Abstract
The introduction of pulsed field ablation (PFA) in electrophysiology marks a significant advancement, promising efficacy comparable to thermal ablation methods while potentially providing safety advantages. Despite a generally favorable safety profile in human trials and postmarket registries, cautious evaluation of PFA's safety is essential. This review provides a comprehensive overview of key safety considerations as we discuss a myriad of considerations ranging from thermal effects, gaseous microbubble formation, muscle contractions, and proarrhythmia to procedural techniques. We explore specific safety concerns with phrenic nerve injury, cerebral lesions, coronary artery spasm, hemolysis and pulmonary bleeding. Vigilance in safety monitoring, coupled with advancements in procedural techniques and understanding of PFA's unique effects, is crucial for optimizing the safe and effective use of PFA.
Keywords: Pulsed field ablation, Atrial fibrillation, Safety considerations
Graphical abstract
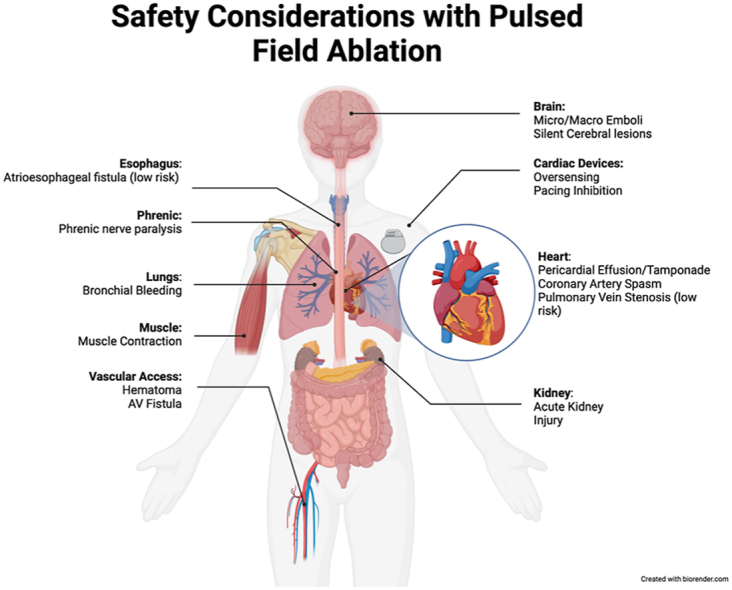
Key Findings.
-
▪
Pulsed field ablation shows limited thermal effects compared with traditional ablation methods, but ongoing research is needed for thermal mitigation.
-
▪
Microbubble formation and muscle contractions are noted with pulsed field ablation, requiring further research and potential solutions like high-frequency irreversible electroporation must be explored.
-
▪
Transient phrenic nerve injury, risks of cerebral lesions from air emboli, and coronary artery spasm during non–pulmonary vein isolation procedures are key concerns needing careful management and mitigation.
Introduction
The arrival of pulsed-field ablation (PFA) in humans has ushered in a new era for electrophysiology. While PFA demonstrates comparable efficacy to thermal ablation methods,1 its potential for less atrial dwell time and reduced risk of collateral tissue damage presents a compelling case for its adoption and use. However, PFA is not immune from safety concerns, and while human trials and postmarket registries have generally showcased a favorable safety profile,2, 3, 4, 5, 6, 7, 8 a cautious approach remains imperative. This review aims to provide a comprehensive overview of the key safety considerations associated with the use of pulsed electric fields for atrial fibrillation ablation. We discuss some general safety principles with PFA, and then specific safety concerns reported in clinical practice.
General safety principles
Thermal
Thermal considerations are critical when considering the safety aspect of PFA. Heating is a particular concern in cardiac ablation, as it can lead to char formation, steam pops, and collateral tissue damage. An ablation energy modality with no heating (or at least biologically insignificant heating) is attractive. Research with radiofrequency (RF) ablation has shown that when cardiac tissue reaches 50 °C, it tends to result in irreversible loss of cellular excitability. Thus, this is largely considered the threshold for tissue death.9 Whenever an electric field is applied to tissue, it generates heat according to Joule's first law.10 However, unlike thermal ablation modalities, the amount of heating is generally assumed to be biologically insignificant. Esophageal temperature studies during PFA delivery report small but insignificant rises in temperature, suggesting at least that there is no clinically significant temperature rise in proximity to the catheter.11,12
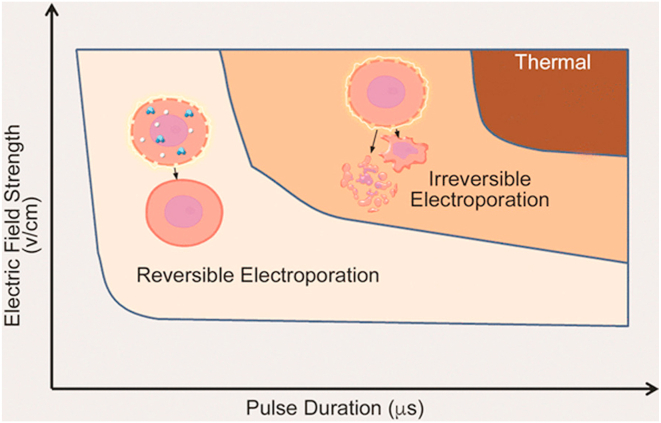
Importantly, although we may not be detecting a significant thermal effect, this does not exclude the possibility that some thermal ablation occurs in close proximity to electrodes. It is established that the greater the electric field applied and the longer the exposure time, the greater the possibility for thermal ablation (Figure 1). Particular considerations need to be further explored, including the impact of electrode size and spacing and electrode orientation relative to the tissue, which could lead to heterogeneous thermal effects. For example, electric field distribution decays more gradually with the use of cylindrical electrodes than with spherical electrodes,13 and employing sequential pulses results in a lower temperature increase than consecutive pulse delivery because the pauses allow cooling.14 While existing data generally provide reassurance about the limited thermal effects of PFA, ongoing research into thermal mitigation strategies remains crucial for ensuring continued safety.
Figure 1.
The impact of an electric pulse can vary, including reversible electroporation, irreversible electroporation, and thermal damage. Longer pulse durations and higher voltages increase the likelihood of causing irreversible damage and potential thermal effects. Reprinted with permission from Maor, Sugrue and colleagues.22
Gaseous microbubbles
Microbubble formation has been observed with PFA on intracardiac echocardiography, particularly at high energy outputs. The mechanism of bubble formation is believed to be related to hydrolysis or possible nitrogen displacement.15 Historically, monitoring for microbubble formation with RF was important, as it could indicate excessive tissue heating and precede steam pops, which are associated with a risk of cerebral emboli, tissue damage, and perforation.16 The impact of microbubbles with PFA is unclear. Further research in this critical safety area is needed, focusing on understanding clinical implications related to microbubbles and developing mitigation strategies.
Muscle contraction
Muscle contractions can lead to patient pain and discomfort and pose safety challenges with catheter stability, vascular access sheaths, and patient positioning. With PFA delivery, muscle contraction can occur through direct muscle or nerve stimulation. At present, clinically, we do not have any tools to prevent this from happening apart from paralysis. Researchers have explored a delivery protocol named high-frequency irreversible electroporation,17 which prevents muscle contractions while still enabling efficient lesion formation. For this protocol, very brief pulses, some as short as <1 μs, at a high frequency of up to 1 MHz, are delivered often in a bipolar manner. Although high-frequency irreversible electroporation has the potential to provide safer delivery through decreased muscle stimulation, ablation efficacy is unknown and further exploration in this space is needed.
Proarrhythmia
Electric field delivery to cardiac tissues risks triggering ventricular fibrillation or other cardiac arrhythmias.18 However, synchronizing pulse delivery with the electrocardiogram (ECG) signal ensures that they are delivered when all myocardial cells are in the absolute refractory period. All PFA treatments administered near the heart incorporate ECG synchronization, which has provided important safety.
Procedural technique and aspects
Vascular access complications
Vascular access complications, including hematoma, retroperitoneal bleeding, arteriovenous fistula, or pseudoaneurysm, are some of the most frequent complications associated with ablation procedures. Using ultrasound can reduce their occurrence; however, about 1.5% of patients still require blood transfusion or surgical repair.19 Currently, most ablations are performed through a deflectable or fixed sheath with a size of 8.5-F for RF and up to 15-F for cryoablation. It may intuitively make sense that with increasing French size, there is an increasing risk of vascular trauma; however, this is not the case with similar vascular access complication rates between cryoablation and RF .
PFA currently requires large groin sheaths for access, and PFA has not been immune to vascular access complications, particularly outside of Investigational Device Exemption trials, with an incidence of 3%, primarily driven by hematoma. Operators must adhere to general access principles and use ultrasound to minimize potential vascular access complications.20
Pericardial effusions and tamponade
Pericardial effusions and tamponade are procedural aspects that warrant close attention, as they can cause significant morbidity and mortality. Cardiac tamponade with PFA occurs in approximately 1% of patients [0.7% in Pulsed Field Ablation to Irreversibly Electroporate Tissue and Treat (PULSED AF PIVITOL) and 1.7% in combined data of A Safety and Feasibility Study of the IOWA Approach Endocardial Ablation System to Treat Atrial Fibrillation (IMPULSE), A Safety and Feasibility Study of the FARAPULSE Endocardial Ablation System to Treat Paroxysmal Atrial Fibrillation (PEFCAT), and Expanded Safety and Feasibility Study of the FARAPULSE Endocardial Multi Ablation System to Treat Paroxysmal Atrial Fibrillation (PEFCAT II)], with approximately 0.2% requiring cardiac surgery. Interestingly, the EUropean real world outcomes with Pulsed field ablatiOn in patients with symptomatic atRIAl fibrillation (EU-PORIA) registry noted a discrepancy in the occurrence of tamponade based on the operator's prior ablation technique. Specifically, in 9 (64%) of 14 instances, pericardial tamponade occurred when the operator's primary ablation method was RF ablation.8 This may be accounted for by several factors. These systems are first-generation PFA catheters using an over-the-wire approach, which requires a different workflow than traditional RF ablation and there is an operator learning curve. Last, in the era of force-sensing catheters, increasing dependence has been placed on using force as a surrogate for contact. In the era of PFA, many approaches do not have any integrated contact or force sensing, and it is quite possible that the lack of a surrogate measure of contact may result in excessive force being applied against the myocardium.
At present, we have limited ability to visualize the PFA catheters in a conventional mapping system. Consequently, the movement of the PFA catheter is primarily driven by fluoroscopy. However, there has been a decrease in the use of fluoroscopy with 3-dimensional mapping systems and contact force catheters. Therefore, without these regular tools, it is important to ensure careful manipulation of the catheter, with particular care near the left atrial appendage and posterior wall. However, we will soon have access to fully integrated mapping and ablation systems, enabling full visualization of the PFA catheter inside the mapping software.
Relying on fluoroscopy also brings further safety considerations, which means a significant increase in fluoroscopy time and radiation dose to the patient and operator. While we wait for the ability to integrate optimally with mapping systems, adhering to the ALARA (as low as reasonably achievable) principles is important.
Atrioesophageal fistula
One of the most devastating consequences of atrial fibrillation ablation is the development of an atrioesophageal fistula. This is associated with a high mortality of 30%. Interestingly, this one ablation complication has not decreased over time and has stayed at a steady incidence of 0.2% to 0.3% (though the true incidence is likely underappreciated).21 Many strategies have been employed to mitigate this risk, including esophageal temperature monitoring, power titration, and a flurry of novel devices ranging from esophageal deviation to esophageal cooling devices. While these techniques have had some success, they do not solve the fundamental issue that drives the problem: thermal energy.
PFA has shown a remarkably good esophageal safety profile across multiple studies of the Boston scientific and Medtronic Food and Drug Administration–approved catheters. The potential safety advantages of PFA are that it seems to be primarily nonthermal (or at least no clinically significant temperature rise), and the biophysics of electrical field ablation offers some potential protection to the esophagus. The esophagus contains both longitudinal and circular muscle fibers. When an electric field is applied between electrodes, the electrode orientation relative to the fiber orientation of the tissue has an important impact on the observed effect.22 The general principle is that when a similar electric field is applied parallel to the tissue fibers, a greater ablation effect will be observed than when the electric field is applied perpendicular to the tissue; therefore, by having both longitudinal and circular muscle fibers, it may provide an innate protection mechanism that can limit esophageal tissue damage.
Phrenic
Phrenic nerve (PN) injury is a well-known complication of thermal ablation, with a prevalence reported to range from 0.3% to 0.48%.23,24 Fortunately, most patients recover, but in those with persistent PN injury, it can be a real cause of significant morbidity.
With PFA, the cell radius is critical in determining the energy requirements to reach a critical threshold for ablation; the larger the cell, the less energy required for ablation. Cardiac muscle cells have a larger diameter than nerve tissue; therefore, on this basic principle, it would be assumed the PN could be spared if energy is titrated appropriately. However, it has been reported that PN paralysis can occur, but this seems primarily transient, with recovery ranging from seconds to 24 hours.25 The Multi-National Survey on the Methods, Efficacy, and Safety on the Post-Approval Clinical Use of Pulsed Field Ablation (MANIFEST-PF) study reported one patient (0.06%) had persistent PN damage.7 In the Pulsed Field Ablation to Irreversibly Electroporate Tissue and Treat AF (PULSED AF) pilot and pivotal trial, there was no reported occurrences of phrenic injury.
Unlike RF, in which pacing can help localize the phrenic and guide ablation lesions to limit close proximity ablation, with PFA, one is currently unable to avoid ablation in regions of close proximity. Further, with delivery in the right pulmonary vein, phrenic stimulation can cause catheter movement and instability, and at least anecdotally, we have seen a preference for paralysis upfront to limit patient movement. This, therefore, can limit the ability to recognize if it has occurred and either alter or limit further delivery.
Cerebral lesions
Symptomatic and silent cerebral lesions (SCLs) are recognized complications of catheter ablation for atrial fibrillation, regardless of ablation technologies. SCLs are common, occur despite anticoagulation and are often associated with persistent AF and procedural factors such as electrical cardioversion. While SCLs are typically asymptomatic, there is a suggestion that they may raise the risk of developing dementia.26 However, the exact influence of these lesions on cognitive decline remains uncertain. It is worth noting that many SCLs resolve within months, leaving questions about their long-term impact. Nonetheless, concerns persist regarding potential subtle cognitive alterations or the cumulative effect of recurrent SCL formation over time.
The creation of gaseous emboli (both macro and micro) has the potential to cause significant complications. The incidence of symptomatic cerebral thrombotic lesions in patients undergoing PVI with PFA is <1%. In the PULSED AF PIVITOL trial, one cerebrovascular accident occurred on the same day as the procedure in a patient with paroxysmal AF. This patient had left lower leg numbness and mild dysphasia that was resolving at the conclusion of the study, zero were reported in the Randomized Controlled Trial for Pulsed Field Ablation versus Standard of Care Ablation for Paroxysmal Atrial Fibrillation (ADVENT) trial. Clinical safety data on asymptomatic cerebral lesions are limited, as it is only reported on small subsets of studies, with varying percentages of 0% to 18.5%.2,27,28 Further research is needed in this area to understand its true incidence and mechanism. One of the key safety aspects is meticulous sheath management, which is critical to minimize the risk of macro air embolism. This is particularly a safety concern with the larger sheaths air ingress is a real risk.
Coronary arteries
The first reported case of spasm was in 2021, coinciding with off-label PFA of the mitral isthmus.29 Since then, there has been a surge in documented cases of coronary artery spasms when performing ablation beyond PVI. Notably, the risk of spasm is minimal with PVI, as evidenced by only 1 (0.06%) reported case in the MANIFEST-PF registry, aligning with the safety profile of current thermal ablation methods. Nevertheless, this emphasizes a genuine safety concern associated with PFA, particularly when utilized outside its intended application of pulmonary vein ablation.
Initial animal studies were encouraging in terms of impact on coronary vessels. PFA delivery on the outer surface of carotid30 and iliac31 arteries resulted in decellularization, connective matrix preservation, and no stenosis. Our group subsequently raised potential safety concerns with coronary spams with PFA in a preclinical study. When delivered intracoronary, regardless of pulse duration, PFA-induced coronary spasm occurred with fixed coronary stenosis developing chronically. Conversely, epicardial application of PFA resulted in temporary coronary spasm without detectable chronic angiographic lesions. Histopathological examination revealed evidence of coronary injury following intracoronary ablation but not after epicardial ablation. Furthermore, longer pulse widths (100 μs) were associated with greater coronary injury than shorter pulses (300 ns).
In human trials, in cavotricuspid isthmus ablation with PFA, patients exhibited severe subtotal vasospasm in the right coronary arterial segment adjacent to the multielectrode PFA catheter and this was alleviated by intracoronary nitroglycerin administration.32 Pretreatment nitroglycerin administration, whether intracoronary or intravenous, mitigated some but not all of the vasospasm. Aside from ECG changes noted in one patient, no clinically significant ST-segment elevations or cardiac arrhythmias were observed. The large footprint of the multielectrode catheter (with subsequently large field) could be a cause of this; however, similar effects have also been seen with a quadripolar focal PFA catheter,33 in which it was further shown that pretreatment with nitroglycerin did decrease the frequency and severity. Mitral isthmus ablation is also not immune from safety concerns, with coronary spasms observed in 7 (41.2%) of 17 patients undergoing PFA.
Therefore, we recommend not using PFA outside its current intended use of PVI and avoiding use proximate to coronary arteries. Further research is needed in this region, and while nitroglycerin may offer a cumbersome short-term solution, the long-term impact has yet to be discovered. The risk of prolonged intraprocedural hypotension in terms of cerebral ischemia, kidney insult, and other complications should be considered.
Aortic wall
It has been reported that injuries to the descending aorta occurred after both PFA and thermal ablation methods.34 Specifically, acute late gadolinium enhancement lesions were frequently observed on the descending aorta following thermal ablation and, to a lesser extent, after PFA. The clinical relevance of these findings remains uncertain.
Lung bleeding
A wire is placed during the catheter ablation into the pulmonary veins to act as a guide and support for many currently approved PFA catheters. Pulmonary bleeding has been reported with straight tip wires, with blood seen in multiple lung segments without signs of active bleeding or the origin of the bleeding. With the use of a J-tip guidewire, no bleeding was seen. Therefore, it is critical that straight wires are avoided to minimize lung injury.35 There was no report of lung bleeding in the PULSED AF pilot and pivotal trial.
Cardiac implantable electronic devices
Limited data regarding the safety and efficacy of PFA in patients with cardiac implantable electronic devices (CIEDs) are available, as clinical trials leading to the widespread adoption of PFA largely excluded patients with CIEDs. Therefore, it is not entirely clear whether energy application can cause physical damage to CIED components (leads and generator) and whether lesion delivery may lead to electromagnetic interference, causing inappropriate device therapies or pacing inhibition.
One bench study with PFA application to CIEDs (<5 cm from the lead tip and <15 cm from the generator) showed no change in device functionality, settings, or damage to leads or the generator.36 However, there was evidence of electromagnetic interference, with some oversensing and pacing inhibition but no effect on tachycardia detection. A small 6-patient study of patients undergoing PFA for pulmonary vein isolation (PVI) and cavotricuspid isthmus ablation with CIEDs found no lead damage or change in device function issues. However, 3 of 6 patients had sensing of PFA application impulses seen.37
Therefore, operators should deactivate therapies in patients with implantable cardioverter-defibrillators, perform pre- and postdevice interrogations, and avoid PFA delivery immediately near the lead tip. Given the possibility of pacing inhibition, programming asynchronous pacing modes in pacemaker-dependent patients could be considered.
Pulmonary vein stenosis
Pulmonary vein stenosis is a complication associated with significant morbidity. Patients can present with pulmonary hypertension, lung infarction, or hemoptysis. Although its incidence is declining, it is still an issue, and any methods to mitigate this risk are a welcome addition. Early animal studies showed a remarkable lack of PV stenosis narrowing with PFA inside the vein. This has translated to human studies, in which no clinically significant incidence of PV stenosis has been reported, and PFA has resulted in less PV narrowing.38
Hemolysis
Pulsed electric fields are known to cause hemolysis of red blood cells. When red blood cells break down, they release cell-free hemoglobin, heme, and iron, triggering immune responses and inflammation. Protective factors like haptoglobin, hemopexin, and apotransferrin help mitigate these effects by scavenging free hemoglobin, heme, and iron, respectively. However, excessive or chronic intravascular hemolysis can imbalance this system. Cell-free hemoglobin and heme are well-recognized potent damage-associated molecular patterns that significantly influence the plasma system, innate immune responses, and endothelial function. These effects are particularly notable in organs such as the kidney but are not exclusively observed there. The kidneys play a crucial role in clearing hemoglobin once natural scavenging systems become overwhelmed, making them particularly vulnerable to injury during hemolysis.
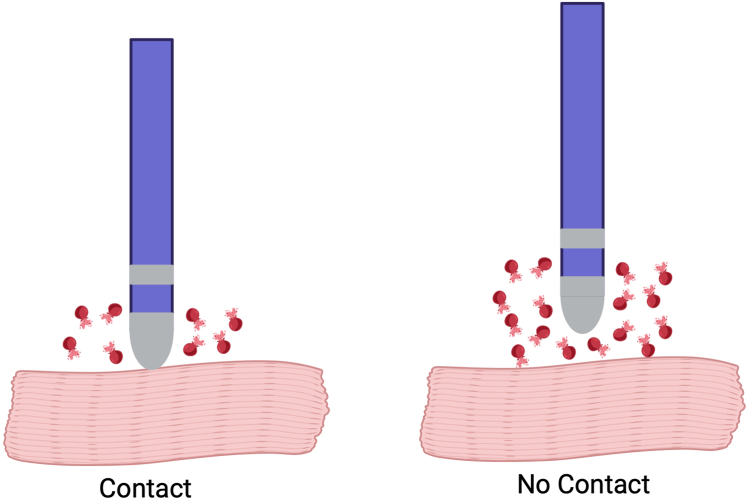
It was reported that 75% of patients postablation experienced hemoglobinuria, with a subsequent increase in lactate dehydrogenase and a decrease in haptoglobin levels in the blood.39 These patients also exhibited a significant increase in postablation creatinine compared with baseline. To mitigate this, planned hydration (average 2 L) was tested, and in contrast, patients who received planned hydration did not show signs of hemoglobinuria or acute kidney injury. Importantly, the number of PFA applications and hydration status were identified as independent predictors of postprocedural renal injury. Specifically, there appears to be a threshold level of the number of applications where this is observed. All patients developing hemolysis-related renal failure requiring hospitalization and transient dialysis in the MANIFEST registry received a high number of PFA lesions (average of 143). Important to note that tissue contact plays an important role, with no contact increasing the risk of hemolysis (Figure 2).
Figure 2.
Risk of hemolysis with tissue contact. With no tissue contact risk of hemolysis increases. Created with BioRender.com
While this offers a relatively straightforward solution, several safety concerns exist. First, as previously discussed, the consequences of hemolysis extending beyond the kidney alone are real. If acute kidney injury following hemolysis is observed, it indicates a significant overwhelming of the body's protective mechanisms and has the potential to lead to other unreported consequences. Second, administering 2 L of hydration is not insignificant, particularly for patients with underlying heart failure. Additionally, it may impact the feasibility of same-day discharge for patients.
Vigilance is not just energy specific, but also catheter specific
As stated previously, it is important to recognize that PFA's efficacy is directly dependent on several factors, including the electrode size, shape, and orientation relative to the cells, as well as specific considerations related to the pulse wave shape, duration, and strength. Thus, it is important to recognize that PFA's safety and efficacy profile may significantly differ between different catheter shapes and sizes. Similar findings were seen in the early days of RF ablation, wherein we recognized that RF ablation's efficacy, char risk, and steam pop may vary by electrode size, shape, and orientation. Thus, it is quite possible and likely that different systems may see different degrees of thermal effect and have differing efficacy in terms of causing durable tissue energy at a specific electric field size and shape. Given this, it is critical that when adopting PFA solutions, it is not assumed that the efficacy and safety profile is directly transferrable between different catheters.
Cable and system durability
When performing PFA, the system has to deliver a very large amount of energy multiple times over a short time frame. These systems have inherent protocols to minimize the risk of a short circuit leading to arcing. However, in the case where a potential risk is identified and therapy aborted, the system automatically “dumps” the energy back into their capacitors. Repeated such events can cause irreparable harm to the intrinsic PFA system and result in system failure. Thus, using systems according to stated approved standards is critical in PFA, including any associated cables and catheters. In turn, any oxidation of exposed wire elements in the cables can result in insufficient energy delivery to the tissue and less predictable tissue injury. These potential issues highlight the importance of paying close attention to labeled use while we continue to learn about how PFA may be safely and effectively delivered for cardiac ablation.
Conclusion
PFA represents a promising ablation modality for the treatment of atrial fibrillation, with overall favorable safety outcomes observed in initial human studies. However, it brings its own issues that need to be considered and addressed. Further research is required to understand better strategies for thermal limitation and microbubble formation. While data on collateral damage, particularly involving the esophagus and PN, are encouraging, there are concerns with microbubbles leading to neurologic sequelae, coronary artery spasm, hemolysis, and potential interaction with cardiac devices. Vigilance in safety monitoring and addressing concerns is paramount moving forward.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
The authors have no conflicts to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Appendix. Supplementary Data
References
- 1.Reddy V.Y., Gerstenfeld E.P., Natale A., et al. Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. N Engl J Med. 2023;389:1660–1671. doi: 10.1056/NEJMoa2307291. [DOI] [PubMed] [Google Scholar]
- 2.Verma A., Haines D.E., Boersma L.V., et al. Pulsed field ablation for the treatment of atrial fibrillation: PULSED AF pivotal trial. Circulation. 2023;147:1422–1432. doi: 10.1161/CIRCULATIONAHA.123.063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma A., Boersma L., Haines D.E., et al. First-in-human experience and acute procedural outcomes using a novel pulsed field ablation system: the PULSED AF pilot trial. Circ Arrythm Electrophysiol. 2022;15 doi: 10.1161/CIRCEP.121.010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy V.Y., Dukkipati S.R., Neuzil P., et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. Clinical Electrophysiology. 2021;7:614–627. doi: 10.1016/j.jacep.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Reddy V.Y., Neuzil P., Koruth J.S., et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol. 2019;74:315–326. doi: 10.1016/j.jacc.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Reddy V.Y., Anic A., Koruth J., et al. Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol. 2020;76:1068–1080. doi: 10.1016/j.jacc.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Turagam M.K., Neuzil P., Schmidt B., et al. Safety and effectiveness of pulsed field ablation to treat atrial fibrillation: one-year outcomes from the MANIFEST-PF registry. Circulation. 2023;148:35–46. doi: 10.1161/CIRCULATIONAHA.123.064959. [DOI] [PubMed] [Google Scholar]
- 8.Kueffer T., Bordignon S., Neven K., et al. Durability of pulmonary vein isolation using pulsed-field ablation: results from the multicenter EU-PORIA registry. JACC Clin Electrophysiol. 2024;10:698–708. doi: 10.1016/j.jacep.2023.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Haines D.E., Watson D.D. Tissue heating during radiofrequency catheter ablation: a thermodynamic model and observations in isolated perfused and superfused canine right ventricular free wall. Pacing Clin Electrophysiol. 1989;12:962–976. doi: 10.1111/j.1540-8159.1989.tb05034.x. [DOI] [PubMed] [Google Scholar]
- 10.Sugrue A., Maor E., Del-Carpio Munoz F., Killu A.M., Asirvatham S.J. Cardiac ablation with pulsed electric fields: principles and biophysics. Europace. 2022;24:1213–1222. doi: 10.1093/europace/euac033. [DOI] [PubMed] [Google Scholar]
- 11.Kirstein B., Heeger C.H., Vogler J., et al. Impact of pulsed field ablation on intraluminal esophageal temperature. J Cardiovasc Electrophysiol. 2024;35:78–85. doi: 10.1111/jce.16096. [DOI] [PubMed] [Google Scholar]
- 12.Grosse Meininghaus D., Freund R., Koerber B., Kleemann T., Matthes H., Geller J.C. Pulsed-field ablation does not induce esophageal and periesophageal injury—a new esophageal safety paradigm in catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2024;35:86–93. doi: 10.1111/jce.16132. [DOI] [PubMed] [Google Scholar]
- 13.Davalos R.V., Rubinsky B. Temperature considerations during irreversible electroporation. Int J Heat Mass Transfer. 2008;51:5617–5622. [Google Scholar]
- 14.Van Den Bos W., Scheffer H.J., Vogel J.A., et al. Thermal energy during irreversible electroporation and the influence of different ablation parameters. J Vasc Interv Radiol. 2016;27:433–443. doi: 10.1016/j.jvir.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 15.van Es R., Groen M.H., Stehouwer M., Doevendans P.A., Wittkampf F.H., Neven K. In vitro analysis of the origin and characteristics of gaseous microemboli during catheter electroporation ablation. J Cardiovasc Electrophysiol. 2019;30:2071–2079. doi: 10.1111/jce.14091. [DOI] [PubMed] [Google Scholar]
- 16.Oh S., Kilicaslan F., Zhang Y., et al. Avoiding microbubbles formation during radiofrequency left atrial ablation versus continuous microbubbles formation and standard radiofrequency ablation protocols: comparison of energy profiles and chronic lesion characteristics. J Cardiovasc Electrophysiol. 2006;17:72–77. doi: 10.1111/j.1540-8167.2005.00300.x. [DOI] [PubMed] [Google Scholar]
- 17.Arena C.B., Sano M.B., Rossmeisl J.H., et al. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed Eng Online. 2011;10:102. doi: 10.1186/1475-925X-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deodhar A., Dickfeld T., Single G.W., et al. Irreversible electroporation near the heart: ventricular arrhythmias can be prevented with ECG synchronization. Am J Roentgenol. 2011;196:W330–W335. doi: 10.2214/AJR.10.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deshmukh A., Patel N.J., Pant S., et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation. 2013;128:2104–2112. doi: 10.1161/CIRCULATIONAHA.113.003862. [DOI] [PubMed] [Google Scholar]
- 20.Calkins H., Hindricks G., Cappato R., et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1–e160. doi: 10.1093/europace/eux274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghia K.K., Chugh A., Good E., et al. A nationwide survey on the prevalence of atrioesophageal fistula after left atrial radiofrequency catheter ablation. J Interv Card Electrophysiol. 2009;24:33–36. doi: 10.1007/s10840-008-9307-1. [DOI] [PubMed] [Google Scholar]
- 22.Maor E., Sugrue A., Witt C., et al. Pulsed electric fields for cardiac ablation and beyond: a state-of-the-art review. Heart Rhythm. 2019;16:1112–1120. doi: 10.1016/j.hrthm.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Kuck K.-H., Brugada J., Fürnkranz A., et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 24.Sacher F., Monahan K.H., Thomas S.P., et al. Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J Am Coll Cardiol. 2006;47:2498–2503. doi: 10.1016/j.jacc.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 25.Pansera F., Bordignon S., Bologna F., et al. Catheter ablation induced phrenic nerve palsy by pulsed field ablation—completely impossible? A case series. Eur Heart J Case Rep. 2022;6 doi: 10.1093/ehjcr/ytac361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calvert P., Kollias G., Pürerfellner H., et al. Silent cerebral lesions following catheter ablation for atrial fibrillation: a state-of-the-art review. Europace. 2023;25 doi: 10.1093/europace/euad151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo F., Wang J., Deng Q., et al. Effects of pulsed field ablation on autonomic nervous system in paroxysmal atrial fibrillation: a pilot study. Heart Rhythm. 2023;20:329–338. doi: 10.1016/j.hrthm.2022.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Reinsch N., Füting A., Höwel D., Bell J., Lin Y., Neven K. Cerebral safety after pulsed field ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2022;19:1813–1818. doi: 10.1016/j.hrthm.2022.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Gunawardene M.A., Schaeffer B.N., Jularic M., et al. Coronary spasm during pulsed field ablation of the mitral isthmus line. Clin Electrophysiol. 2021;7:1618–1620. doi: 10.1016/j.jacep.2021.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Maor E., Ivorra A., Leor J., Rubinsky B. The effect of irreversible electroporation on blood vessels. Technol Cancer Res Treat. 2007;6:307–312. doi: 10.1177/153303460700600407. [DOI] [PubMed] [Google Scholar]
- 31.Maor E., Ivorra A., Mitchell J.J., Rubinsky B. Vascular smooth muscle cells ablation with endovascular nonthermal irreversible electroporation. J Vasc Interv Radiol. 2010;21:1708–1715. doi: 10.1016/j.jvir.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy V.Y., Petru J., Funasako M., et al. Coronary arterial spasm during pulsed field ablation to treat atrial fibrillation. Circulation. 2022;146:1808–1819. doi: 10.1161/CIRCULATIONAHA.122.061497. [DOI] [PubMed] [Google Scholar]
- 33.Malyshev Y., Neuzil P., Petru J., et al. Nitroglycerin to ameliorate coronary artery spasm during focal pulsed-field ablation for atrial fibrillation. JACC Clin Electrophysiol. 2024;10:885–896. doi: 10.1016/j.jacep.2023.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Cochet H., Nakatani Y., Sridi-Cheniti S., et al. Pulsed field ablation selectively spares the oesophagus during pulmonary vein isolation for atrial fibrillation. Europace. 2021;23:1391–1399. doi: 10.1093/europace/euab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Füting A., Reinsch N., Brokkaar L., et al. Bronchial safety after pulsed field ablation for paroxysmal atrial fibrillation. Circ Arrythm Electrophysiol. 2023;16 doi: 10.1161/CIRCEP.122.011547. [DOI] [PubMed] [Google Scholar]
- 36.Lennerz C., Oconnor M., Schaarschmidt C., et al. Pulsed-field ablation in patients with permanent pacemaker or implantable cardioverter defibrillator: Is it safe? Europace. 2023;25 [Google Scholar]
- 37.Winkelmann S., Lemoine M., Wuerger T., et al. Safety of pulsed-field ablation in patients with cardiac implantable electronic devices. A single-center pilot study. Europace. 2022;24 [Google Scholar]
- 38.Mansour M., Gerstenfeld E.P., Patel C., et al. Pulmonary vein narrowing after pulsed field versus thermal ablation. Europace. 2024;26 doi: 10.1093/europace/euae038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohanty S., Casella M., Compagnucci P., et al. Acute kidney injury resulting from hemoglobinuria after pulsed-field ablation in atrial fibrillation: is it preventable? JACC Clin Electrophysiol. 2024;10:709–715. doi: 10.1016/j.jacep.2023.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.