Abstract
Introduction and importance
Mucinous cystadenoma is a benign cystic ovarian tumor arising from the surface epithelium of the ovary that usually presents with vague, unspecified abdominal symptoms. If not detected early, this tumor has the potential to grow to a substantial size and can present with huge abdominal distention leading to various compression symptoms. The reported incidence of giant ovarian cystadenoma in postmenopausal women is relatively unknown due to the widespread use of ultrasound and other radiological imaging modalities.
Case presentation
Here, we present a rare case of giant borderline mucinous cystadenoma in a 67-year-old female 10 years postmenopausal from Northern Tanzania with multiple comorbidities including hypertension and type 2 diabetes mellitus. She presented with abdominal distention which was of gradual onset for 1 year. Abdominal CT scan with contrast revealed a huge septated cystic mass occupying the entirety of the abdominal and pelvic cavities causing a mass effect on adjacent intra-abdominal structures. An intact 23-kg left ovarian cyst was removed, and a total abdominal hysterectomy (TAH) along with bilateral salpingo-oophorectomy (BSO) was performed. Post-operative recovery was excellent and the patient was discharged. The final histopathological report showed a borderline ovarian mucinous cystadenoma and the patient was managed conservatively after surgery.
Clinical discussion
In this case report, we discuss the condition's rarity especially in postmenopausal women, related reports in the literature, and the numerous difficulties clinicians face when encountering a patient with borderline mucinous cystadenoma. The rarity of borderline mucinous cystadenomas in postmenopausal women makes these cases clinically unusual, challenging diagnostic and therapeutic expectations.
Conclusion
This case report highlights the importance of thoroughly evaluating women with vague abdominal symptoms. While this condition is rare in postmenopausal women, its massive form can be dangerous if not diagnosed and managed promptly, with the potential to progress to malignancy. Greater awareness of this condition could lead to earlier detection and reporting of more cases.
Keywords: Giant ovarian cyst, Mucinous cystadenoma, Bilateral salpingo-oophorectomy, Postmenopausal, Borderline, Histopathological
Highlights
-
•
Mucinous cystadenomas occur most commonly in the third to sixth decades of life, are rare at the extremities of age, before puberty, and after menopause.
-
•
The surgical management of these masses is associated with many life threatening complications.
-
•
In postmenopausal women, CA 125 should be used for primary evaluation because it allows the Risk of Malignancy Index of ovarian cysts to be calculated.
1. Introduction
The occurrence of borderline mucinous cystadenomas in postmenopausal women is particularly unusual due to the rarity of such tumors in this age group, the different hormonal environment, and the altered clinical expectations for ovarian masses in older women. The case raises important considerations about the pathogenesis, diagnosis, and management of borderline ovarian tumors in postmenopausal women. Borderline ovarian tumors (BOTs) are histologically characterized by cellular proliferation and nuclear atypia without destructive stromal invasion. Taylor, in 1929, described BOTs as ovarian neoplasms with a favorable prognosis despite peritoneal involvement and called these tumors “semi-malignant” ovarian tumors [1]. The term ‘” borderline tumors” was used for the first time by the World Health Organization (WHO) in 2003 [2]. In 2016, the Japan Society of Obstetrics and Gynecology (JSOG) and the Japanese Society of Pathology defined epithelial ovarian tumors of borderline malignancy as tumors that have “histologic characteristics intermediate between benign and malignant tumors. Patients with these tumors have a better prognosis because of their low malignancy.” [3]. Ovarian tumors are termed “giant” if they are bigger than 20 cm and are rare these days because of early diagnosis and treatment. Ovarian tumors are the most common gynecological neoplasms, with a prevalence of 2.5–6.6 % in a woman's lifetime, and are the 8th leading cause of cancer mortality among women worldwide [4,5,14]. Among them, mucinous cystadenoma is a benign cystic ovarian tumor originating from the surface epithelium of the ovary, characterized by mucin production and classified into benign (80 %), borderline or low malignant potential (10 %), and invasive (10 %) subtypes [6]. Mucinous cystadenomas occur most commonly in the third to sixth decades of life, but benign ovarian mucinous tumors are rare at the extremities of age, before puberty, and after menopause [5,6,14]. Borderline subtypes tend to be more common than invasive tumors, accounting for 67 % of mucinous neoplasm versus less than 2.4 % in primary mucinous carcinomas [7]. Most ovarian tumors are now detected on ultrasound and other radiological imaging due to their widespread use [8,14]. Mucinous cystadenomas are unilateral in 95 % of cases and can present with vague symptoms like progressive abdominal distention, fullness, bloating, an adnexal mass, vague pelvic or abdominal pain, and gastrointestinal symptoms [8,14]. The surgical management of these masses is associated with many life-threatening complications, which arise predominantly after surgery owing to rapid changes in body circulation, and pulmonary edema. The former include severe hypotension, increased venous return, cardiac failure, respiratory failure, and intestinal enlargement [9]. We report a rare case of giant borderline mucinous cystadenoma diagnosed in postmenopausal woman from Northern Tanzania with multiple comorbidities. This work has been reported in line with the SCARE criteria [15].
2. Case report
A 67-year-old P2L2 10 years postmenopausal woman presented to our clinic with complaints of massive abdominal distention which started gradually 1 year ago. This was associated with vaginal bleeding on and off for more than 3 months. She reported a loss of appetite, nausea, early satiety with recurrent heartburn, a history of perceiving incomplete rectal emptying with increased urinary frequency and urgency, weight loss, on and off a history of vomiting, and more recently, difficulty breathing especially on lying supine and on ambulation. She is a known hypertensive and diabetic patient, on regular medication for more than 5 years, but otherwise has a negative personal and family history of ovarian, uterine, bowel, and breast cancers.
On examination, she was pale and afebrile with a BP of 145/86 mmHg, a PR of 126 bpm, a respiratory rate of 20 bpm, a SP02: 99 % in room air, and an RBG of 5 mmol/L. She weighed 90 kg, and was 160 cm in height with an abdominal circumference of 125 cm at the level of the umbilicus, with bilateral lower limb pitting edema, the abdomen was grossly distended revealing a large mass extending corresponding with 36 weeks of gravid uterus. The mass was mobile, smooth, firm, and hard in consistency. On vaginal examination, the cervix appeared normal, and both fornices were full. Examination of the chest revealed bilaterally decreased air entry in both lungs. The examination of other systems was unremarkable.
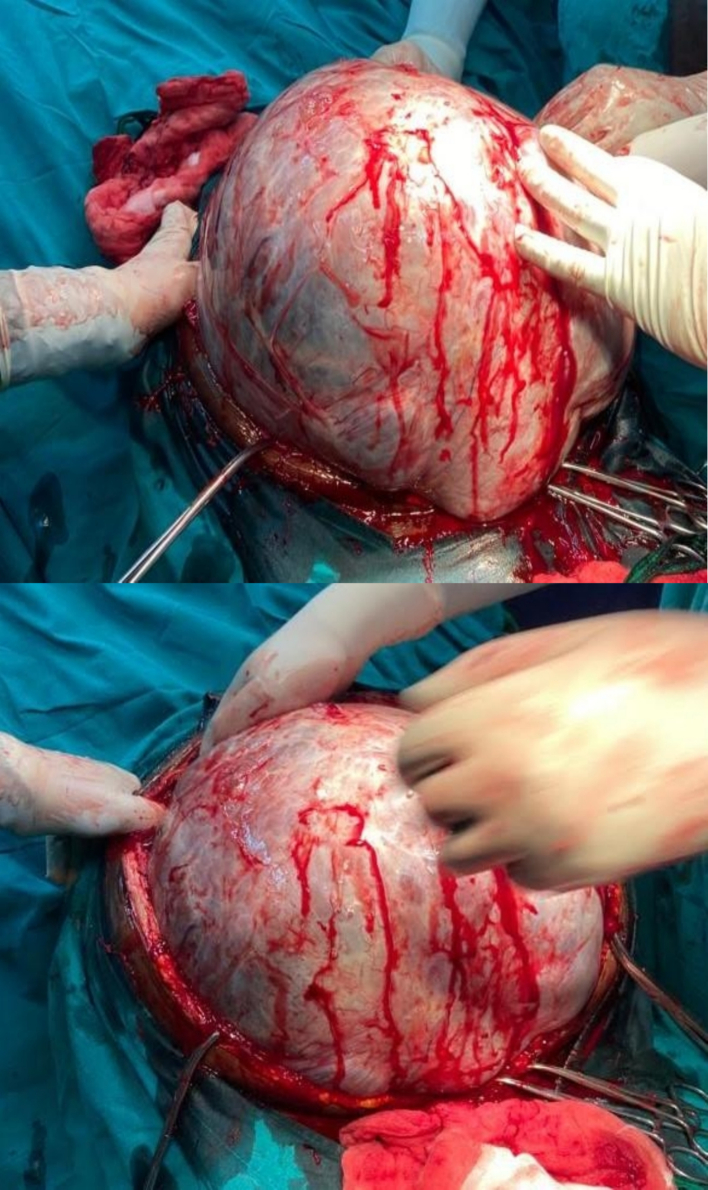
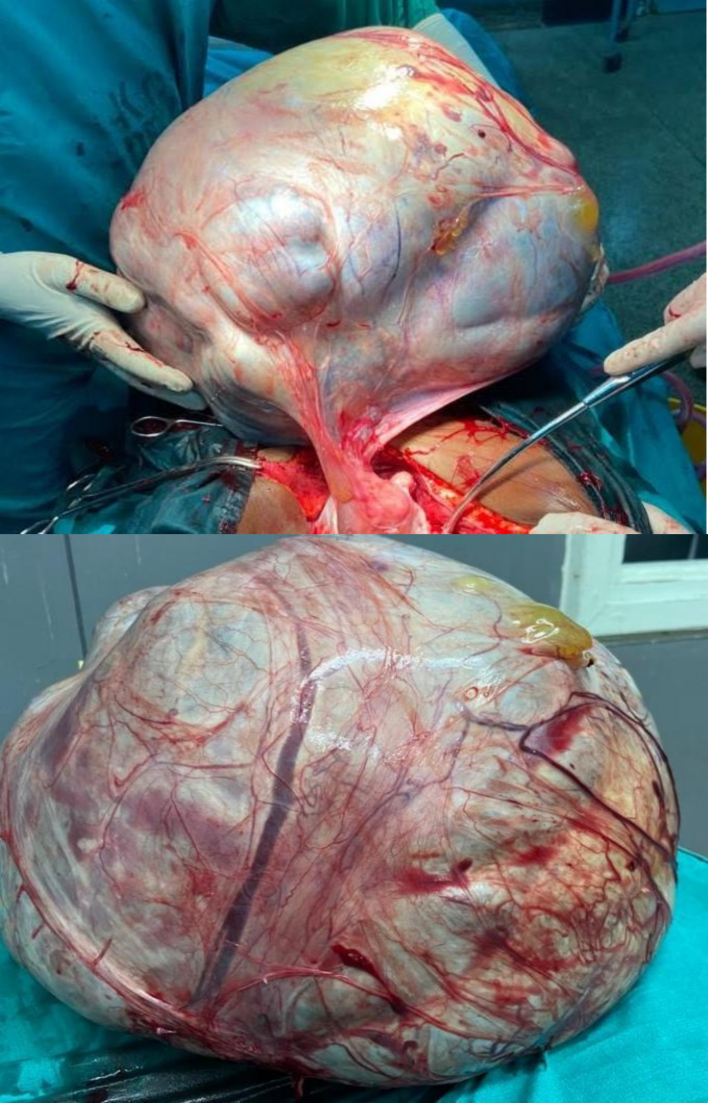
The leading differential diagnoses at this time included serous cystadenoma, retroperitoneal tumors, and mucinous cystadenocarcinoma. Routine laboratory investigations were performed within normal limits. Tumor marker CA 125 was elevated at 66 U/mL (ref range 0.000–35.000) with normal CEA at 2.358 ng/mL (ref range 0.00–5.093), and beta HCG and alpha-fetoprotein were within normal limits. A transabdominal ultrasound scan (USS) showed a huge mass with septa, as well as bilateral mild hydronephrosis, with enhanced abdominal CT with contrast showing huge septated cystic lesion occupying the whole of the abdominal and pelvic cavities causing mass effect on adjacent intra-abdominal structures measuring 35.6 cm × 34.8 cm × 32 cm (Fig. 1). The left ovary was not appreciated, and explorative laparotomy was performed under general anesthesia, with a left lateral tilt to the operating table. The abdomen was opened in layers with an extended umbilical midline incision. Minimal ascites were present in the peritoneal cavity, a grossly whitish glistening capsule of the tumor was identified which extended up to the xiphisternum (Fig. 2), and size of the mass was identified as 36 cm × 30 cm × 18 cm weighing 23 kg (Fig. 3) occupying the abdominal cavity and arising from the left ovary and adhering to the anterior part of the abdominal wall. The liver, spleen, and hemidiaphragms looked normal. A total abdominal hysterectomy and bilateral salpingo-oophorectomy were done, and hemostasis was achieved. The Postoperative recovery was uneventful. Histopathological examination showed ovarian cystic lesion sections showing mucinous complex cysts with atypical proliferating glands with low or no cytologic atypia and the impression was borderline mucinous cystadenoma (Fig. 4).
Fig. 1.
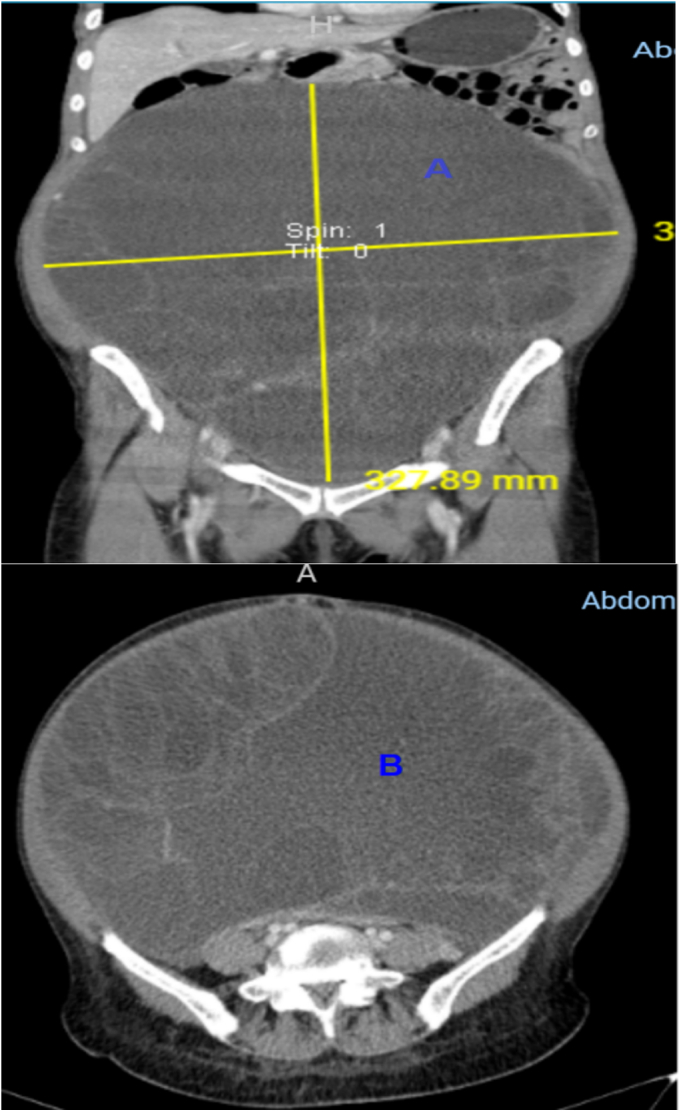
CT with contrast A (coronal view) and B (Axial view) showing huge septated cystic lesion occupying the whole of the abdominal and pelvic cavities causing mass effect on adjacent intra-abdominal structures and displacement of the great vessels posteriorly measuring 35.6 cm × 34.8 cm × 32 cm.
Fig. 2.
Intraoperative findings upon abdominal entry.
Fig. 3.
Gross pathology of completely removed giant ovarian tumor. Postoperative cyst weighing 23 kg.
Fig. 4.
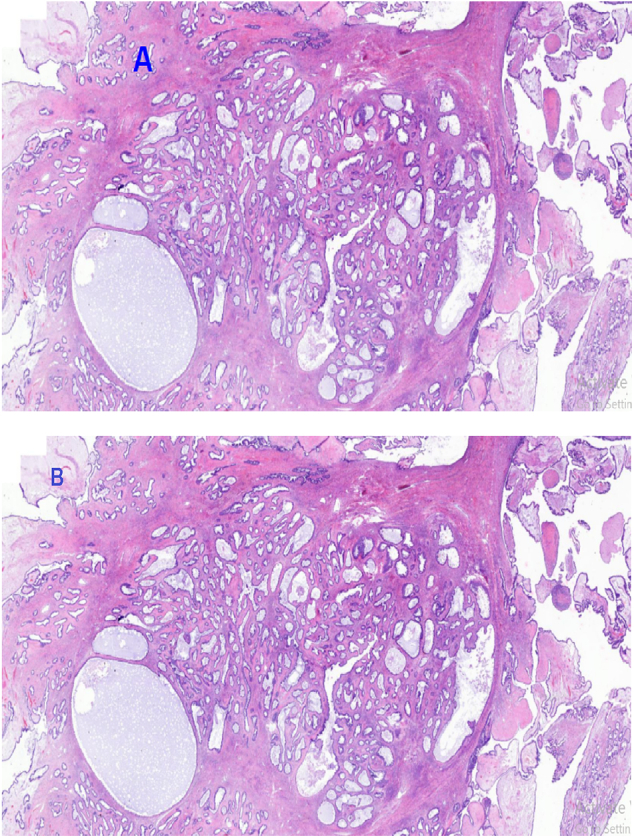
A. Histopathology of atypical proliferative (borderline) tumor demonstrates a cystic lesion with mucinous epithelium. Much of the lesion has the appearance of a mucinous cystadenoma, with flat, bland epithelium, but there are focal proliferative changes, with epithelial stratification and tufting, consistent with a borderline tumor; H&E staining at 20 x original magnification.
B. Photomicroscopy of the mucinous borderline tumor showing complex neoplastic glands of various sizes displaying proliferation, H&E staining at 40 x original magnification.
The patient developed mild hypotension following the decompression of the mass and she was managed with head down and IV fluids. She had received 2 units of blood, 1 unit of fresh frozen plasma, and 3.5 l of Ringer's lactate alternated with normal saline intraoperatively. After 3 h of surgery, she was nursed in the general ward for postoperative care. She received intravenous antibiotics and was put on analgesia. She was discharged on the 6th postoperative day and returned to the clinic after 2 weeks for follow-up with the gynaecologist and she will be followed up every 3 months to see the progress. To date, regular follow-up evaluation has shown no evidence of tumor recurrence, with negative radiologic findings, including transvaginal ultrasound, chest and abdominal pelvic CT scan, and also her elevated serum CA 125 level resolved immediately.
3. Discussion
The present study describes a rare case of borderline ovarian mucinous cystadenoma in a postmenopausal female. Given the rarity of the condition worldwide, and particularly in our setting in northern Tanzania, our initial clinical impression was an ovarian tumor. However, following surgical intervention, we were able to definitively confirm the diagnosis of BOTs on histopathology and thus, pursue conservative management. In this report, we discuss the rarity of this particular condition, the literature surrounding similar reports, and the management of the condition.
Benign ovarian mucinous cystadenomas make up the majority (81 %) of mucinous tumors. These tumors can grow to extremely large sizes and are among the largest of any recorded tumor in the body [10], as was the case with our patient, the mass weight was 23 kg. In postmenopausal women, however, the incidence of ovarian borderline tumors significantly decreases therefore quite uncommon. Mucinous tumors tend to occur in the fifth or sixth decade of life, although their actual incidence in postmenopausal women remains underreported due to early detection, as our patient was 67 year old of age postmenopausal. The aging ovary is more susceptible to malignant transformations, making the diagnosis of a borderline, non-invasive tumor in this demographic a deviation from the expected clinical presentation [5,6]. Giant ovarian tumors generally pose a risk due to their location and pressure effects on surrounding structures. They can undergo a malignant transformation as well. This case is particularly unusual because it presents a low-grade, borderline tumor in a population where ovarian malignancies are more common. Even though their symptomatology is vague they can cause serious complications such as torsion, rupture, and ascites and due to their expanding size lead to higher grades of dyspnea [11]. Among the tumor markers measured in our patient, the carcinoembryonic antigen (CEA) was normal, and cancer antigen 125 (CA 125) was elevated. Benign conditions like pelvic inflammatory disease and endometriosis can also cause CA 125 to elevate. In postmenopausal women, CA 125 should be used for primary bnevaluation because it allows the Risk of Malignancy Index of ovarian cysts to be calculated. This patient had an RMI of 198 well below the cut-off level of 200 [12]. However, CA 125 should not be used in isolation, because it is nonspecific for ovarian cancer. Abdominal pelvic ultrasound is the imaging study of choice for the evaluation of any adnexal mass which allows accurate identification in approximately 90 % of cases [8,13,14]. A computed tomography (CT) scan is preferred to pelvic ultrasonography for preoperative assessment as it also aids in evaluating the extent of the disease. CT scans in our case showed a huge septated cystic lesion occupying the whole of the abdominal and pelvic cavities with the size described earlier. The gold standard of treatment for any suspected ovarian mass includes intact removal of the involved adnexa with intraoperative pathological evaluation, typically laparotomy, total hysterectomy, bilateral salpingo-oophorectomy, and staging procedures, including lymphadenectomy. [7,13]. To reduce the risk of other malignancies, a total hysterectomy and bilateral salpingo-oophorectomy are usually considered in postmenopausal women, regardless of the histology [7]. In our case, we were able to remove the tumor intact without the need for intraoperative drainage. Many case reports have shown that slow removal can adequately prevent such hemodynamic instability, which we also demonstrated in our case by gently removing the tumor. After conservative surgery, patients must be closely monitored for recurrence, particularly if the tumor was not fully removed during the procedure. Careful follow-up is essential to detect any signs of regrowth or progression early, allowing for timely intervention [16].
4. Conclusion
The rarity of borderline mucinous cystadenomas in postmenopausal women makes such cases particularly notable due to their atypical presentation, the altered risk profile, and the challenges in clinical management. This case report highlights the importance of thoroughly evaluating women with vague abdominal symptoms. While this condition is rare in postmenopausal women, its massive form can be dangerous if not diagnosed and managed promptly, with the potential to progress to malignancy. Greater awareness of this condition could lead to earlier detection and reporting of more cases.
Consent
Written informed consent was obtained from the patient to publish this case report and accompanying images. On request, a copy of the written consent is available for review by the Editor in-Chief of this journal.
Ethical approval
This case report is exempt from ethical approval in our institute (Kilimanjaro Christian Medical Centre). Case reports are exempt from ethical approval in our University Hospital.
This case report was approved by the authors' institution review board committee.
The patient's relative provided written informed consent to allow for her de-identified medical information to be used in this publication. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Funding
This work did not receive any fund from any source.
Author contribution
John Lugata: Conceptualization, study design, prepared initial manuscript version and approved the final manuscript draft.
Glory Maringo: Involved in the patient management, provided supervision and reviewed, and approved the final manuscript draft.
Alex Mremi: Conceptualization, and also performed histopathological analysis and prepared the final manuscript draft.
Nasra Batchu: Conceptualization, study design, prepared initial manuscript version and approved the final manuscript draft.
Bariki Mchome: A lead Obstetrician and Gynaecologist, provided expertise throughout the entire process and revised and approved the final draft.
Fredrick Mbise: A lead Obstetrician and Gynaecologist, provided expertise throughout the entire process and revised and approved the final draft.
Guarantor
Dr. John Lugata.
Research registration number
-
1.
Name of the registry: N/A.
-
2.
Unique identifying number or registration ID: N/A.
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): N/A.
Conflict of interest statement
All authors have declared that no competing interests exist.
Acknowledgement
The authors would like to thank the patient for allowing us to use her medical information for learning purposes. We equally appreciated the contributions, support and input from Epifania Venance, Mwanaasha Mustafa, and Doreen Mwandike in this work.
References
- 1.Hauptmann S., Friedrich K., Redline R., et al. Ovarian borderline tumors in the 2014 WHO classification: evolving concepts and diagnostic criteria. Virchows Arch. 2017;470:125–142. doi: 10.1007/s00428-016-2040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavassolo F.A., Devilee P., editors. WHO Classification of Tumors: Pathology and Genetics of the Tumors of the Breast and Female Genital Organs. IARC Press; Lyon, France: 2003. pp. 113–202. [Google Scholar]
- 3.Japan Society of Obstetrics and Gynecology, The Japanese Society of Pathology . 1 ed. Kanehara Shuppan; Kyoto, Japan: 2016. The General Rules for Clinical and Pathological Management of Ovarian Tumor, Fallopian Tube Cancer, and Primary Peritoneal Cancer Pathological Edition; pp. 15–65. [Google Scholar]
- 4.Borgfeldt C., Andolf E. Transvaginal sonographic ovarian findings in a random sample of women 25-40 years old. Ultrasound Obstet. Gynecol. May 1999;13(5):345–350. doi: 10.1046/j.1469-0705.1999.13050345.x. (PMID: 10380300) [DOI] [PubMed] [Google Scholar]
- 5.Torre L.A., Islami F., Siegel R.L., Ward E.M., Jemal A. Global cancer in women: burden and trends. Cancer Epidemiol. Biomarkers Prev. Apr 2017;26(4):444–457. doi: 10.1158/1055-9965.EPI-16-0858. Epub 2017 Feb 21. PMID: 28223433. [DOI] [PubMed] [Google Scholar]
- 6.Kumar V., Abbas A.K., Aster J.C. Ninth edition. Elsevier/Saunders; Philadelphia, PA: 2015. Robbins and Cotran Pathologic Basis of Disease; pp. 696–698. (Google Scholar) [Google Scholar]
- 7.Brown J., Frumovitz M. Mucinous tumors of the ovary: current thoughts on diagnosis and management. Curr. Oncol. Rep. Jun 2014;16(6):389. doi: 10.1007/s11912-014-0389-x. PMID: 24777667; PMCID: PMC4261626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers E.R., Bastian L.A., Havrilesky L.J., Kulasingam S.L., Terplan M.S., Cline K.E., Gray R.N., McCrory D.C. Management of adnexal mass. Evid. Rep. Technol. Assess. (Full Rep) 2006 Feb;(130):1–145. PMID: 17854238; PMCID: PMC4781260. [PMC free article] [PubMed] [Google Scholar]
- 9.Pilone V., Tramontano S., Picarelli P., Monda A., Romano M., Renzulli M., Cutolo C. Giant mucinous ovarian borderline tumor. A good lesson from an asymptomatic case. Int. J. Surg. Case Rep. 2018;50:25–27. doi: 10.1016/j.ijscr.2018.07.016. Epub 2018 Jul 26. PMID: 30071377; PMCID: PMC6080572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine D.A., Lin L., Gaillard S. Lippincott Williams & Wilkins; 2020 Mar 11. Handbook for Principles and Practice of Gynecologic Oncology. [Google Scholar]
- 11.Posabella A., Galetti K., Engelberger S., Giovannacci L., Gyr T., Rosso R. A huge mucinous cystadenoma of ovarian: a rare case report and review of the literature. Rare Tumors. Jun 3 2014;6(2):5225. doi: 10.4081/rt.2014.5225. PMID: 25002945; PMCID: PMC4083665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appendix: risk of malignancy index (RMII) Mar; 2022. https://www.nice.org.uk/guidance/cg122/chapter/appendix-risk-of-malignancy-index-rmi-i 2022.
- 13.Gwanzura C., Muyotcha A.F., Magwali T., et al. Giant mucinous cystadenoma: a case report. J Med Case Reports. 2019;13:181. doi: 10.1186/s13256-019-2102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akhras L.N., Akhras L.N., Faroog S., AlSebay L. A 27-kg Giant ovarian mucinous cystadenoma in a 72-year-old postmenopausal patient: a case report. Am. J. Case Rep. Nov 2019;1(20):1601–1606. doi: 10.12659/AJCR.917490. PMID: 31672957; PMCID: PMC6849502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A. The SCARE 2023 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. Lond. Engl. 2023;109(5):1136. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozgun M.T., Turkyilmaz C. A giant ovarian mucinous cystadenoma in an adolescent: a case report. Arch. Med. Sci. 2009;5(2):281–283. [Google Scholar]