Abstract
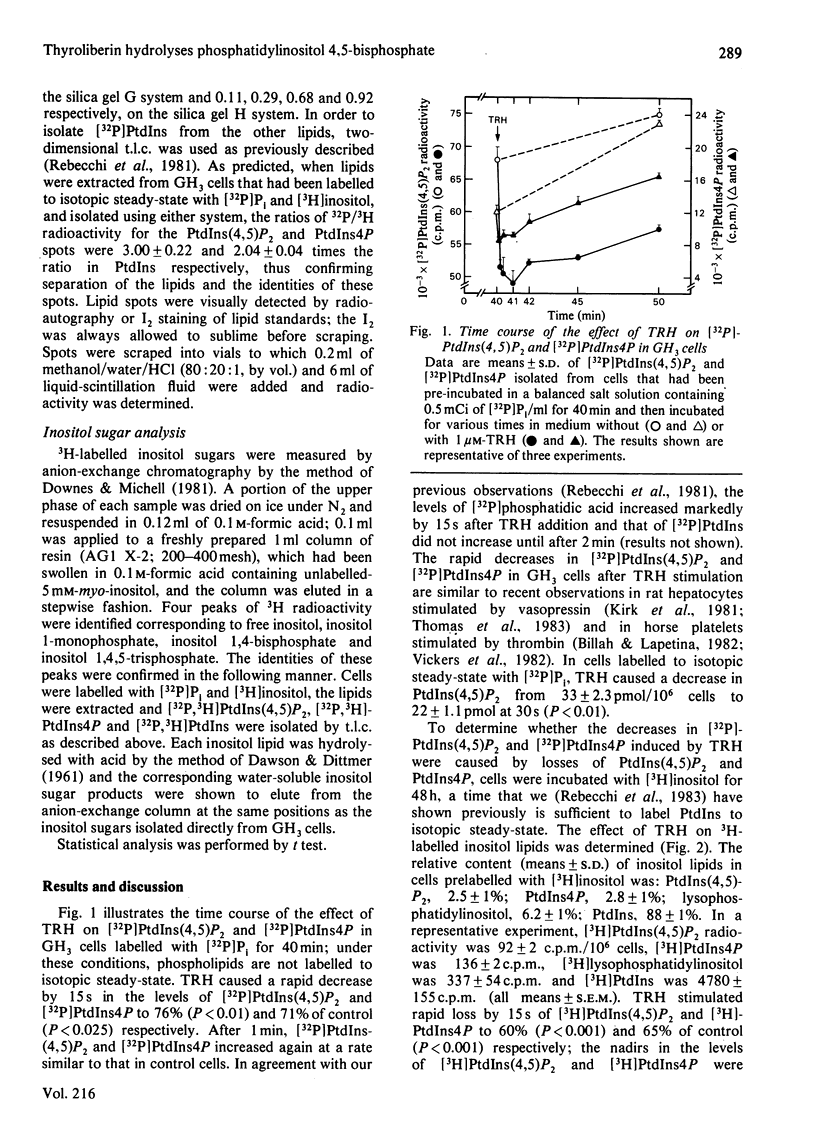
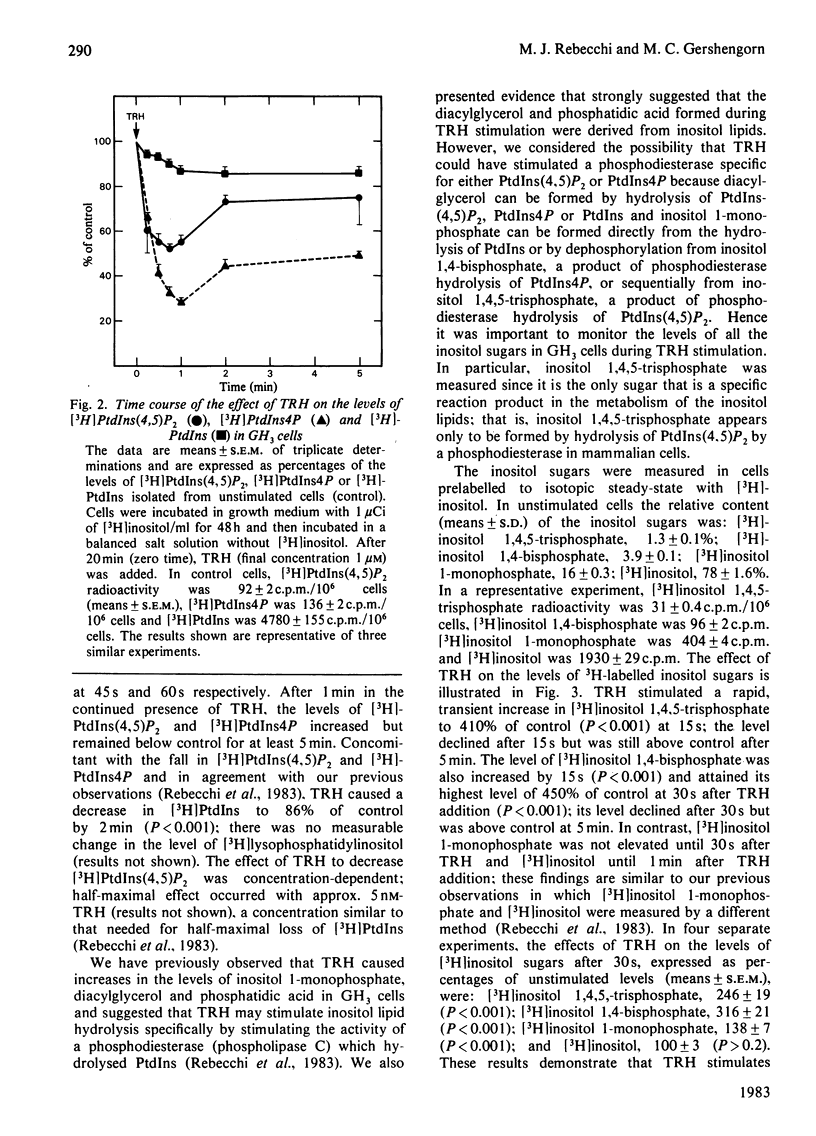
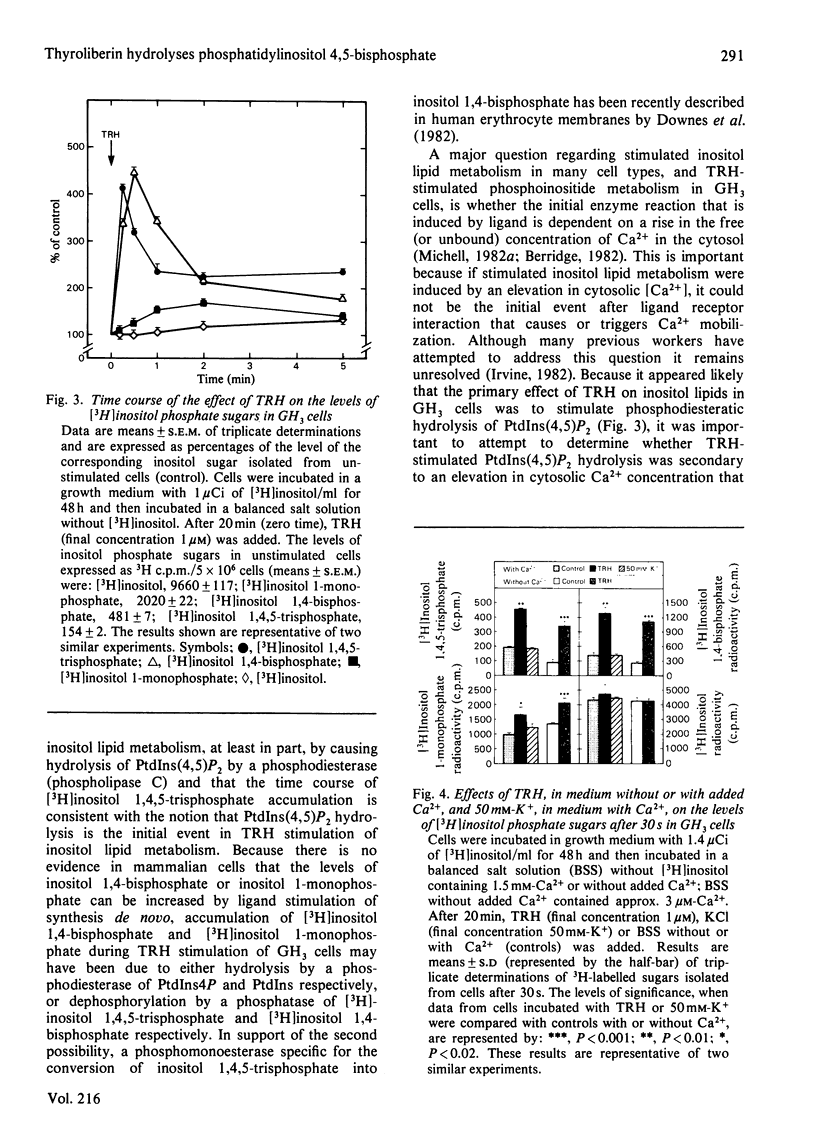
Thyrotropin-releasing hormone (TRH; thyroliberin) stimulated rapid hydrolysis of phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] by a phosphodiesterase (phospholipase C) in GH3 cells, a prolactin-secreting rat pituitary tumour cell line. TRH caused a rapid decrease in the level of PtdIns(4,5)P2 to 60% of control and stimulated a marked transient increase in inositol 1,4,5-trisphosphate, the unique product of phosphodiesteratic hydrolysis of PtdIns(4,5)P2, to a peak of 410% of control at 15 s. TRH also caused decreases in phosphatidylinositol 4-monophosphate (PtdIns4P) and phosphatidylinositol (PtdIns) to 65% and 93% of control at 15 s respectively. Inositol 1,4-bisphosphate was increased to a peak of 450% at 30 s; inositol 1-monophosphate and inositol were not elevated until 30 s and 1 min respectively after TRH addition. To study whether PtdIns(4,5)P2 hydrolysis may be caused by an elevation in cytosolic Ca2+ concentration, the changes induced by TRH in the levels of inositol sugars were compared with the effects of membrane depolarization by high extracellular [K+]. The elevation in cytosolic [Ca2+] induced by K+ depolarization did not change the level of inositol 1,4,5-trisphosphate. These data suggest that phosphodiesteratic hydrolysis of PtdIns(4,5)P2 may be the initial event in TRH stimulation of inositol lipid metabolism in GH3 cells and that PtdIns(4,5)P2 hydrolysis is not stimulated by an elevation in cytosolic Ca2+ concentration. The decreases in PtdIns4P and PtdIns may be due to enhanced conversion of PtdIns into PtdIns4P into PtdIns(4,5)P2 or to their direct hydrolysis by phosphomonoesterases and/or phosphodiesterases. These results are consistent with the hypothesis that TRH-stimulated PtdIns(4,5)P2 breakdown causes Ca2+ mobilization leading to prolactin secretion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W., Murthy P., Seguin E. B. Thrombin-induced phosphodiesteratic cleavage of phosphatidylinositol bisphosphate in human platelets. J Biol Chem. 1983 Feb 25;258(4):2076–2078. [PubMed] [Google Scholar]
- Akhtar R. A., Abdel-Latif A. A. Requirement for calcium ions in acetylcholine-stimulated phosphodiesteratic cleavage of phosphatidyl-myo-inositol 4,5-bisphosphate in rabbit iris smooth muscle. Biochem J. 1980 Dec 15;192(3):783–791. doi: 10.1042/bj1920783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. 5-Hydroxytryptamine stimulation of phosphatidylinositol hydrolysis and calcium signalling in the blowfly salivary gland. Cell Calcium. 1982 Oct;3(4-5):385–397. doi: 10.1016/0143-4160(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Rapid decrease of phosphatidylinositol 4,5-bisphosphate in thrombin-stimulated platelets. J Biol Chem. 1982 Nov 10;257(21):12705–12708. [PubMed] [Google Scholar]
- DAWSON R. M., DITTMER J. C. Evidence for the structure of brain triphosphoinositide from hydrolytic degradation studies. Biochem J. 1961 Dec;81:540–545. doi: 10.1042/bj0810540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes P., Michell R. H. Phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: lipids in search of a function. Cell Calcium. 1982 Oct;3(4-5):467–502. doi: 10.1016/0143-4160(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Drust D. S., Martin T. F. Thyrotropin-releasing hormone rapidly and transiently stimulates cytosolic calcium-dependent protein phosphorylation in GH3 pituitary cells. J Biol Chem. 1982 Jul 10;257(13):7566–7573. [PubMed] [Google Scholar]
- Gershengorn M. C., Hoffstein S. T., Rebecchi M. J., Geras E., Rubin B. G. Thyrotropin-releasing hormone stimulation of prolactin release from clonal rat pituitary cells: evidence for action independent of extracellular calcium. J Clin Invest. 1981 Jun;67(6):1769–1776. doi: 10.1172/JCI110216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershengorn M. C., Marcus-Samuels B. E., Geras E. Estrogens increase the number of thyrotropin-releasing hormone receptors on mammotropic cells in culture. Endocrinology. 1979 Jul;105(1):171–176. doi: 10.1210/endo-105-1-171. [DOI] [PubMed] [Google Scholar]
- Gershengorn M. C. Thyrotropin releasing hormone. A review of the mechanisms of acute stimulation of pituitary hormone release. Mol Cell Biochem. 1982 Jun 25;45(3):163–179. doi: 10.1007/BF00230085. [DOI] [PubMed] [Google Scholar]
- Griffin H. D., Hawthorne J. N. Calcium-activated hydrolysis of phosphatidyl-myo-inositol 4-phosphate and phosphatidyl-myo-inositol 4,5-bisphosphate in guinea-pig synaptosomes. Biochem J. 1978 Nov 15;176(2):541–552. doi: 10.1042/bj1760541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F. The enzymology of stimulated inositol lipid turnover. Cell Calcium. 1982 Oct;3(4-5):295–309. doi: 10.1016/0143-4160(82)90018-5. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Creba J. A., Downes C. P., Michell R. H. Hormone-stimulated metabolism of inositol lipids and its relationship to hepatic receptor function. Biochem Soc Trans. 1981 Oct;9(5):377–379. doi: 10.1042/bst0090377. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Is phosphatidylinositol really out of the calcium gate? Nature. 1982 Apr 8;296(5857):492–493. doi: 10.1038/296492a0. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Stimulated inositol lipid metabolism: an introduction. Cell Calcium. 1982 Oct;3(4-5):285–294. doi: 10.1016/0143-4160(82)90017-3. [DOI] [PubMed] [Google Scholar]
- Moriarty C. M., Leuschen M. P. Role of calcium in acute stimulated release of prolactin from neoplastic GH3 cells. Am J Physiol. 1981 Jun;240(6):E705–E711. doi: 10.1152/ajpendo.1981.240.6.E705. [DOI] [PubMed] [Google Scholar]
- Rawyler A. J., Roelofsen B., Wirtz K. W., Op den Kamp J. A. (poly) Phosphoinositide phosphorylation is a marker for plasma membrane in Friend erythroleukaemic cells. FEBS Lett. 1982 Nov 1;148(1):140–144. doi: 10.1016/0014-5793(82)81260-x. [DOI] [PubMed] [Google Scholar]
- Rebecchi M. J., Gerry R. H., Gershengorn M. C. Thyrotropin-releasing hormone causes loss of cellular calcium without calcium uptake by rat pituitary cells in culture. Studies using arsenazo III for direct measurement of calcium. J Biol Chem. 1982 Mar 25;257(6):2751–2753. [PubMed] [Google Scholar]
- Rebecchi M. J., Kolesnick R. N., Gershengorn M. C. Thyrotropin-releasing hormone stimulates rapid loss of phosphatidylinositol and its conversion to 1,2-diacylglycerol and phosphatidic acid in rat mammotropic pituitary cells. Association with calcium mobilization and prolactin secretion. J Biol Chem. 1983 Jan 10;258(1):227–234. [PubMed] [Google Scholar]
- Rebecchi M. J., Monaco M. E., Gershengorn M. C. Thyrotropin releasing hormone rapidly enhances [32P]orthophosphate incorporation into phosphatidic acid in cloned GH3 cells. Biochem Biophys Res Commun. 1981 Jul 16;101(1):124–130. doi: 10.1016/s0006-291x(81)80019-8. [DOI] [PubMed] [Google Scholar]
- Ronning S. A., Heatley G. A., Martin T. F. Thyrotropin-releasing hormone mobilizes Ca2+ from endoplasmic reticulum and mitochondria of GH3 pituitary cells: characterization of cellular Ca2+ pools by a method based on digitonin permeabilization. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6294–6298. doi: 10.1073/pnas.79.20.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel W., Roduit C., Zahnd G. Thyrotropin releasing hormone stimulates metabolism of phosphatidyl inositol in GH3 cells. A possible mechanism in stimulus-response coupling. FEBS Lett. 1981 Nov 2;134(1):47–49. doi: 10.1016/0014-5793(81)80547-9. [DOI] [PubMed] [Google Scholar]
- Sutton C. A., Martin T. F. Thyrotropin-releasing hormone (TRH) selectively and rapidly stimulates phosphatidylinositol turnover in GH pituitary cells: a possible second step of TRH action. Endocrinology. 1982 Apr;110(4):1273–1280. doi: 10.1210/endo-110-4-1273. [DOI] [PubMed] [Google Scholar]
- Tan K. N., Tashjian A. H., Jr Receptor-mediated release of plasma membrane-associated calcium and stimulation of calcium uptake by thyrotropin-releasing hormone in pituitary cells in culture. J Biol Chem. 1981 Sep 10;256(17):8994–9002. [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Thaw C., Wittlin S. D., Gershengorn M. C. Tetracaine, propranolol and trifluoperazine inhibit thyrotropin releasing hormone-induced prolactin secretion from GH3 cells by displacing membrane calcium: further evidence that TRH acts to mobilize cellular calcium. Endocrinology. 1982 Dec;111(6):2138–2140. doi: 10.1210/endo-111-6-2138. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Marks J. S., Coll K. E., Williamson J. R. Quantitation and early kinetics of inositol lipid changes induced by vasopressin in isolated and cultured hepatocytes. J Biol Chem. 1983 May 10;258(9):5716–5725. [PubMed] [Google Scholar]
- Vickers J. D., Kinlough-Rathbone R. L., Mustard J. F. Changes in phosphatidylinositol-4,5-bisphosphate 10 seconds after stimulation of washed rabbit platelets with ADP. Blood. 1982 Dec;60(6):1247–1250. [PubMed] [Google Scholar]


