Abstract
Despite effective antiretroviral therapy, cognitive impairment remains prevalent among people with HIV (PWH) and decrements in executive function are particularly prominent. One component of executive function is cognitive flexibility, which integrates a variety of executive functions to dynamically adapt one’s behavior in response to changing contextual demands. Though substantial work has illuminated HIV-related aberrations in brain function, it remains unclear how the neural oscillatory dynamics serving cognitive flexibility are affected by HIV-related alterations in neural functioning. Herein, 149 participants (PWH: 74; seronegative controls: 75) between the ages of 29–76 years completed a perceptual feature matching task that probes cognitive flexibility during high-density magnetoencephalography (MEG). Neural responses were decomposed into the time-frequency domain and significant oscillatory responses in the theta (4–8 Hz), alpha (10–16 Hz), and gamma (74–98 Hz) spectral windows were imaged using a beamforming approach. Whole-brain voxel-wise comparisons were then conducted on these dynamic functional maps to identify HIV-related differences in the neural oscillatory dynamics supporting cognitive flexibility. Our findings indicated group differences in alpha oscillatory activity in the cingulo-opercular cortices, and differences in gamma activity were found in the cerebellum. Across all participants, alpha and gamma activity in these regions were associated with performance on the cognitive flexibility task. Further, PWH who had been treated with antiretroviral therapy for a longer duration and those with higher current CD4 counts had alpha responses that more closely resembled those of seronegative controls, suggesting that optimal clinical management of HIV infection is associated with preserved neural dynamics supporting cognitive flexibility.
Keywords: Hierarchical cognitive control, Perceptual feature matching, Higher-order cognition, Oscillations, Magnetoencephalography
1. Introduction
HIV is known to cross the blood-brain barrier and affect neural processes early in the course of infection, with a large literature supporting structural and functional changes in the brain that are detrimental to cognition (Alagaratnam and Winston, 2022; González-Scarano and Martín-García, 2005; Manji and Miller, 2004). Viral suppression of HIV is associated with significantly improved health, including performance on cognitive tasks and activities of daily living (Anderson et al., 2019). Since the advent of effective antiretroviral therapies (ART), the prevalence of the most severe forms of HIV-associated neurocognitive disorders (HAND) has substantially diminished (Cysique and Brew, 2009; Heaton et al., 2015). Despite this clinical success, people with HIV (PWH) remain at a much higher risk of developing deficits in cognitive function relative to seronegative individuals (Antinori et al., 2007; Cysique and Brew, 2009; Dastgheyb et al., 2019; Gannon et al., 2011; Grant et al., 2014; Heaton et al., 2010, 2011; Moschopoulos et al., 2024; Robertson et al., 2007; Rubin and Maki, 2019; Simioni et al., 2010; Sundermann et al., 2018; Trunfio et al., 2024; Winston and Spudich, 2020). These deficits span across multiple cognitive domains, with phenotypes of cognitive impairment among virally suppressed PWH being largely driven by deficits in attention and executive functions (Dastgheyb et al., 2019; Wang et al., 2017; Winston and Spudich, 2020), which can have substantial ramifications given the importance of intact executive functions for day-to-day behavior, including managing one’s healthcare-related needs and adhering to medications such as ART (Ettenhofer et al., 2010).
Broadly, executive functions support goal-directed behaviors via top-down effects on posterior and subcortical regions (Diamond, 2013; Miller, 2000; Norman and Shallice, 1986; Yuan and Raz, 2014), which are implemented through distributed network-level interactions involving the prefrontal, cingulate, and parietal cortices (Cabeza and Nyberg, 1997; Cavanagh and Frank, 2014; Pastötter et al., 2013; Yuan and Raz, 2014). In particular, oscillations in the theta, alpha, and gamma bands in association cortices are critical for supporting executive functions (Arif et al., 2021; Busch et al., 2009; Cavanagh and Frank, 2014; Dietz et al., 2023; Dijk et al., 2008; Forte et al., 1999; Gupta et al., 2012; Händel et al., 2011; Jensen et al., 2012; Klimesch, 2012; Klimesch et al., 1998; Landau and Fries, 2012; McDonald et al., 2024; Rajan et al., 2019; Sauseng et al., 2010; Schantell et al., 2024b, 2024a, 2024c, 2022a; Spaak et al., 2014; Wiesman et al., 2017). Furthermore, task difficulty may modulate these oscillatory responses during task performance (Gevins et al., 1997; Sauseng et al., 2002).
One component of executive function is cognitive flexibility, which refers to the ability to dynamically adapt one’s behavior in response to changing environmental demands (Armbruster et al., 2012; Dajani and Uddin, 2015; Scott, 1962). The architecture and dynamic coordination of neural oscillatory responses subserving cognitive flexibility are likely to be particularly vulnerable to HIV-related neurological changes, as prior studies have shown widespread reductions in gray matter, in conjunction with differences in functional connectivity in the temporo-parietal regions relative to seronegative individuals (Becker et al., 2012a; Lew et al., 2021; O’Connor et al., 2018; Sanford et al., 2017; Schantell et al., 2021). Many studies have shown that PWH exhibit aberrations in oscillatory theta, alpha, beta, and gamma dynamics serving basic sensory processing across a range of modalities, as well as higher-order attention and other cognitive processes (Arif et al., 2020; Becker et al., 2012b; Casagrande et al., 2021; Lew et al., 2018; Schantell et al., 2022b; Spooner et al., 2022, 2020, 2018; Wiesman et al., 2018; Wilson et al., 2013a, 2013b). Such oscillatory aberrations scale with key clinical indices of HIV health and behavioral performance on cognitive tasks. These data suggest that PWH who exhibit altered CNS structure and function may use compensatory processes to meet task demands (Casagrande et al., 2021; Chang et al., 2008; Ernst et al., 2009; Lew et al., 2018; Schantell et al., 2022b; Spooner et al., 2022; Wiesman et al., 2018); however, whether such findings relate to cognitive flexibility remains unknown.
In the current study, we leveraged a novel perceptual feature matching task and the excellent spatiotemporal precision of magnetoencephalography (MEG) to investigate the neural oscillatory dynamics serving cognitive flexibility in a large sample of PWH and demographically similar seronegative controls. In addition to quantifying the neural dynamics, we examined the relationship between oscillatory signatures of HIV-related neural alterations, behavioral metrics, and clinical indices of HIV. We hypothesized that PWH would exhibit aberrant oscillatory activity during the cognitive flexibility task and that these differences would scale with clinical measures of HIV disease and behavioral indices.
2. Materials and methods
2.1. Participants
A total of 149 adults (74 PWH, 75 seronegative controls) were enrolled as part of a study examining the Research Domain Criteria (RDoC) framework in the context of neuroHIV (R01-MH118013; see Table 1). PWH were required to be on an effective ART regimen and have an HIV-1 RNA viral load of less than 50 copies/mL within three months of participation in the study. All controls were confirmed seronegative using the OraQuick ADVANCE® Rapid HIV-1/2 Antibody Test at the time of neuropsychological testing. Exclusion criteria for the study included any major diagnosed neurological or psychiatric disorder (except HAND), any medical illness associated with CNS dysfunction (other than HIV), history of head trauma, current substance use disorder, the presence of metallic implants that could affect MEG signal quality or be an MRI safety hazard, and pregnancy. Additionally, we excluded participants who had artifactual or missing MEG data, or had incidental findings (e.g., tumor).
Table 1.
Participant demographics and neuropsychological profiles.
| HIV (n = 64) |
Controls (n = 73) |
Effect size |
P value |
|
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 51.28 (11.80) | 51.36 (10.01) | d = −0.007 | .968 |
| Sex at Birth (n) | 59 Male / 5 Female | 64 Male / 9 Female | V = 0.074 | .384 |
| Race (n) | 49 White / 10 Black / 5 Other | 63 White / 6 Black / 4 Other | V = 0.160 | .477 |
| Ethnicity (n) | 57 Not Hispanic / 7 Hispanic / 0 Unknown | 68 Not Hispanic / 4 Hispanic / 1 Unknown | V = 0.127 | .332 |
| Smoking Status (n) | 21 Current Smokers / 16 Past Smokers / 26 Nonsmokers / 1 Unknown | 3 Current Smokers / 25 Past Smokers / 42 Nonsmokers / 3 Unknown | V = 0.380 | < .001 |
| HIV Clinical Metrics | ||||
| CD4 Nadir (cells/μL) | 268.88 (169.51) | – | – | – |
| Current CD4 (cells/μL) | 800.31 (351.01) | – | – | – |
| Years Since HIV Diagnosis | 16.93 (10.20) | – | – | – |
| Years on ART | 14.31 (8.54) | – | – | – |
| Neuropsychological Performance | ||||
| Learning T-Score | 48.53 (8.79) | 50.31 (7.50) | d = −0.218 | .206 |
| Memory T-Score | 48.53 (8.18) | 49.88 (7.60) | d = −0.171 | .327 |
| Executive Function T-Score | 52.11 (7.03) | 55.25 (5.96) | d = −0.484 | .006 |
| Processing Speed T-Score | 47.45 (7.03) | 49.15 (8.01) | d = −0.225 | .194 |
| Attention T-Score | 48.47 (7.10) | 54.61 (8.79) | d = −0.764 | < .001 |
| Language T-Score | 52.42 (7.74) | 50.41 (9.74) | d = 0.227 | .192 |
| Motor Dexterity T-Score | 48.05 (8.97) | 51.20 (9.97) | d = −0.331 | .058 |
Note. Means and standard deviations for the final sample are displayed for each continuous variable. Cohen’s d is the effect size reported for continuous variables, and Cramér’s V is reported for categorical variables. Domain T-scores were calculated by averaging the T-scores from the individual assessments comprising each cognitive domain. ART: Antiretroviral therapy.
2.2. Standard Protocol Approvals and Participant Consents
The Institutional Review Board at Boys Town National Research Hospital reviewed and approved this investigation. Participants provided written informed consent following a detailed description of the study, which was conducted in accordance with the Declaration of Helsinki.
2.3. Neuropsychological Assessment
Cognitive function was measured using a neuropsychological battery that assessed premorbid function and functionality across seven domains (i.e., learning, memory, executive function, attention, processing speed, language, and motor dexterity). The battery included the following tests for each domain: learning (Wechsler Memory Scale [WMS-IV] Logical Memory I (Wechsler, 2009), Brief Visuospatial Memory Test – Revised [BVMT-R] Total Recall (Benedict, 1997), Hopkins Verbal Learning Test – Revised [HVLT-R] Total Recall (Brandt and Benedict, 2001)); memory (WMS-IV Logical Memory II (Wechsler, 2009), HVLT-R Delayed Recall (Brandt and Benedict, 2001), and BVMT-R Delayed Recall (Benedict, 1997)); executive function (Stroop Color Word Test Interference Trial (Golden and Freshwater, 2002) and Trail Making Test Part B (Heaton et al., 2004)); processing speed (Stroop Color Word Test, Color and Word Trials (Golden and Freshwater, 2002), Wechsler Adult Intelligence Scale [WAIS-IV] Coding (Wechsler, 2008), and Trail Making Test Part A (Heaton et al., 2004)); attention (WAIS-IV Letter-Number Sequencing (Wechsler, 2008), WAIS-IV Digit Span (Wechsler, 2008)); language (Boston Naming Test (Heaton et al., 2004; Kaplan et al., 2001), phonemic (FAS) and semantic (Animals) verbal fluencies (Heaton et al., 2004); and motor dexterity (Grooved Pegboard, dominant and non-dominant hands (Heaton et al., 2004; Matthews and Klove, 1964)). Further, premorbid function was assessed using the Wide Range Achievement Test, Fourth Edition (WRAT-4) Word Reading (Wilkinson and Robertson, 2006). Finally, perceived functional impairment was quantified using a modified version of the Lawton and Brody Activities of Daily Living Scale (Lawton and Brody, 1969).
Demographically corrected scores (T-scores) for each assessment were obtained using published normative data (Benedict, 1997; Brandt and Benedict, 2001; Golden and Freshwater, 2002; Heaton et al., 2004; Kaplan et al., 2001; Matthews and Klove, 1964; Wechsler, 2009, 2008; Wilkinson and Robertson, 2006). Domain composite scores were calculated by averaging the T-scores of assessments that comprised each respective cognitive domain (Table 1). Cognitive impairment was assigned per the Frascati criteria (Antinori et al., 2007) by a board-certified clinical neuropsychologist using the composite domain T-scores.
2.4. Experimental Paradigm
Participants completed a 15-min executive function task aimed at assessing cognitive flexibility by matching stimuli based on perceptual feature cues (Fig. 1). Participants were seated in a magnetically-shielded room and were instructed to fixate on a centrally located crosshair with a variable ISI (range: 1500–1900 ms) followed by the presentation of a set of three images for 2700 ms, one of which was presented centrally above the crosshair and surrounded by either a square or a diamond. The instructions pertaining to the meaning of the surrounding squares versus diamonds were counterbalanced across conditions and groups (χ2 = 0.02, p = .900) to eliminate potential confounding effects. For one set of instructions, the squares indicated that participants should select which of the bottom two images matched the top image based on its shape, while the diamonds indicated that participants should select which of the bottom two images matched the top image based on its pattern. In the other set of instructions, the meaning of diamonds and squares was reversed. Participants selected the bottom image on the left by clicking a button with their right index finger and that on the right using their right middle finger. Trials were equally divided between rule sets and their presentation was pseudorandomized. Reaction time and accuracy data were collected and used for behavioral analysis. Independent samples t-tests were used to assess differences in reaction time and accuracy between seronegative controls and PWH, and Pearson correlations were used to examine possible associations between reaction time, accuracy, and the duration in which participants had been diagnosed with HIV and other clinical metrics.
Fig. 1. Illustration of the perceptual feature matching task.
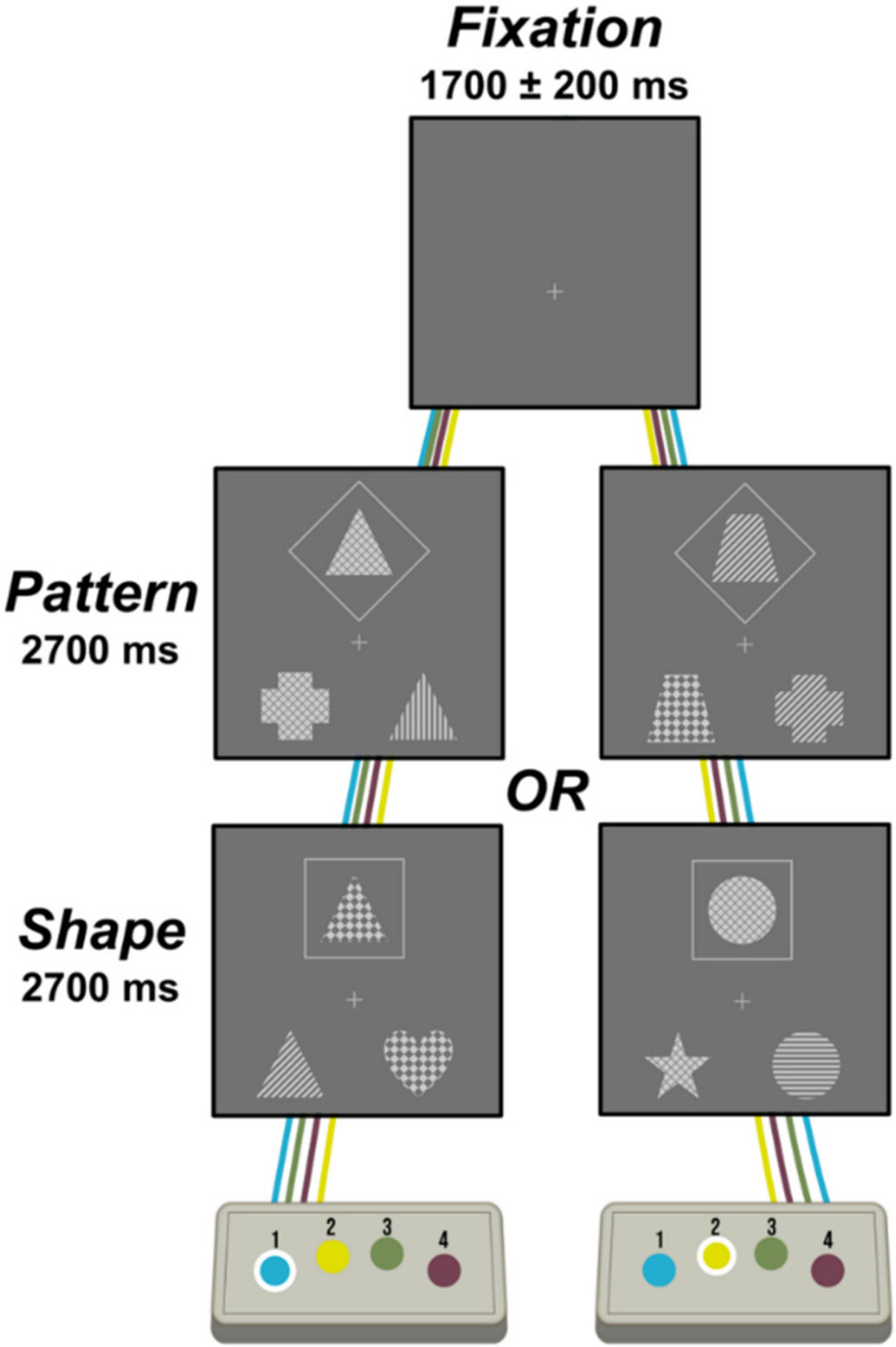
Trials had a fixation period lasting an average of 1700 ms and a stimulus presentation period of 2700 ms, during which, a set of three images were displayed. Participants matched the top image to one of the two bottom images based on either its pattern or shape, which was dictated by whether the top image was surrounded by a diamond or square. The meaning of diamond versus square was counterbalanced within each participant group. Participants indicated their choice via button press.
2.5. MEG Data Acquisition
Our MEG data acquisition, structural coregistration, preprocessing, and sensor–/source-level analyses closely followed previously reported pipelines (Wiesman and Wilson, 2020). Functional MEG data were collected using a MEGIN TRIUX Neo MEG system (Helsinki, Finland) equipped with 306 sensors (204 planar gradiometers, 102 magnetometers) using a 1 kHz sampling rate and an acquisition bandwidth of 0.1–330 Hz in a two-layer magnetically shielded VACOSHIELD room (Vacuumschmelze, Hanau, Germany). Participants were monitored during data acquisition via real-time audio-video feeds from inside the shielded room. MEG data were subjected to environmental noise reduction and corrected for head motion using the signal space separation method with a temporal extension (Taulu and Simola, 2006). Only data from the 204 planar gradiometers were used for further analysis.
2.6. Structural MRI Processing and MEG Coregistration
Prior to MEG acquisition, five coils were attached to the participant’s head and localized along with fiducial and scalp surface points using a three-dimensional (3D) digitizer (FASTRAK, Polhemus Navigator Sciences, Colchester, Vermont). Once the participants were positioned for MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the five coils, thus inducing a measurable magnetic field and thereby allowing each coil to be localized in reference to the MEG sensor array throughout the recording session. MEG data were coregistered with structural T1-weighted MRI data using BESA MRI (Version 3.0) prior to source-space analysis. These structural T1-weighted images were acquired using a Siemens Prisma 3T MRI scanner with a 32-channel head coil and a 3D MP-RAGE sequence with the following parameters: TR = 2400 ms; TE = 2.05 ms; flip angle = 8°; FOV = 256 mm; slice thickness = 1 mm; voxel size = 1 mm3. The structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into standardized space.
2.7. MEG Preprocessing
Cardiac and ocular artifacts were removed from the MEG data using signal space projection (SSP) (Uusitalo and Ilmoniemi, 1997). The continuous magnetic time series was then filtered with a 60 Hz notch filter and divided into 4200 ms epochs (−1500 to 2700 ms), with 0.0 ms defined as stimulus onset. Only trials with correct responses were considered for further analysis. Epochs containing artifacts were rejected using a fixed threshold method that was set per participant and supplemented with visual inspection. Briefly, in MEG, the raw signal amplitude is strongly affected by the distance between the brain and the MEG sensor array, as the magnetic field strength falls off sharply as the distance from the current source (i.e., brain) increases. To account for this source of variance across participants, as well as other sources of variance (e.g., head size), we used an individualized threshold based on the signal distribution for both amplitude and gradient to reject artifacts. The average amplitude threshold across all participants was 1064.36 (SD = 547.72) fT/cm, the average gradient threshold was 245.39 (SD = 195.34) fT/(cm*ms), and an average of 162.71 (SD = 13.84) trials out of the original 200 were used for further analysis. The number of trials included in the final MEG analyses did not statistically differ by group (t = 1.12, p = .263).
2.8. MEG Time-Frequency Transformation and Sensor-Level Statistics
Artifact-free epochs were transformed into the time-frequency domain (resolution: 2 Hz, 25 ms) using complex demodulation (Kovach and Gander, 2016; Papp and Ktonas, 1977), and the resulting spectral power estimations per sensor were averaged across trials to generate time-frequency plots of mean spectral density. These sensor-level data were then normalized with respect to the mean baseline power (i.e., −700 to 0 ms). The time-frequency windows used for subsequent source imaging were identified using a stringent two-stage statistical approach that utilized paired-sample t-tests against baseline on each pixel in the spectrogram (per sensor) at the first stage, followed up with cluster-based nonparametric permutation testing at the second level. This testing was conducted across all participants and the entire frequency range (4–100 Hz) and used an initial cluster threshold of p < .05 and 5000 permutations. These methods are described in depth in our recent publications (Wiesman et al., 2021; Wiesman and Wilson, 2020). All MEG and MRI data were processed in BESA (Research: V7.1; MRI: V3.0; Statistics: V2.1).
2.9. MEG Source Analysis
Time-frequency resolved source images were computed using the dynamic imaging of coherent sources (DICS) beamformer to image oscillatory activity within the time-frequency windows of interest per participant (Dalal et al., 2006; Gross et al., 2001; Van Veen et al., 1997). We used task and baseline periods of equal duration and bandwidth (Hillebrand et al., 2005) to compute noise-normalized source power per voxel, with the resulting pseudo-t units reflecting power differences (i.e., active versus baseline) per voxel (resolution: 4 × 4 × 4 mm). The resulting beamformer maps were then transformed into standardized space and spatially resampled by applying the same transform that was applied to the native space structural images per participant.
2.10. Statistical Analyses
To assess for differences in neural oscillatory activity serving task performance among PWH and seronegative controls, the spectrally specific whole-brain maps per participant and time-frequency component were used for whole-brain, voxel-wise independent samples t-tests. A significance threshold of p < .005 was used for the identification of significant clusters in all whole-brain statistical maps, accompanied by a cluster (k) threshold of at least 10 contiguous voxels (i.e., 640 mm3 of brain tissue) based on the theory of Gaussian random fields (Poline et al., 1995; Worsley et al., 1999, 1996). All whole-brain statistical analyses were computed using a custom function in MATLAB (MathWorks; Natick, Massachusetts) and other statistical analyses were conducted in IBM SPSS v.29. Of note, sensitivity analyses were conducted to evaluate whether the main findings held after adjusting for tobacco dependence as well as after removing females from statistical analyses.
3. Results
3.1. Participant Characteristics and Behavioral Results
Of the 149 participants enrolled, six participants were excluded from further analysis due to poor task performance (accuracy <60 % correct; 5 PWH, 1 seronegative control), four PWH were excluded for HIV viral loads >50 copies/mL, one PWH was excluded due to excessive noise during MEG recording, and one control was withdrawn from the study resulting in a final sample of 137 participants (64 PWH, 73 seronegative controls). The groups were similar in demographic characteristics such as age, sex, race, and ethnicity (Table 1).
In terms of neuropsychological performance, PWH performed more poorly on the attention (t = −4.50 [95 % CI: −8.84, −3.41], Cohen’s d = −0.76 [95 % CI: −1.11, −0.41], p < .001) and executive function (t = −2.81 [95 % CI: −5.36, −0.93], Cohen’s d = −0.48 [95 % CI: −0.83, −0.14], p = .006) domains relative to seronegative controls, though both groups performed similarly on the learning, memory, processing speed, language, and motor domains (all p-values > .05; Table 1). Based on the Frascati criteria (Antinori et al., 2007), 13 PWH (20.3 %) met criteria for HAND (asymptomatic neurocognitive impairment (ANI): 11; mild neurocognitive disorder (MND): 1; HIV-associated dementia (HAD): 1). Further, behavioral cognitive flexibility performance on the MEG perceptual feature matching task was worse among PWH, with PWH performing less accurately (PWH: M = 91.73 %, SD = 7.51 %; Controls: M = 96.05 %, SD = 3.83 %; t = 4.16 [95 % CI: −6.39, −2.26], Cohen’s d = −0.74 [95 % CI: −1.09, −0.39], p < .001; Fig. 2A) and responding more slowly (M = 1642.56 ms, SD = 293.65) than controls (M = 1514.40 ms, SD = 245.33; t = −2.78 [95 % CI: 35.92, 220.42], Cohen’s d = 0.48 [95 % CI: 0.14, 0.82], p = .006; Fig. 2B). Further, those with longer HIV disease durations tended to have slower reaction times, above and beyond the effects of age (r = 0.30 [95 % CI: 0.04, 0.51], p = .023; Fig. 2C). Next, we probed whether these group differences in task behavior held after adjusting for scores on the attention domain and HAND classifications, which revealed that group differences in accuracy held after adjusting for attention scores (F(2,135) = 11.38, p < .001) and HAND classifications (F(2,135) = 15.54, p < .001), while group differences in reaction time held after adjusting for HAND classifications (F(2,135) = 5.46, p = .021), but not after adjusting for attention domain scores (F(2,135) = 1.02, p = .314). Of note, there were no significant group-by-condition interactions in terms of reaction time (F(1,135) = 0.09, p = .770) or accuracy (F(1,135) = 0.83, p = .363).
Fig. 2. Behavioral performance on the MEG perceptual feature matching task.
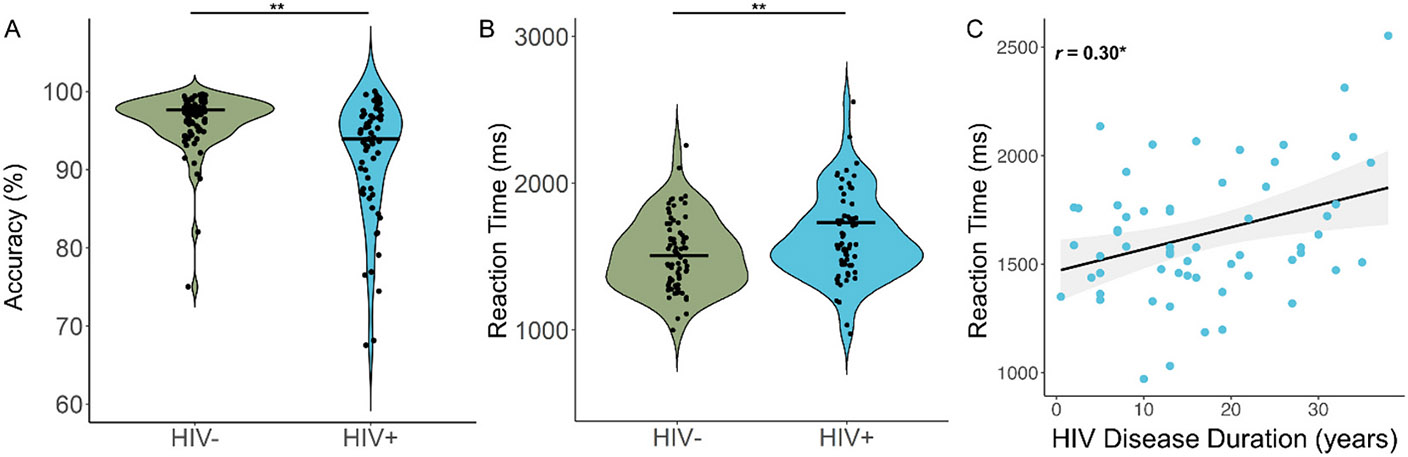
People with HIV (PWH) were less accurate (A) and responded slower (B) on the perceptual feature matching task compared to controls. Accuracy, expressed as the percent correct, appears on the y-axis of (A) and reaction time, in ms, is displayed on the y-axis of (B), with group on the x-axes of both panels. Violin plots show the probability density per group, with the black bars indicating the group means. (C) Reaction time (y-axis) was also significantly correlated with HIV disease duration in PWH, with longer HIV disease durations (in years, x-axis) being associated with slower reaction times, controlling for the effect of age. The gray shading surrounding the trendline represents the standard error of the mean. **p < .01, *p < .05.
Finally, to help validate the task’s assessment of cognitive flexibility, we evaluated relationships between raw scores of individual tests comprising the executive function and attention domains. As expected, faster reaction time and higher accuracy were most strongly associated with a faster completion time during the Trail Making Test Part B (Reaction time: r = 0.51 [95 % CI: 0.37, 0.62], Pcorrected < .001; Accuracy: r = −0.34 [95 % CI: −0.48, −0.19], pcorrected < −001), and were also associated with more optimal performance on the WAIS-IV Digit Span Test (Reaction time: r = −0.46 [95 % CI: −0.58, −0.32], Pcorrected < −001; Accuracy: r = 0.37 [95 % CI: 0.21, 0.50], Pcorrected = .007), WAIS-IV Letter-Number Sequencing Test (Reaction time: r = −0.39 [95 % CI: −0.53, −0.24], Pcorrected < .001; Accuracy: r = 0.22 [95 % CI: 0.06, 0.38], Pcorrected = .072), and the Stroop Interference Trial (Reaction time: r = −0.25 [95 % CI: −0.40, −0.08], pcorrected = .032; Accuracy: r = 0.11 [95 % CI: −0.06, 0.28], Pcorrected > .99) after adjusting for multiple comparisons using a Bonferroni correction. These findings provide compelling evidence that our MEG task is assessing cognitive flexibility.
3.2. Oscillatory Neural Responses
We observed robust neural oscillatory responses in four temporally and spectrally defined windows serving cognitive flexibility during the perceptual feature matching task (Fig. 3). These included statistically significant increases in power relative to the baseline period in the theta band (50–450 ms; 4–8 Hz), a decrease in power in the alpha band (10–16 Hz) from 200 to 500 ms and from 650 to 1350 ms, and an increase in power in the gamma band (100–250 ms; 74–98 Hz). All responses were significant at p < .05 following multiple comparisons correction using nonparametric cluster-based permutation testing. At the cortex level, the theta, alpha, and gamma responses originated from the bilateral occipital regions and extended into the higher-order association cortices.
Fig. 3. Neural oscillatory responses during the perceptual feature matching task.
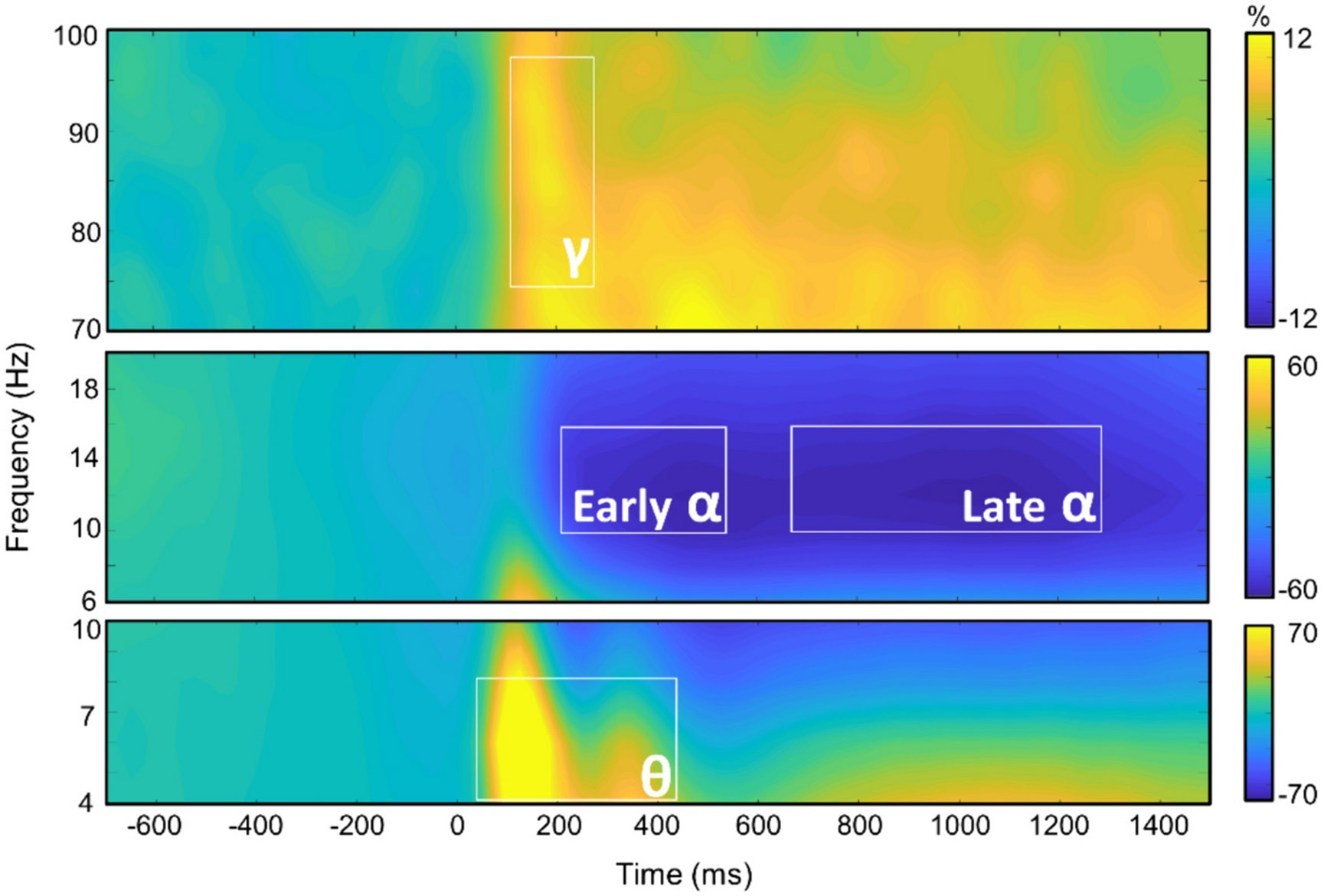
Grand-averaged time-frequency spectrograms of MEG sensors exhibiting one or more significant oscillatory responses. Shown from top to bottom: gamma, early and late alpha, and theta activity. In each spectrogram, frequency (Hz) is shown on the y-axis, and time (ms) is shown on the x-axis. Signal power data are expressed as a percent difference from the baseline period, with color legends shown to the right of each spectrogram.
3.3. Group Differences in Oscillatory Activity
To address our primary hypotheses, we assessed the effects of HIV status on these neural responses by conducting whole-brain independent samples t-tests separately for the theta, early alpha, late alpha, and gamma responses. There were no significant differences in oscillatory theta activity among PWH and controls. However, in the early alpha band, PWH had stronger alpha oscillations in the right PFC (i.e., more negative relative to baseline; t = −3.19 [95 % CI: −3.79, −0.89], Cohen’s d = −0.55 [95 % CI: −0.90, −0.21], p < .005; Fig. 4A). Likewise, in the late alpha band, several peaks were found in the left insula (t = −2.74 [95 % CI: −6.70, −1.08], Cohen’s d = −0.48 [95 % CI: −0.83, −0.13], p < .005; Fig. 4B), left anterior cingulate cortex (ACC; t = −2.99 [95 % CI: −5.74, −1.17], Cohen’s d = −0.53 [95 % CI: −0.88, −0.18], p < .005; Fig. 4C), and the left ventral temporal cortex (t = −2.85 [95 % CI: −5.00, −0.91], Cohen’s d = −0.50 [95 % CI: −0.85, −0.15], p < .005; Fig. 4D), with PWH exhibiting stronger oscillatory alpha activity relative to controls in all of these regions. In contrast, PWH exhibited reduced oscillatory gamma responses in the right cerebellum relative to controls (t = −3.02 [95 % CI: −1.97, −0.41], Cohen’s d = −0.53 [95 % CI: −0.88, −0.18], p < .005; Fig. 4E).
Fig. 4. People with HIV exhibit altered oscillatory alpha and gamma activity during performance of the MEG task.
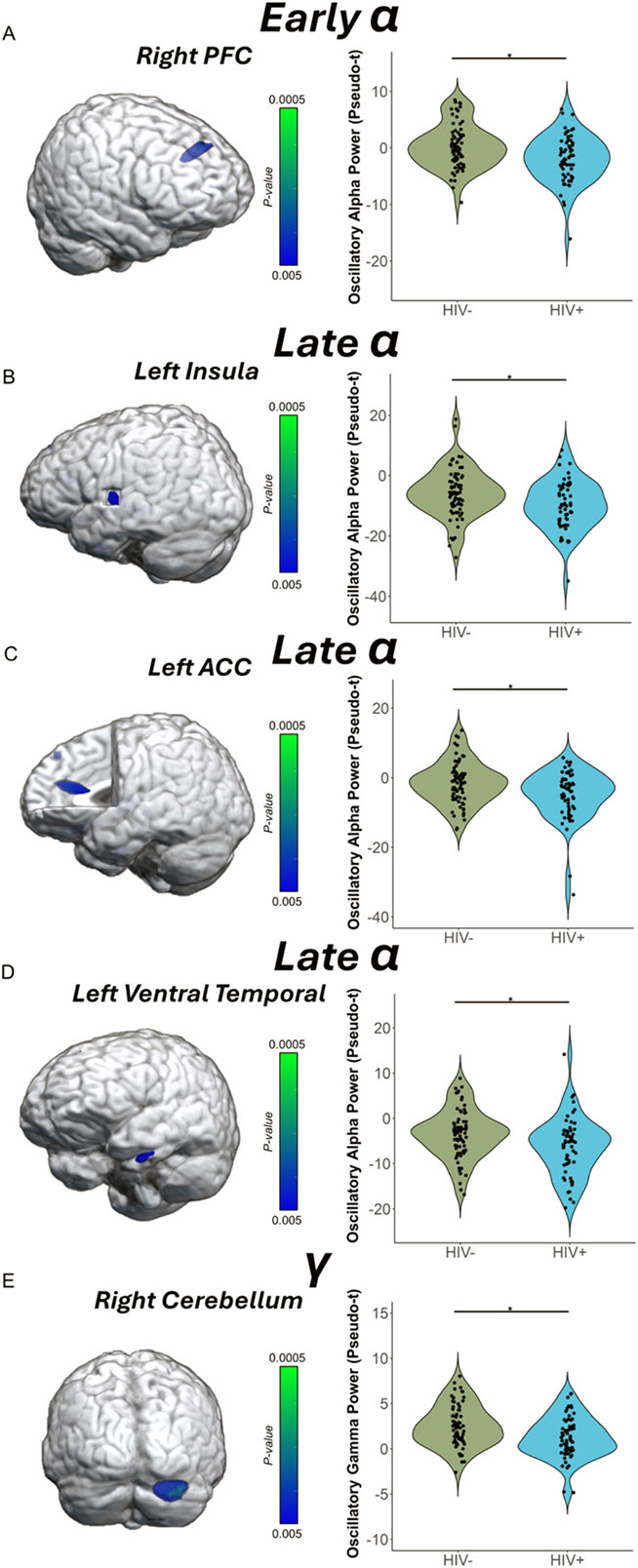
(A) The brain image on the left represents the voxel-wise group difference in oscillatory alpha activity during the early time window, with stronger alpha responses (i.e., more negative relative to baseline) in the right prefrontal cortex (PFC) of PWH compared to controls. (B—D) Similarly, voxel-wise group differences in oscillatory alpha activity during the late window were observed in the (B) left insula, (C) left anterior cingulate cortex (ACC), and in the (D) left ventral temporal cortex, with PWH having stronger oscillatory alpha activity relative to controls in all regions. (E) Group differences in gamma oscillations were also identified in the right cerebellum, with controls exhibiting stronger responses than PWH. In each panel, the peak voxel power values were extracted from the cluster depicted in the 3D rendition and are plotted to the right in the accompanying violin plots. *p < .005.
We then probed how these distinct neural oscillatory responses were related to clinical indices of HIV (i.e., CD4 nadir, current CD4 count, duration on ART, disease duration) and behavioral performance on the MEG task (i.e., reaction time, accuracy). Pseudo-t values were extracted from peak voxels identified in the whole-brain analyses and analyzed with Pearson correlations. We found that those who had been treated with ART for a longer duration of time had weaker (i.e., more optimal) oscillatory alpha responses in the left ventral temporal cortex, after adjusting for age (r = 0.29 [95 % CI: 0.001, 0.55], p = .035; Fig. 5A). Further, higher current CD4 counts (cells/μL) were associated with weaker oscillatory alpha responses (i.e., more optimal) in the left anterior cingulate (r = 0.30 [95 % CI: 0.01, 0.54], p = .042; Fig. 5B). Additionally, across all participants and independent of HIV-status, stronger gamma power (i.e., more optimal) in the right cerebellum was associated with faster reaction times during the MEG perceptual feature matching task (r = −0.36 [95 % CI: −0.50, −0.20], p < .001; Fig. 6A), while stronger late alpha responses (i.e., less optimal) in the left ACC (r = −0.21 [95 % CI: −0.36, −0.03], p = .019; Fig. 6B) and left ventral temporal cortex (r = −0.24 [95 % CI: −0.39, −0.07], p = .007; Fig. 6C) were associated with slower reaction times. Further, stronger alpha oscillations in the left insula (r = 0.21 [95 % CI: 0.04, 0.37], p = .015; Fig. 6D) and left ventral temporal cortex (r = 0.21 [95 % CI: 0.04, 0.37], p = .017; Fig. 6E) were associated with poorer task accuracy. Finally, to assess whether our primary findings were biased by the sex of participants and/or were sensitive to group differences in tobacco use, we conducted sensitivity analyses which revealed that all main findings remained statistically significant after adjusting for tobacco dependence and after removing females from the statistical analyses.
Fig. 5. Clinical HIV metrics are related to alpha oscillations.
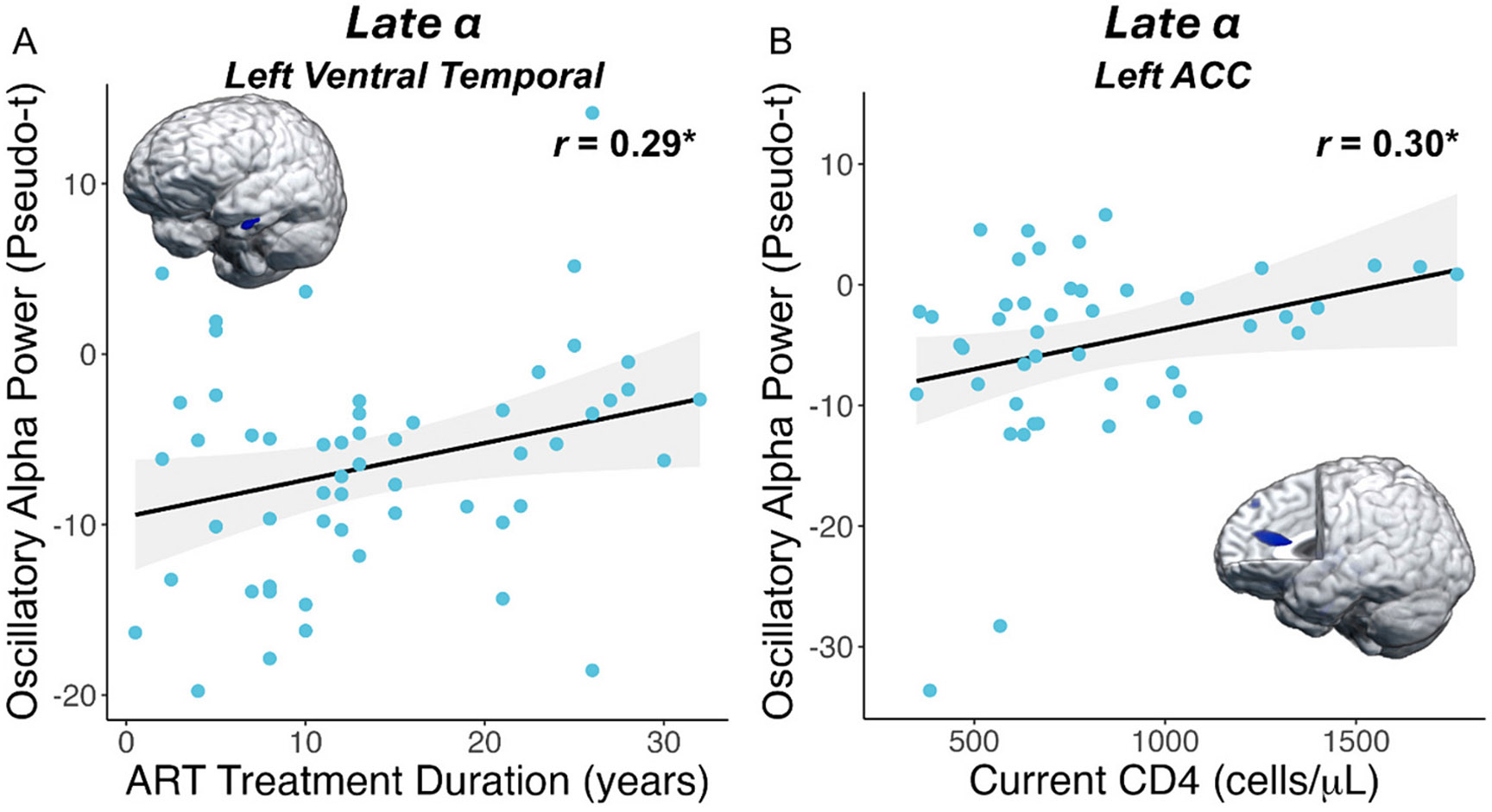
(A) People with HIV (PWH) who had been treated with ART for a longer duration had weaker (i.e., less negative; more optimal) oscillatory alpha responses in the left ventral temporal cortex, above and beyond the effects of age. (B) Higher current CD4 counts in PWH were associated with weaker alpha oscillations (i.e., more optimal) in the anterior cingulate cortex (ACC). The gray shading surrounding the trendline represents the standard error of the mean. *p < .05.
Fig. 6. Oscillatory gamma and alpha power scale with behavioral measures.
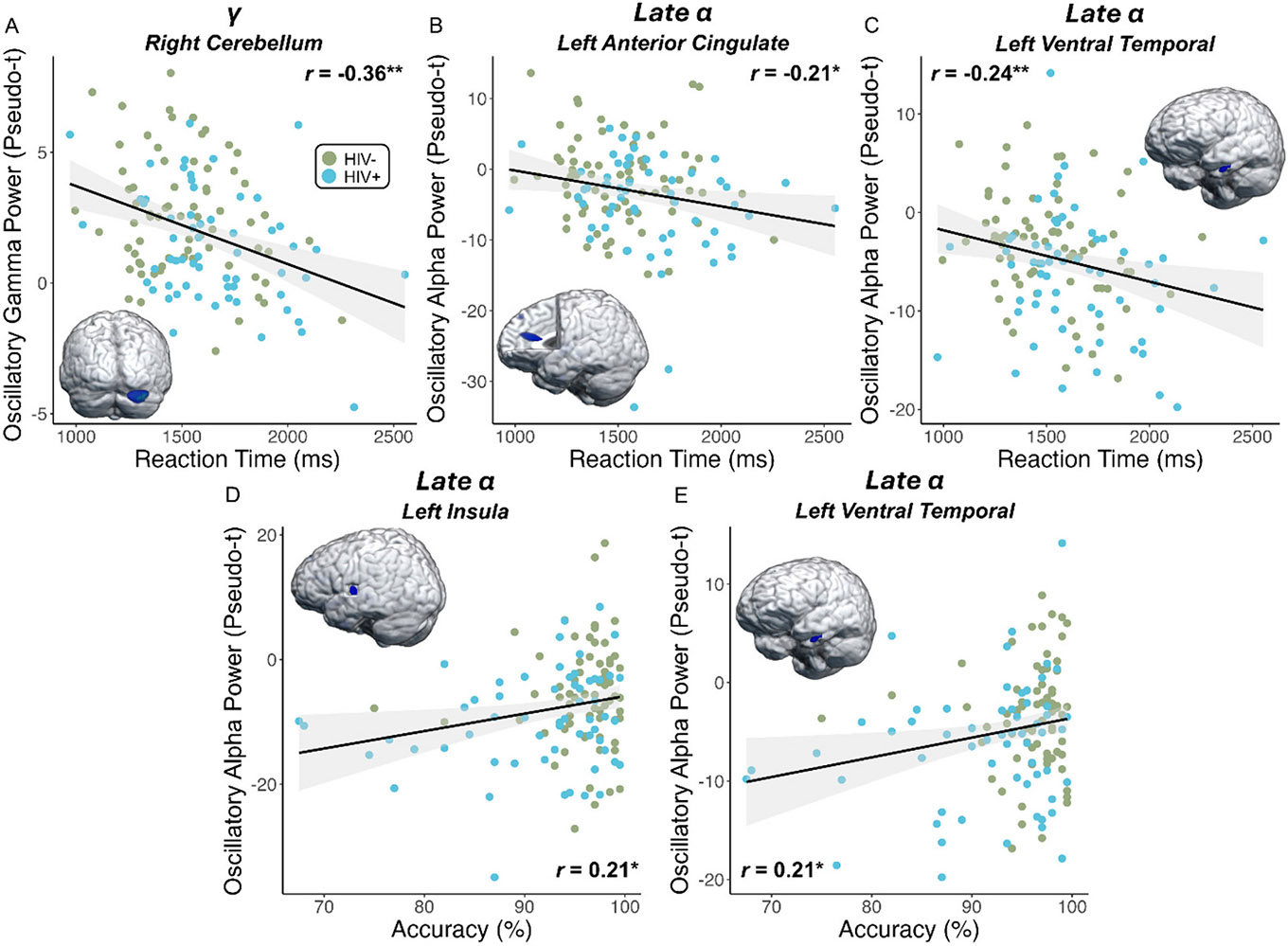
(A) Regardless of HIV status, stronger gamma oscillations in the right cerebellum were associated with faster responses during the MEG task. (B-E) In contrast, stronger (i.e., more negative) alpha responses in the (B) left anterior cingulate and (C) left ventral temporal cortex were associated with slower reaction times, and stronger alpha oscillations in the (D) left insula and (E) left ventral temporal cortex were associated with lower accuracy on the task. Note that controls exhibited weaker alpha activity compared to PWH, and thus in all cases, poorer performance was associated with the neural response patterns observed in PWH. The gray shading surrounding the trendline represents the standard error of the mean. *p < .05, **p < .01.
4. Discussion
Previous research has established that PWH experience higher rates of neurocognitive impairment compared to seronegative controls, with deficits in executive function being especially common (Dastgheyb et al., 2019; Heaton et al., 2011; Winston and Spudich, 2020). Unfortunately, the neurobiological underpinnings of these impairments remain incompletely understood, especially among virally suppressed individuals. In the current study, we exploited the spatiotemporal precision of MEG and a novel cognitive flexibility task to identify the neural oscillatory dynamics underlying such executive dysfunction in PWH. Our findings indicated the expected pattern of impaired behavioral performance on the perceptual feature matching MEG task in PWH, as well as altered alpha and gamma oscillations within higher-order cortices that are known to play a critical role in executive function. Additionally, we found that alterations in these oscillatory responses scaled with key behavioral metrics and clinical indices of HIV health.
In terms of task behavior, PWH performed less accurately and responded more slowly compared to controls, which corroborates many studies of higher-order cognitive function in PWH (Lew et al., 2018; Schantell et al., 2022b, 2022a; Wiesman et al., 2018). Interestingly, PWH who had been diagnosed with HIV for a longer duration had slower reaction times during the task, even after controlling for age. This finding is of particular interest, as our sample of PWH was virally suppressed, and all had been receiving ART for the vast majority of time since acquiring HIV. Therefore, these results suggest that the neurocognitive impacts of HIV persist despite viral suppression (Schantell et al., 2022b).
Beyond these behavioral findings, we demonstrated that PWH have alterations in the neural dynamics serving cognitive flexibility. In the high-frequency gamma range, PWH had reduced oscillatory power in the right cerebellum relative to controls. Across both groups, stronger oscillatory gamma activity in this region was associated with faster reaction times during task performance, indicating that gamma oscillations in the right cerebellar cortices are a critical component of the neurophysiological network serving cognitive flexibility and may scale with the efficiency of task performance (Bellebaum and Daum, 2007; Kirschen et al., 2005). Gamma oscillations are tightly coupled to local GABA activity (Edden et al., 2009; Gaetz et al., 2011; Kujala et al., 2015; Muthukumaraswamy et al., 2009), which suggests that reduced neuronal inhibition in PWH contributes to their altered performance on this task and, more broadly, in tasks and environments that require cognitive flexibility.
In addition, we found alterations in oscillatory alpha activity in PWH. Specifically, we found that PWH had stronger (i.e., more negative relative to baseline) alpha oscillations in the right PFC during the early time window, as well as stronger alpha responses in the left insula, ACC, and ventral temporal regions during the late window. These regions have been linked with hierarchical cognitive control and cognitive flexibility (Barredo et al., 2015; Brockett et al., 2020; Miller, 2000; Varjacic et al., 2018), which helps highlight their importance and likely role in the current context. Further, we identified several relationships between oscillatory alpha activity in these regions and behavioral performance. These included stronger alpha oscillations (i.e., more negative) in the left ACC, left insula, and left ventral temporal cortex being associated with poorer behavioral performance in terms of accuracy and reaction time across all participants, which would be expected based on the direction of the neural findings. Further supporting these findings, we found that weaker alpha oscillations in the left ventral temporal cortex (i.e., more similar to controls) were associated with receiving ART for a longer duration of time, and the same oscillatory pattern in the left ACC was associated with higher current CD4 T-cell counts. Overall, these findings suggest that alpha oscillations in these regions are directly tied to clinical indices of HIV health and are critical for task performance in our study and, by extension, cognitive flexibility (Schantell et al., 2022b).
Previous work has suggested that PWH may exhaust their cognitive resources during performance of higher-order cognitive tasks, especially when task demands are high (Chang et al., 2008; Ernst et al., 2009). The compensation-related utilization of neural circuits hypothesis, or CRUNCH (Reuter-Lorenz and Cappell, 2008), postulates that people recruit more neural resources as task demands increase, and those experiencing cognitive decline may recruit greater resources at lower task demands to compensate for this decline. In this context, compensation refers to the recruitment of additional neural resources to address higher cognitive load (Schantell et al., 2022b; Schneider-Garces et al., 2010). As described above, across multiple regions that play a putative role in executive function processes, PWH exhibited stronger alpha oscillations during task performance relative to controls. This suggests that PWH may differentially engage neural resources as an attempt to compensate for processing deficits and/or inefficiencies in order to meet the demands of the task at hand. While this strategy may work when task demands are relatively lower, as demands rise, these compensatory processes eventually become exhausted and performance declines (Arif et al., 2020; Schantell et al., 2022b; Schneider-Garces et al., 2010). Given the relationships we observed between alpha oscillations in several brain regions and behavioral performance, we believe such a breaking point may have been reached in the current study, as the stronger alpha activity observed in PWH was associated with lower accuracy and slower reaction times, indicating an exhausted attempt to compensate.
Before closing, it is important to note the limitations of this study. First, due to the variety of ART regimens used across our sample and potential medication changes among our patients during the course of their treatment, we were not able to analyze the impact of using different ART regimens on task performance or neural oscillatory dynamics. Several ART treatments are known to cross the blood-brain barrier and are associated with neurocognitive impairment (Robertson et al., 2012), and thus, future work should consider calculating a CNS Penetration Effectiveness score to provide further insight into the mechanisms of HIV-related CNS dysfunction (Letendre et al., 2008). Second, this study did not investigate persistent inflammation. Despite viral suppression during ART, PWH continue to experience damaging levels of chronic systemic inflammation (French et al., 2009). This is a relevant avenue to explore in the future to fully understand the neuro-biology of cognitive decrements observed in PWH. Third, we focused on one facet of executive function (i.e., cognitive flexibility), and future studies should extend our findings to the other core functions (Diamond, 2013). Next, substance use among participants included in our sample was low, and participants had relatively minimal psychiatric symptomatology, which is important to consider as our findings may not generalize to populations of PWH at-large as substance use disorders and other psychiatric conditions are more common in PWH. Note that this was by design in the current study, as these comorbidities are known to independently affect neural dynamics and brain function more generally. Finally, our sample reflected the demographics of the local HIV population, and therefore, we were underpowered to investigate the influence of sex on our findings. Future work should consider evaluating the interaction between sex and HIV status on the neural responses serving cognitive flexibility.
To close, this study provides clear evidence that virally suppressed PWH exhibit robust deficits in cognitive flexibility compared to seronegative controls. These deficits appear to be related to altered alpha and gamma oscillations in higher-order association cortices, with such neurophysiological markers tightly coupled to behavioral performance on the task, as well as to clinical indices of HIV health. Although future work is clearly needed, this study provides novel insight on the neural oscillatory dynamics and brain regions that may be of critical importance to understanding the origins of deficits in cognitive flexibility observed in virally suppressed PWH.
Acknowledgments
The authors thank the participants for generously volunteering their time to participate in the study. The authors also wish to specifically thank Dr. Susan Swindells for her extensive help with the clinical aspects of the study and Nichole Knott for helping with the MEG recordings.
Funding
This work was supported by the National Institutes of Health [R01-MH116782 (TWW), R01-MH118013 (TWW and JTB), S10-OD028751 (TWW), P20-GM144641 (TWW), F31-DA056296 (MS), F30-MH134713 (JJS), F30-MH130150 (ADK)]. The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Footnotes
CRediT authorship contribution statement
Katherine K. Landler: Writing – review & editing, Writing – original draft, Visualization, Investigation, Formal analysis, Data curation, Conceptualization. Mikki Schantell: Writing – review & editing, Writing – original draft, Visualization, Validation, Investigation, Formal analysis, Data curation, Conceptualization. Ryan Glesinger: Formal analysis, Data curation. Lucy K. Home: Formal analysis, Data curation. Christine M. Embury: Writing – review & editing, Validation, Investigation, Formal analysis. Jake J. Son: Writing – review & editing, Validation, Investigation, Formal analysis. Yasra Arif: Writing – review & editing, Validation, Investigation, Formal analysis. Anna T. Coutant: Formal analysis, Data curation. Grant M. Garrison: Formal analysis, Data curation. Kellen M. McDonald: Writing – review & editing, Validation, Investigation, Formal analysis. Jason A. John: Formal analysis, Data curation. Hannah J. Okelberry: Formal analysis, Data curation. Thomas W. Ward: Writing – review & editing, Validation, Investigation, Formal analysis. Abraham D. Killanin: Writing – review & editing, Validation, Investigation, Formal analysis. Maureen Kubat: Formal analysis, Data curation. Renae A. Furl: Formal analysis, Data curation. Jennifer O’Neill: Formal analysis, Data curation. Sara H. Bares: Writing – review & editing, Validation, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. Pamela E. May-Weeks: Writing – review & editing, Validation, Supervision, Project administration, Methodology, Investigation, Formal analysis. James T. Becker: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Tony W. Wilson: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
SHB reports scientific advisory to Gilead Sciences and research grants to her institution from ViiV Healthcare and Janssen. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
De-identified data have been made available to the public through the Collaborative Informatics and Neuroimaging Suite (COINS) database. Data requests can also be fulfilled via the corresponding author.
References
- Alagaratnam J, Winston A, 2022. Molecular neuroimaging of inflammation in HIV. Clin. Exp. Immunol 210, 14–23. 10.1093/cei/uxab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AM, Pérez-Santiago J, Zheng Z, Huang E, Franklin D, Iudicello J, Moore DJ, Ellis RJ, Heaton RK, Letendre SL, 2019. Better executive function is independently associated with full HIV suppression during combination therapy. AIDS 33, 2309–2316. 10.1097/QAD.0000000000002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE, 2007. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69, 1789–1799. 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif Y, Wiesman AI, O’Neill J, Embury C, May PE, Lew BJ, Schantell MD, Fox HS, Swindells S, Wilson TW, 2020. The age-related trajectory of visual attention neural function is altered in adults living with HIV: a cross-sectional MEG study. EBioMedicine 61, 103065. 10.1016/j.ebiom.2020.103065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif Y, Spooner RK, Heinrichs-Graham E, Wilson TW, 2021. High-definition transcranial direct current stimulation modulates performance and alpha/beta parieto-frontal connectivity serving fluid intelligence. J. Physiol 599, 5451–5463. 10.1113/JP282387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster DJN, Ueltzhöffer K, Basten U, Fiebach CJ, 2012. Prefrontal cortical mechanisms underlying individual differences in cognitive flexibility and stability. J. Cogn. Neurosci 24, 2385–2399. 10.1162/jocn_a_00286. [DOI] [PubMed] [Google Scholar]
- Barredo J, Öztekin I, Badre D, 2015. Ventral fronto-temporal pathway supporting cognitive control of episodic memory retrieval. Cereb. Cortex 25, 1004–1019. 10.1093/cercor/bht291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Bajo R, Fabrizio M, Sudre G, Cuesta P, Aizenstein HJ, Lopez OL, Wolk D, Parkkonen L, Maestu F, Bagic A, 2012a. Functional connectivity measured with magnetoencephalography identifies persons with HIV disease. Brain Imaging Behav. 6, 366–373. 10.1007/s11682-012-9149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Fabrizio M, Sudre G, Haridis A, Ambrose T, Aizenstein HJ, Eddy W, Lopez OL, Wolk DA, Parkkonen L, Bagic A, 2012b. Potential utility of resting-state magnetoencephalography as a biomarker of CNS abnormality in HIV disease. J. Neurosci. Methods 206, 176–182. 10.1016/j.jneumeth.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellebaum C, Daum I, 2007. Cerebellar involvement in executive control. Cerebellum 6, 184–192. 10.1080/14734220601169707. [DOI] [PubMed] [Google Scholar]
- Benedict RHB, 1997. Brief Visuospatial Memory Test - Revised. Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- Brandt J, Benedict RHB, 2001. Hopkins Verbal Learning Test – Revised: Professional Manual. Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- Brockett AT, Tennyson SS, deBettencourt CA, Gaye F, Roesch MR, 2020. Anterior cingulate cortex is necessary for adaptation of action plans. Proc. Natl. Acad. Sci. USA 117, 6196–6204. 10.1073/pnas.1919303117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R, 2009. The phase of ongoing EEG oscillations predicts visual perception. The Journal of neuroscience : the official journal of the Society for Neuroscience 29, 7869–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L, 1997. Imaging cognition: an empirical review of PET studies with Normal subjects. J. Cogn. Neurosci 9, 1–26. 10.1162/jocn.1997.9.1.1. [DOI] [PubMed] [Google Scholar]
- Casagrande CC, Lew BJ, Taylor BK, Schantell M, O’Neill J, May PE, Swindells S, Wilson TW, 2021. Impact of HIV-infection on human somatosensory processing, spontaneous cortical activity, and cortical thickness: a multimodal neuroimaging approach. Hum. Brain Mapp 42, 2851–2861. 10.1002/hbm.25408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, 2014. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci 18, 414–421. 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Nakama H, Stokes B, Ernst T, 2008. Antiretroviral treatment is associated with increased attentional load-dependent brain activation in HIV patients. Journal of Neuroimmune Pharmacology : The Official Journal of the Society on Neuroimmune Pharmacology 3, 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ, 2009. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol. Rev 19, 169–185. 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- Dajani DR, Uddin LQ, 2015. Demystifying cognitive flexibility: implications for clinical and developmental neuroscience. Trends Neurosci. 38, 571–578. 10.1016/j.tins.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal SS, Sekihara K, Nagarajan SS, 2006. Modified beamformers for coherent source region suppression. IEEE Trans. Biomed. Eng 53, 1357–1363. 10.1109/TBME.2006.873752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastgheyb RM, Sacktor N, Franklin D, Letendre S, Marcotte T, Heaton R, Grant I, McArthur JC, Rubin LH, Haughey NJ, 2019. Cognitive trajectory phenotypes in human immunodeficiency virus-infected patients. J. Acquir. Immune Defic. Syndr 82, 61–70. 10.1097/QAI.0000000000002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., 2013. Executive functions. Annu. Rev. Psychol 64, 135–168. 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz SM, Schantell M, Spooner RK, Sandal ME, Mansouri A, Arif Y, Okelberry HJ, John JA, Glesinger R, May PE, Heinrichs-Graham E, Case AJ, Zimmerman MC, Wilson TW, 2023. Elevated CRP and TNF-α levels are associated with blunted neural oscillations serving fluid intelligence. Brain Behav. Immun 114, 430–437. 10.1016/j.bbi.2023.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk H, Schoffelen JM, Oostenveld R, Jensen O, 2008. Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 28, 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RAE, Muthukumaraswamy SD, Freeman TCA, Singh KD, 2009. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J. Neurosci 29, 15721–15726. 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, Ricardo-Dukelow ML, Chang L, 2009. Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Ann. Neurol 65, 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenhofer ML, Foley J, Castellon SA, Hinkin CH, 2010. Reciprocal prediction of medication adherence and neurocognition in HIV/AIDS. Neurology 74, 1217–1222. 10.1212/WNL.0b013e3181d8c1ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte J, Hogben JH, Ross J, 1999. Spatial limitations of temporal segmentation. Vis. Res 39, 4052–4061. [DOI] [PubMed] [Google Scholar]
- French MA, King MS, Tschampa JM, da Silva BA, Landay AL, 2009. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J. Infect. Dis 200, 1212–1215. 10.1086/605890. [DOI] [PubMed] [Google Scholar]
- Gaetz W, Edgar JC, Wang DJ, Roberts TPL, 2011. Relating MEG measured motor cortical oscillations to resting γ-aminobutyric acid (GABA) concentration. Neuroimage 55, 616–621. 10.1016/j.neuroimage.2010.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon P, Khan MZ, Kolson DL, 2011. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr. Opin. Neurol 24, 275–283. 10.1097/WCO.0b013e32834695fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D, 1997. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cereb. Cortex 7, 374–385. 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Golden C, Freshwater SM, 2002. Stroop Color and Word Test: Revised Examiner’s Manual. Stoelting, Wood Dale, IL. [Google Scholar]
- González-Scarano F, Martín-García J, 2005. The neuropathogenesis of AIDS. Nat. Rev. Immunol 5, 69–81. 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Grant I, Franklin DR, Deutsch R, Woods SP, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Collier AC, Marra CM, Clifford DB, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Smith DM, Heaton RK, CHARTER Group, 2014. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology 82, 2055–2062. 10.1212/WNL.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R, 2001. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc. Natl. Acad. Sci. USA 98, 694–699. 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AS, Meer MA, Touretzky DS, Redish AD, 2012. Segmentation of spatial experience by hippocampal θ sequences. Nat. Neurosci 15, 1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Händel BF, Haarmeier T, Jensen O, 2011. Alpha oscillations correlate with the successful inhibition of unattended stimuli. J. Cogn. Neurosci 23, 2494–2502. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I, 2004. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, 2010. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy. Neurology 75, 2087–2096. 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CHARTER Group, HNRC Group, 2011. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neuro-Oncol 17, 3–16. 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Deutsch R, Letendre S, Ellis RJ, Casaletto K, Marquine MJ, Woods SP, Vaida F, Atkinson JH, Marcotte TD, McCutchan JA, Collier AC, Marra CM, Clifford DB, Gelman BB, Sacktor N, Morgello S, Simpson DM, Abramson I, Gamst AC, Fennema-Notestine C, Smith DM, Grant I, CHARTER Group, 2015. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin. Infect. Dis 60, 473–480. 10.1093/cid/ciu862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR, 2005. A new approach to neuroimaging with magnetoencephalography. Hum. Brain Mapp 25, 199–211. 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Bonnefond M, VanRullen R, 2012. An oscillatory mechanism for prioritizing salient unattended stimuli. Trends Cogn. Sci 16, 200–206. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S, 2001. Boston Naming Test, Second edition. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- Kirschen MP, Chen SHA, Schraedley-Desmond P, Desmond JE, 2005. Load- and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: an fMRI study. Neuroimage 24, 462–472. 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Klimesch W, 2012. α-Band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci 16, 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T, Schwaiger J, 1998. Induced alpha band power changes in the human EEG and attention. Neurosci. Lett 244, 73–76. [DOI] [PubMed] [Google Scholar]
- Kovach CK, Gander PE, 2016. The demodulated band transform. J. Neurosci. Methods 261, 135–154. 10.1016/j.jneumeth.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala J, Jung J, Bouvard S, Lecaignard F, Lothe A, Bouet R, Ciumas C, Ryvlin P, Jerbi K, 2015. Gamma oscillations in V1 are correlated with GABA(a) receptor density: a multi-modal MEG and Flumazenil-PET study. Sci. Rep 5, 16347. 10.1038/srep16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau AN, Fries P, 2012. Attention samples stimuli rhythmically. Current biology : CB 22, 1000–1004. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM, 1969. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9, 179–186. [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ, 2008. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch. Neurol 65, 65–70. 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew BJ, McDermott TJ, Wiesman AI, O’Neill J, Mills MS, Robertson KR, Fox HS, Swindells S, Wilson TW, 2018. Neural dynamics of selective attention deficits in HIV-associated neurocognitive disorder. Neurology 91, e1860–e1869. 10.1212/WNL.0000000000006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew BJ, Schantell MD, O’Neill J, Morsey B, Wang T, Ideker T, Swindells S, Fox HS, Wilson TW, 2021. Reductions in gray matter linked to epigenetic HIV-associated accelerated aging. Cereb. Cortex 31, 3752–3763. 10.1093/cercor/bhab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji H, Miller R, 2004. The neurology of HIV infection. J. Neurol. Neurosurg. Psychiatry 75 (Suppl. 1), i29–i35. 10.1136/jnnp.2003.034348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews CG, Klove K, 1964. Instruction Manual for the Adult Neuropsychology Test Battery. University of Wisconsin Medical School, Madison, WI. [Google Scholar]
- McDonald KM, Schantell M, Horne LK, John JA, Rempe MP, Glesinger R, Okelberry HJ, Coutant AT, Springer SD, Mansouri A, Embury CM, Arif Y, Wilson TW, 2024. The neural oscillations serving task switching are altered in cannabis users. J. Psychopharmacol 2698811241235204. 10.1177/02698811241235204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, 2000. The prefrontal cortex and cognitive control. Nat. Rev. Neurosci 1, 59–65. 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Moschopoulos CD, Alford K, Antoniadou A, Vera JH, 2024. Cognitive impairment in people living with HIV: mechanisms, controversies, and future perspectives. Trends Mol. Med S1471–4914 (24). 10.1016/j.molmed.2024.06.005, 00163-1. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD, 2009. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc. Natl. Acad. Sci. USA 106, 8356–8361. 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman DA, Shallice T, 1986. Attention to action. In: Davidson RJ, Schwartz GE, Shapiro D (Eds.), Consciousness and Self-Regulation: Advances in Research and Theory, Springer US, vol. 4. Boston, MA, pp. 1–18. 10.1007/978-1-4757-0629-1_1. [DOI] [Google Scholar]
- O’Connor EE, Zeffiro Timothy A., Zeffiro Thomas A., 2018. Brain structural changes following HIV infection: Meta-analysis. AJNR Am. J. Neuroradiol 39, 54–62. 10.3174/ajnr.A5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp N, Ktonas P, 1977. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed. Sci. Instrum 13, 135–145. [PubMed] [Google Scholar]
- Pastötter B, Dreisbach G, Bäuml K-HT, 2013. Dynamic adjustments of cognitive control: oscillatory correlates of the conflict adaptation effect. J. Cogn. Neurosci 25, 2167–2178. 10.1162/jocn_a_00474. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Holmes AP, Frackowiak RS, Friston KJ, 1995. Estimating smoothness in statistical parametric maps: variability of p values. J. Comput. Assist. Tomogr 19, 788–796. 10.1097/00004728-199509000-00017. [DOI] [PubMed] [Google Scholar]
- Rajan A, Siegel SN, Liu Y, Bengson J, Mangun GR, Ding M, 2019. Theta Oscillations Index Frontal Decision-Making and Mediate Reciprocal Frontal–Parietal Interactions in Willed Attention. Cereb. Cortex 29, 2832–2843. 10.1093/cercor/bhy149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA, 2008. Neurocognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci 17, 177–182. 10.1111/j.1467-8721.2008.00570.x. [DOI] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ, 2007. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 21, 1915–1921. 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Robertson K, Liner J, Meeker RB, 2012. Antiretroviral neurotoxicity. J. Neuro-Oncol 18, 388–399. 10.1007/s13365-012-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Maki PM, 2019. HIV, depression, and cognitive impairment in the era of effective antiretroviral therapy. Curr. HIV/AIDS Rep 16, 82–95. 10.1007/s11904-019-00421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford R, Fernandez Cruz AL, Scott SC, Mayo NE, Fellows LK, Ances BM, Collins DL, 2017. Regionally specific brain volumetric and cortical thickness changes in HIV-infected patients in the HAART era. J. Acquir. Immune Defic. Syndr 74, 563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gruber W, Doppelmayr M, Stadler W, Schabus M, 2002. The interplay between theta and alpha oscillations in the human electroencephalogram reflects the transfer of information between memory systems. Neurosci. Lett 324, 121–124. 10.1016/s0304-3940(02)00225-2. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Griesmayr B, Freunberger R, Klimesch W, 2010. Control mechanisms in working memory: a possible function of EEG theta oscillations. Neurosci. Biobehav. Rev 34, 1015–1022. [DOI] [PubMed] [Google Scholar]
- Schantell M, Taylor BK, Lew BJ, O’Neill JL, May PE, Swindells S, Wilson TW, 2021. Gray matter volumes discriminate cognitively impaired and unimpaired people with HIV. Neuroimage Clin 31, 102775. 10.1016/j.nicl.2021.102775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantell M, Springer SD, Arif Y, Sandal ME, Willett MP, Johnson HJ, Okelberry HJ, O’Neill JL, May PE, Bares SH, Wilson TW, 2022a. Regular cannabis use modulates the impact of HIV on the neural dynamics serving cognitive control. J. Psychopharmacol 36, 1324–1337. 10.1177/02698811221138934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantell M, Taylor BK, Spooner RK, May PE, O’Neill J, Morsey BM, Wang T, Ideker T, Bares SH, Fox HS, Wilson TW, 2022b. Epigenetic aging is associated with aberrant neural oscillatory dynamics serving visuospatial processing in people with HIV. Aging (Albany NY) 14. 10.18632/aging.204437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantell M, Glesinger R, Coutant AT, Okelberry HJ, John JA, Dietz SM, Springer SD, Arif Y, Wilson TW, 2024a. Stress and psychosocial distress scale with blunted oscillatory dynamics serving abstract reasoning. Depress. Anxiety 4720803. 10.1155/2024/4720803. [DOI] [Google Scholar]
- Schantell M, John JA, Coutant AT, Okelberry HJ, Horne LK, Glesinger R, Springer SD, Mansouri A, May-Weeks PE, Wilson TW, 2024b. Chronic cannabis use alters the spontaneous and oscillatory gamma dynamics serving cognitive control. Hum. Brain Mapp 45, e26787. 10.1002/hbm.26787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantell M, Taylor BK, Mansouri A, Arif Y, Coutant AT, Rice DL, Wang Y-P, Calhoun VD, Stephen JM, Wilson TW, 2024c. Theta oscillatory dynamics serving cognitive control index psychosocial distress in youth. Neurobiology of Stress 29, 100599. 10.1016/j.ynstr.2023.100599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, Maclin EL, Gratton G, Fabiani M, 2010. Span, CRUNCH, and beyond: working memory capacity and the aging brain. J. Cogn. Neurosci 22, 655–669. 10.1162/jocn.2009.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott WA, 1962. Cognitive complexity and cognitive flexibility. Sociometry 25, 405–414. 10.2307/2785779. [DOI] [Google Scholar]
- Simioni S, Cavassini M, Annoni J-M, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave J-P, Giacobini E, Hirschel B, Du Pasquier RA, 2010. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 24, 1243–1250. 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Spaak E, Lange FP, Jensen O, 2014. Local entrainment of α oscillations by visual stimuli causes cyclic modulation of perception. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 3536–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, Mills MS, O’Neill J, Robertson KR, Fox HS, Swindells S, Wilson TW, 2018. Aberrant oscillatory dynamics during somatosensory processing in HIV-infected adults. Neuroimage Clin 20, 85–91. 10.1016/j.nicl.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, O’Neill J, Schantell MD, Fox HS, Swindells S, Wilson TW, 2020. Prefrontal gating of sensory input differentiates cognitively impaired and unimpaired aging adults with HIV. Brain Commun 2, fcaa080. 10.1093/braincomms/fcaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Taylor BK, Ahmad IM, Dyball K, Emanuel K, O’Neill J, Kubat M, Swindells S, Fox HS, Bares SH, Stauch KL, Zimmerman MC, Wilson TW, 2022. Mitochondrial redox environments predict sensorimotor brain-behavior dynamics in adults with HIV. Brain Behav. Irnmun 107, 265–275. 10.1016/j.bbi.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann EE, Heaton RK, Pasipanodya E, Moore RC, Paolillo EW, Rubin LH, Ellis R, Moore DJ, HNRP Group, 2018. Sex differences in HIV-associated cognitive impairment. AIDS 32, 2719–2726. 10.1097/QAD.0000000000002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Simola J, 2006. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol 51, 1759–1768. 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Trunfio M, Vuaran E, Vai D, Quarta C, Di Stefano A, Imperiale D, Cinnirella G, Bonora S, Di Perri G, Letendre SL, Calcagno A, 2024. Symptomatic and asymptomatic neurocognitive impairment, ART adherence and HIV control: a 4-year observational study. AIDS Behav. 10.1007/s10461-024-04440-w. [DOI] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ, 1997. Signal-space projection method for separating MEG or EEG into components. Med. Biol. Eng. Comput 35, 135–140. 10.1007/BF02534144. [DOI] [PubMed] [Google Scholar]
- Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A, 1997. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng 44, 867–880. 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Varjačić A, Mantini D, Levenstein J, Slavkova ED, Demeyere N, Gillebert CR, 2018. The role of left insula in executive set-switching: lesion evidence from an acute stroke cohort. Cortex 107, 92–101. 10.1016/j.cortex.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-Q, Pan Y, Zhu S, Wang Y-G, Shen Z-H, Wang K, 2017. Selective impairments of alerting and executive control in HIV-infected patients: evidence from attention network test. Behav. Brain Funct 13. 10.1186/s12993-017-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D., 2008. Wechsler Adult Intelligence Scale, Fourth edition. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wechsler D., 2009. Wechsler Memory Scale, Fourth edition. Pearson, San Antonio, TX. [Google Scholar]
- Wiesman AI, Wilson TW, 2020. Attention modulates the gating of primary somatosensory oscillations. NeuroImage 211, 116610. 10.1016/j.neuroimage.2020.116610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Heinrichs-Graham E, Proskovec AL, McDermott TJ, Wilson TW, 2017. Oscillations during observations: dynamic oscillatory networks serving visuospatial attention: oscillations during observations. Hum. Brain Mapp 38, 5128–5140. 10.1002/hbm.23720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, O’Neill J, Mills MS, Robertson KR, Fox HS, Swindells S, Wilson TW, 2018. Aberrant occipital dynamics differentiate HIV-infected patients with and without cognitive impairment. Brain 141, 1678–1690. 10.1093/brain/awy097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Christopher-Hayes NJ, Eastman JA, Heinrichs-Graham E, Wilson TW, 2021. Response certainty during bimanual movements reduces gamma oscillations in primary motor cortex. NeuroImage 224, 117448. 10.1016/j.neuroimage.2020.117448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G, Robertson G, 2006. Wide Range Achievement Test, Fourth Edition, Professional Manual, Fourth, ed. Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- Wilson TW, Fox HS, Robertson KR, Sandkovsky U, O’Neill J, Heinrichs-Graham E, Knott NL, Swindells S, 2013a. Abnormal MEG oscillatory activity during visual processing in the prefrontal cortices and frontal eye-fields of the aging HIV brain. PLoS One 8 e66241. doi: 10.137/journal.pone.0066241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Robertson KR, Sandkovsky U, O’Neill J, Knott NL, Fox HS, Swindells S, 2013b. Functional brain abnormalities during finger-tapping in HIV-infected older adults: a magnetoencephalography study. J. Neurolmmune Pharmacol 8, 965–974. 10.1007/s11481-013-9477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston A, Spudich S, 2020. Cognitive disorders in people living with HIV. Lancet HIV 7, e504–e513. 10.1016/S2352-3018(20)30107-7. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC, 1996. A unified statistical approach for determining significant signals in images of cerebral activation. Hum. Brain Mapp 4, 58–73. . [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Andermann M, Koulis T, MacDonald D, Evans AC, 1999. Detecting changes in nonisotropic images. Hum. Brain Mapp 8, 98–101. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Raz N, 2014. Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neurosci. Biobehav. Rev 42, 180–192. 10.1016/j.neubiorev.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified data have been made available to the public through the Collaborative Informatics and Neuroimaging Suite (COINS) database. Data requests can also be fulfilled via the corresponding author.


