Abstract
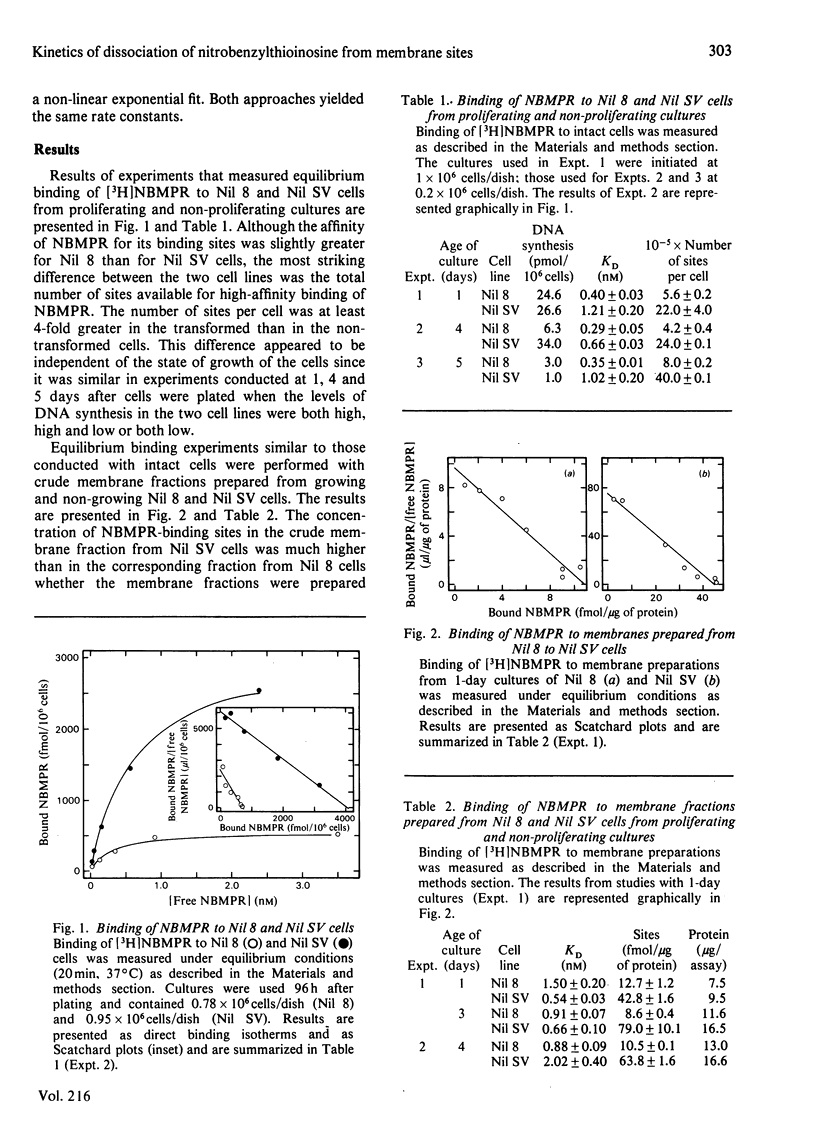
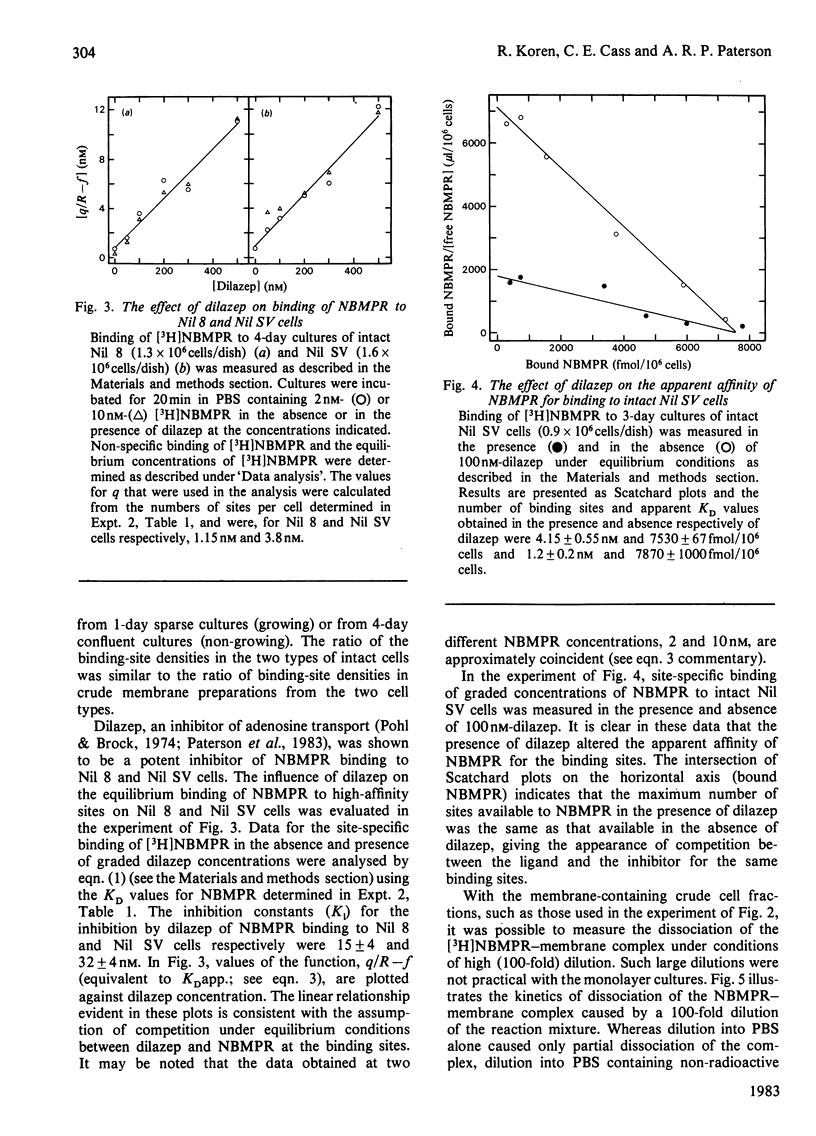
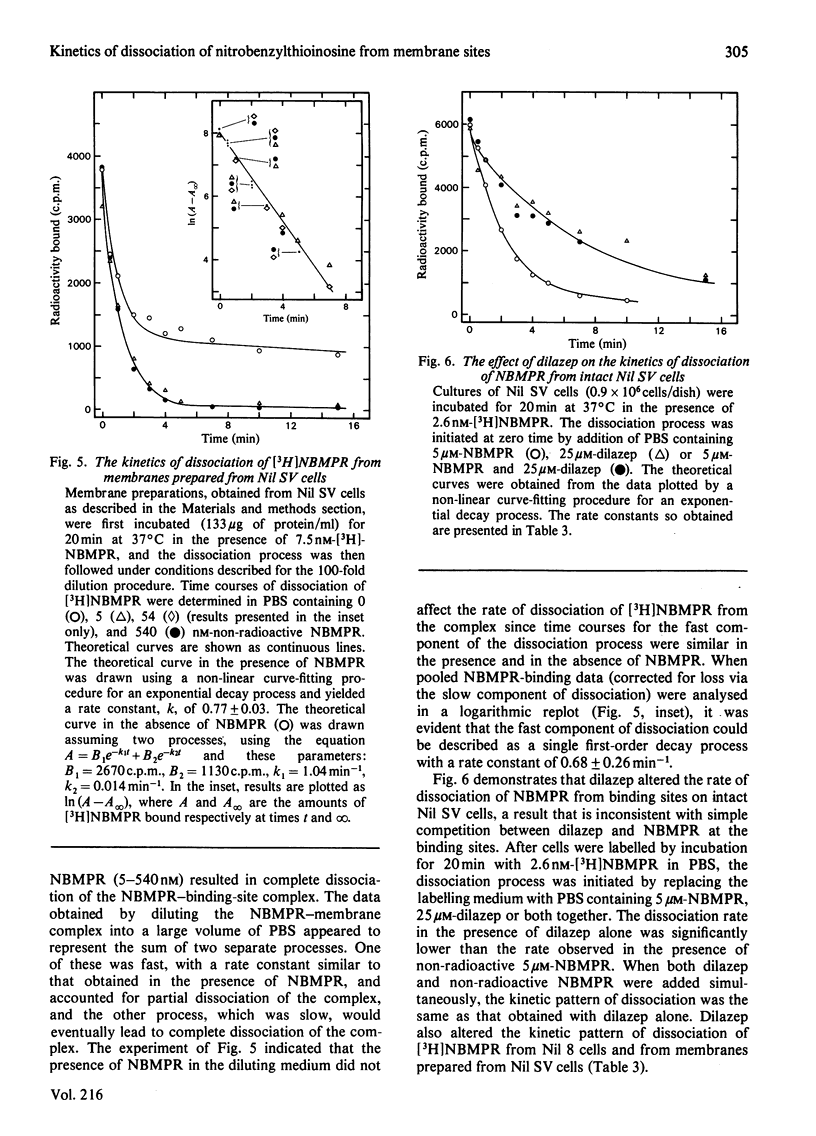
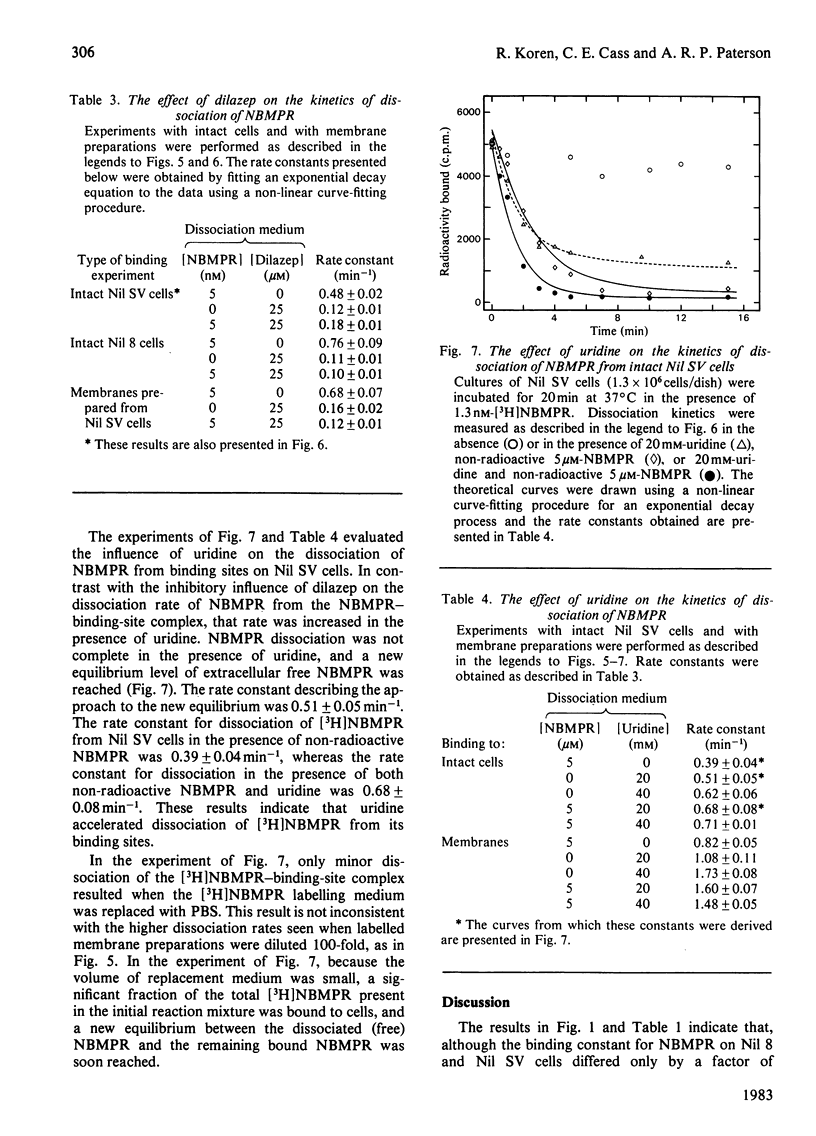
Nucleoside transport in various types of animal cells is inhibited by the binding of nitrobenzylthioinosine (NBMPR) to a set of high-affinity sites on the plasma membrane. This work examined the binding of [3H]NBMPR to the nucleoside transporters of cultured Nil 8 hamster fibroblasts and of cells of a virus-transformed clone (Nil SV) derived from Nil 8. Experiments conducted with intact Nil 8 and Nil SV cells and with membrane preparations indicated that the two lines differed significantly in the cellular content of binding sites and only slightly in the affinities of these sites for NBMPR. Nil 8 and Nil SV cells possessed (4.2-8.0) X 10(5) and (2.0-4.0) X 10(6) sites per cell respectively, whereas the dissociation constants of site-bound NBMPR obtained with intact cells and with membrane preparations were similar, ranging from 0.29 to 1.5 nM. Dilazep, a potent inhibitor of nucleoside transport that is structurally unrelated to NBMPR, appeared to compete with NBMPR for binding to the high-affinity sites when tested under equilibrium conditions with Ki values for inhibition of NBMPR binding to Nil 8 and Nil SV cells respectively of 15 +/- 4 and 32 +/- 4 nM. The dissociation of NBMPR from the binding site--NBMPR complex of Nil SV membrane preparations was a first-order decay process with a rate constant of 0.68 +/- 0.26 min-1. The rate of dissociation of NBMPR from the binding-site complex of membrane preparations and intact cells was decreased significantly in the presence of dilazep and increased in the presence of the permeant uridine. These results suggest that the apparent competitive-inhibition kinetics obtained for dilazep under equilibrium conditions should not be interpreted as binding of dilazep to the same site as NBMPR but rather as binding of the two inhibitors to closely associated sites on the nucleoside transporter. Similarly, uridine also appears to bind to a site separate from the NBMPR-binding site.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Gaudette L. A., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Specific binding of the inhibitor nitrobenzylthioinosine to nucleoside transport sites in the erythrocyte membrane. Biochim Biophys Acta. 1974 Apr 12;345(1):1–10. doi: 10.1016/0005-2736(74)90239-9. [DOI] [PubMed] [Google Scholar]
- Cass C. E., Kolassa N., Uehara Y., Dahlig-Harley E., Harley E. R., Paterson A. R. Absence of binding sites for the transport inhibitor nitrobenzylthioinosine on nucleoside transport-deficient mouse lymphoma cells. Biochim Biophys Acta. 1981 Dec 21;649(3):769–777. doi: 10.1016/0005-2736(81)90182-6. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M., Hammond J. R., Paterson A. R., Clanachan A. S. Species differences in nucleoside transport. A study of uridine transport and nitrobenzylthioinosine binding by mammalian erythrocytes. Biochem J. 1982 Oct 15;208(1):83–88. doi: 10.1042/bj2080083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Nucleoside translocation in sheep reticulocytes and fetal erythrocytes: a proposed model for the nucleoside transporter. J Physiol. 1982 Mar;324:47–66. doi: 10.1113/jphysiol.1982.sp014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S. M., Young J. D. Nucleoside transport in human and sheep erythrocytes. Evidence that nitrobenzylthioinosine binds specifically to functional nucleoside-transport sites. Biochem J. 1980 Aug 15;190(2):377–383. doi: 10.1042/bj1900377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzon G. J., Paterson A. R. Binding of the nucleoside transport inhibitor nitrobenzylthioinosine to HeLa cells. Mol Pharmacol. 1977 Sep;13(5):883–891. [PubMed] [Google Scholar]
- Pande S. V. Liquid scintillation counting of aqueous samples using triton-containing scintillants. Anal Biochem. 1976 Jul;74(1):25–34. doi: 10.1016/0003-2697(76)90306-7. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Kolassa N., Cass C. E. Transport of nucleoside drugs in animal cells. Pharmacol Ther. 1981;12(3):515–536. doi: 10.1016/0163-7258(81)90096-6. [DOI] [PubMed] [Google Scholar]
- Paterson A. R., Lau E. Y., Dahlig E., Cass C. E. A common basis for inhibition of nucleoside transport by dipyridamole and nitrobenzylthioinosine? Mol Pharmacol. 1980 Jul;18(1):40–44. [PubMed] [Google Scholar]
- Paterson A. R., Naik S. R., Cass C. E. Inhibition of uridine uptake in HeLa cells by nitrobenzylthioinosine and related compounds. Mol Pharmacol. 1977 Nov;13(6):1014–1023. [PubMed] [Google Scholar]
- Paterson A. R., Oliver J. M. Nucleoside transport. II. Inhibition by p-nitrobenzylthioguanosine and related compounds. Can J Biochem. 1971 Feb;49(2):271–274. doi: 10.1139/o71-039. [DOI] [PubMed] [Google Scholar]
- Paul B., Chen M. F., Paterson A. R. Inhibitors of nucleoside transport. A structure-activity study using human erythrocytes. J Med Chem. 1975 Oct;18(10):968–973. doi: 10.1021/jm00244a003. [DOI] [PubMed] [Google Scholar]
- Pickard M. A., Brown R. R., Paul B., Paterson A. R. Binding of the nucleoside transport inhibitor 4-nitrobenzylthioinosine to erythrocyte membranes. Can J Biochem. 1973 May;51(5):666–672. doi: 10.1139/o73-083. [DOI] [PubMed] [Google Scholar]
- Pohl V. P., Brock N. Vergleichende Untersuchungen zur Hemmung des Adenosinabbaus in vitro durch Dilazep. Arzneimittelforschung. 1974 Nov;24(11A):1901–1905. [PubMed] [Google Scholar]
- Shohami E., Koren R. S-(N-dansylaminoethyl)-6-mercaptoguanosine as a fluorescent probe for the uridine transport system in human erythrocytes. Biochem J. 1979 Feb 15;178(2):271–277. doi: 10.1042/bj1780271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlhueter R. M., Marz R., Plagemann P. G. Properties of the thymidine transport system of Chinese hamster ovary cells as probed by nitrobenzylthioinosine. J Membr Biol. 1978 Sep 19;42(3):247–264. doi: 10.1007/BF01870361. [DOI] [PubMed] [Google Scholar]


