Dear Editor
Cystic fibrosis (CF) is a debilitating genetic disorder marked by chronic lung infections and persistent inflammation, significantly impacting patient quality of life and longevity. While well-known pathogens such as Pseudomonas aeruginosa and Staphylococcus aureus have been extensively studied, the emergence of Pandoraea sputorum (P. sputorum) as a formidable pathogen in CF warrants urgent attention.
P. sputorum, a member of the Burkholderia cepacia complex, is increasingly associated with severe, treatment-resistant infections, highlighting the need for innovative therapeutic approaches. P. sputorum has been linked to a range of clinical challenges in CF patients, including declining lung function, chronic colonization, and heightened morbidity. The pathogen's ability to form biofilms within the viscous CF mucus significantly contributes to its persistence and resistance to conventional antibiotic therapies. Moreover, P. sputorum exhibits phenotypic antimicrobial tolerance, which complicates eradication efforts and often leads to recurrent infections [1]. Understanding these mechanisms is crucial for developing effective treatments. Biofilm formation is a key survival strategy for P. sputorum, providing a protective niche that shields it from both the host immune system and antibiotic treatments (Fig. 1) [2].
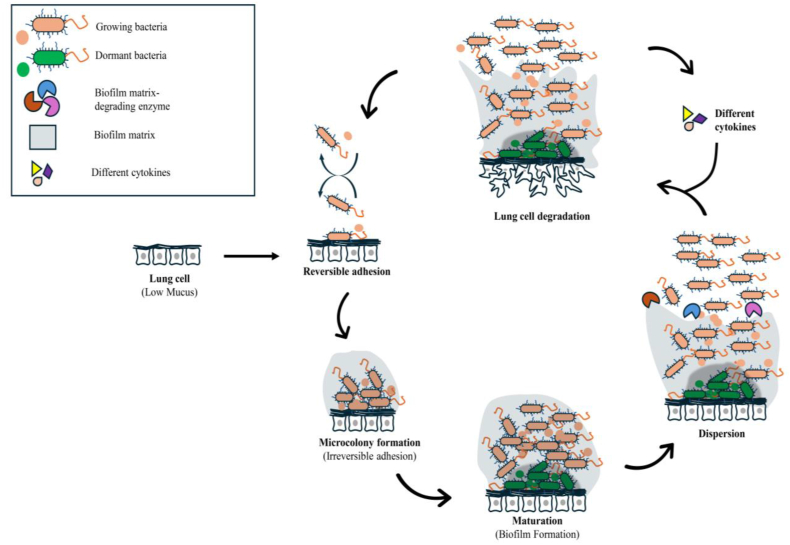
Fig. 1.
Pathogenesis of P. sputorum in cystic fibrosis patients:P. sputorum adhere to lung cell surface by adhesins via reversible adhesion process that leads to microcolony formation through irreversible adhesion process, biofilm formation and dispersion. These events trigger a significant pro-inflammatory response with the role of virulence factors including proteases and lipases result in lung tissue deterioration by altering ion transport and osmosis. Beside virulence factors, different sorts of cytokines activate immune responses including inflammation.
P. sputorum also employs sophisticated immune evasion strategies, manipulating host immune responses to promote chronic inflammation and tissue damage, thereby hindering bacterial clearance. These strategies are likely similar to those used by related Burkholderia species, though direct studies on P. sputorum are limited. Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which are characteristic of CF, exacerbate the colonization and persistence of P. sputorum. Defective CFTR leads to thick, dehydrated mucus in the lungs, creating an environment conducive to bacterial growth. The pathogen's ability to adhere to lung epithelial cells via adhesins further facilitates colonization and infection [3]. Moreover, P. sputorum can induce a strong pro-inflammatory response in CF patients, exacerbating lung dysfunction and damage.
The intrinsic antimicrobial resistance of P. sputorum poses a significant therapeutic challenge in managing persistent infections. This pathogen displays resistance to multiple classes of antibiotics, including β-lactams, aminoglycosides, and polymyxins [2]. This multi-drug resistance severely limits treatment options and necessitates the use of last-resort antibiotics with potential adverse side effects. Furthermore, the lack of standardized treatment guidelines for P. sputorum infections complicates clinical decision-making. Current management often relies on susceptibility testing and antibiotic combinations tailored to individual cases, with outcomes remaining variable.
Given the significant challenges posed by P. sputorum, there is an urgent need for novel therapeutic strategies. One promising approach is the development of treatments that target biofilm disruption and immune modulation. Specifically, the use of synergistic drug combinations that exploit P. sputorum's biofilm vulnerabilities could enhance treatment efficacy. For instance, combining conventional antibiotics with agents that disrupt biofilm matrix components or inhibit biofilm formation could prevent the establishment of chronic infections [4]. Additionally, exploring immunomodulatory therapies that enhance the host's ability to clear infections without exacerbating inflammation could be beneficial. For example, utilizing anti-inflammatory agents alongside antibiotics may reduce tissue damage and improve overall treatment outcomes [5].
P. sputorum represents a significant and underappreciated threat to CF patients. Addressing this challenge requires a multifaceted approach, combining epidemiological research, mechanistic studies, and the development of innovative therapeutic strategies. Prioritizing research on biofilm dynamics, resistance mechanisms, and immune evasion strategies will be critical. By exploring novel drug combinations and immunomodulatory therapies, we can hope to improve outcomes for CF patients facing persistent infections with P. sputorum.
CRediT authorship contribution statement
Riyan Al Islam Reshad: Writing – original draft, Methodology, Conceptualization. Roni Mia: Writing – original draft, Conceptualization. Yusha Araf: Writing – original draft, Methodology. Anandha Mozumder: Formal analysis, Data curation. Sharmin Akter: Writing – review & editing. Sukumar Saha: Writing – review & editing. Muzahed Uddin Ahmed: Writing – review & editing. Chirojit Debnath: Writing – review & editing, Conceptualization. Mohammad Kamruzzaman Khan: Writing – review & editing. Chitta Ranjan Debnath: Writing – review & editing, Supervision. Mamun Al Mahtab: Writing – review & editing, Supervision. Tofazzal Islam: Writing – review & editing, Supervision. Md. Golzar Hossain: Writing – review & editing, Supervision.
CRediT authorship contribution statement
Riyan Al Islam Reshad: Writing – original draft, Methodology, Conceptualization. Roni Mia: Writing – original draft, Conceptualization. Yusha Araf: Writing – original draft, Methodology. Anandha Mozumder: Formal analysis, Data curation. Sharmin Akter: Writing – review & editing. Sukumar Saha: Writing – review & editing. Muzahed Uddin Ahmed: Writing – review & editing. Chirojit Debnath: Writing – review & editing, Conceptualization. Mohammad Kamruzzaman Khan: Writing – review & editing. Chitta Ranjan Debnath: Writing – review & editing, Supervision. Mamun Al Mahtab: Writing – review & editing, Supervision. Tofazzal Islam: Writing – review & editing, Supervision. Md. Golzar Hossain: Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors affirm no known financial conflicts or personal relationships that might have influenced the work presented in this paper.
Handling Editor: Patricia Schlagenhauf
References
- 1.Van Acker H., Van Dijck P., Coenye T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014;22:326–333. doi: 10.1016/j.tim.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Martina P.F., Martínez M., Frada G., Alvarez F., Leguizamón L., Prieto C., Barrias C., Bettiol M., Lagares A., Bosch A., et al. First time identification of Pandoraea sputorum from a patient with cystic fibrosis in Argentina: a case report. BMC Pulm Med. 2017;17:33. doi: 10.1186/s12890-017-0373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caraher E., Collins J., Herbert G., Murphy P.G., Gallagher C.G., Crowe M.J., Callaghan M., McClean S. Evaluation of in vitro virulence characteristics of the genus Pandoraea in lung epithelial cells. J Med Microbiol. 2008;57:15–20. doi: 10.1099/jmm.0.47544-0. [DOI] [PubMed] [Google Scholar]
- 4.Ciofu O., Tolker-Nielsen T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents-how P. aeruginosa can escape antibiotics. Front Microbiol. 2019;10:913. doi: 10.3389/fmicb.2019.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernández-Olmos A., Morosini M.I., Lamas A., García-Castillo M., García-García L., Cantón R., Máiz L. Clinical and microbiological features of a cystic fibrosis patient chronically colonized with Pandoraea sputorum identified by combining 16S rRNA sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2012;50:1096–1098. doi: 10.1128/jcm.05730-11. [DOI] [PMC free article] [PubMed] [Google Scholar]