJMoa2310234. [DOI] [PubMed] [Google Scholar]
- 11.Shurrab M., Elitzur Y., Healey J.S., et al. VDD vs DDD pacemakers: a meta-analysis. Can J Cardiol. 2014;30:1385–1391. doi: 10.1016/j.cjca.2014.04.035. [DOI] [PubMed] [Google Scholar]