Abstract
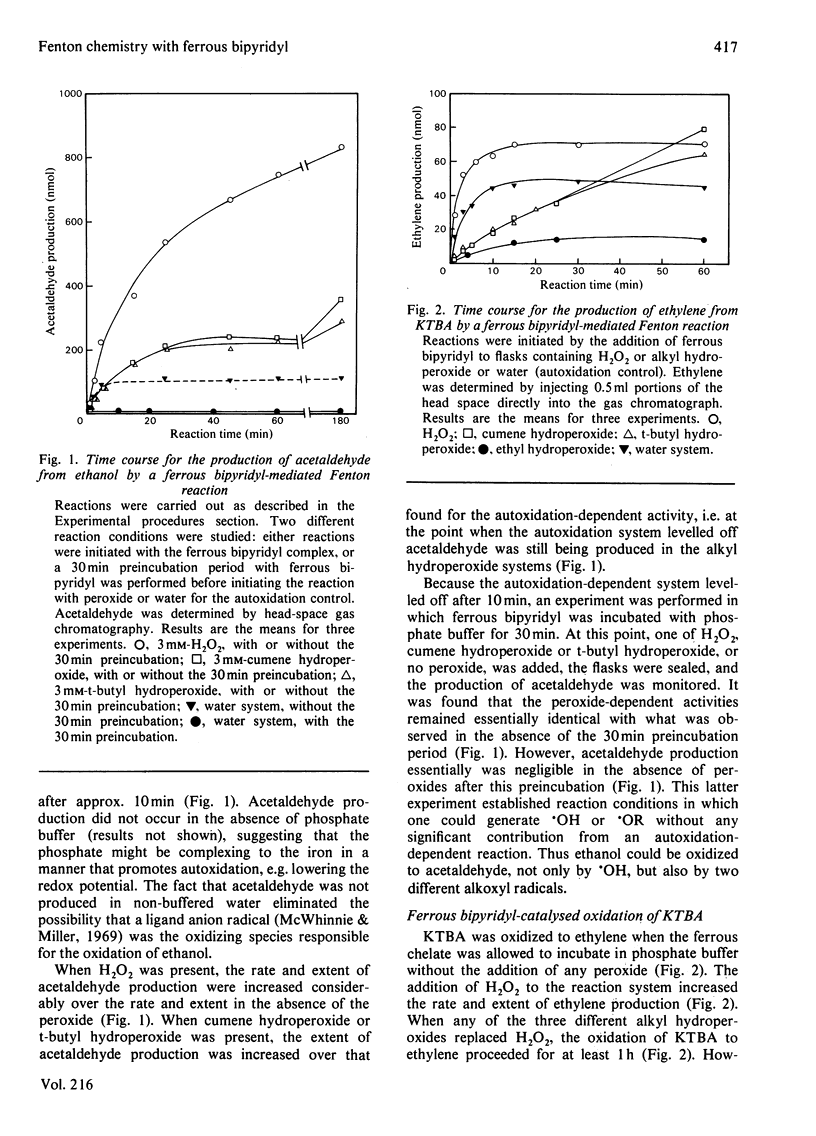
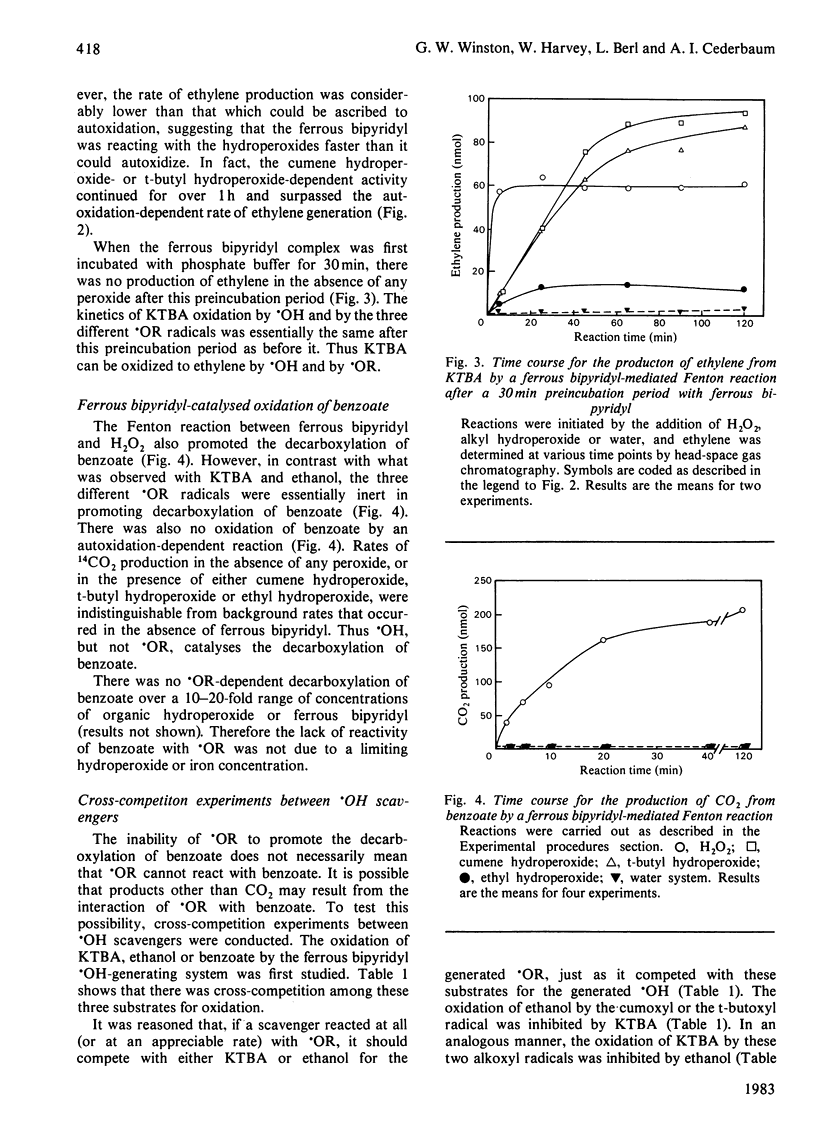
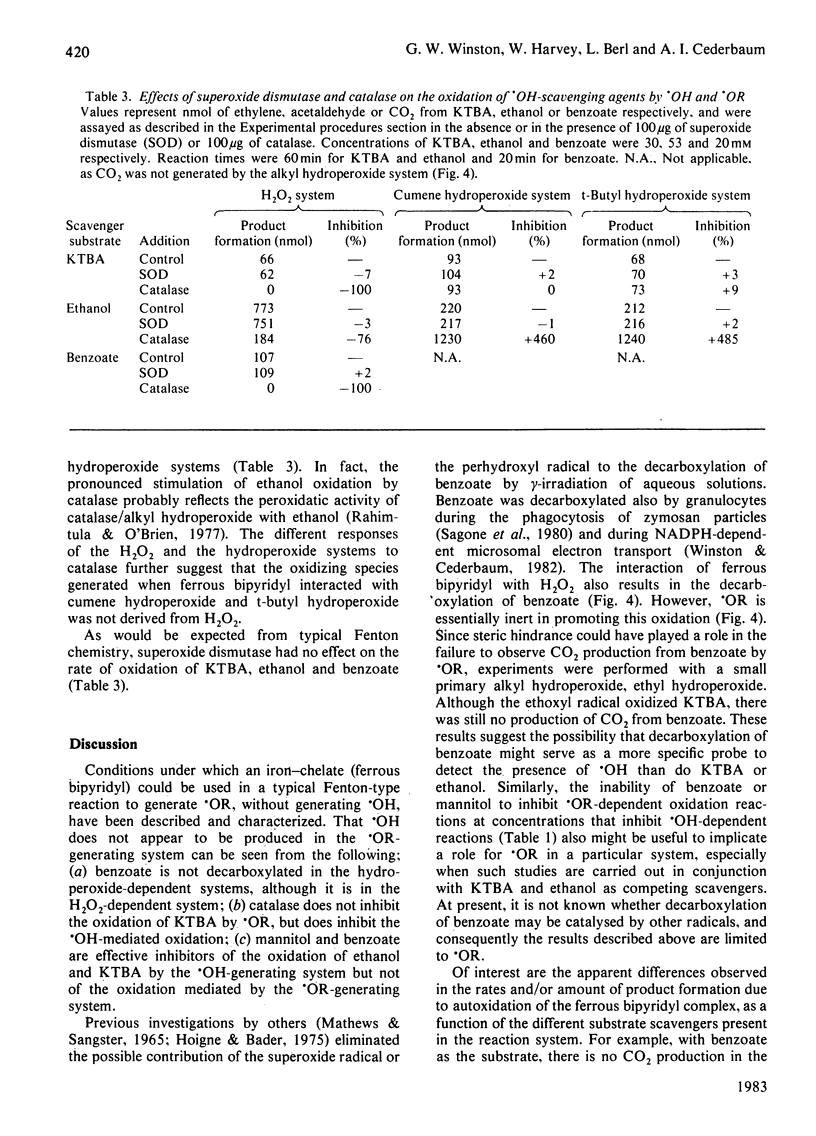
Reaction conditions by which the iron-chelate ferrous bipyridyl can be used as a Fenton reagent to generate specifically alkoxyl radical (.OR) from its corresponding alkyl hydroperoxide (ROOH) without producing hydroxyl radical (.OH) as a result of autoxidation are described. In this manner, the relative ability of common .OH-scavenging agents to react with .OH and various .OR species could be assessed. When .OH was generated from H2O2, 4-methylmercapto-2-oxobutyrate, ethanol and benzoate all were oxidized. When .OR (cumoxyl radical, t-butoxyl radical or ethoxyl radical) was generated specifically, each was found to oxidize 4-methylmercapto-2-oxobutyrate and ethanol. In contrast with .OH, however, none of the .OR radicals mediated the decarboxylation of benzoate. Cross-competition studies with the scavengers showed that, in contrast with the .OH-dependent reaction, the .OR-dependent oxidation of 4-methylmercapto-2-oxobutyrate and ethanol was not inhibited by benzoate. Rate constants for ferrous bipyridyl oxidation by ROOH and by H2O2 were found to be essentially the same, and therefore the differential oxidation of the various scavengers was not a reflection of iron-peroxide interaction, but rather an interaction between generated oxy radicals and the scavengers. In contrast with the H2O2 system, catalase did not inhibit the oxidation of 4-methylmercapto-2-oxobutyrate or ethanol by either the cumene hydroperoxide or the t-butyl hydroperoxide system, suggesting that the oxidizing species was not derived from H2O2. These results suggest that benzoate decarboxylation might serve as a more specific probe to detect the presence of .OH than either 4-methylmercapto-2-oxobutyrate or ethanol, which react readily with .OR.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Bors W., Saran M., Lengfelder E., Michel C., Fuchs C., Frenzel C. Detection of oxygen radicals in biological reactions. Photochem Photobiol. 1978 Oct-Nov;28(4-5):629–638. doi: 10.1111/j.1751-1097.1978.tb06982.x. [DOI] [PubMed] [Google Scholar]
- Cederbaum A. I., Dicker E., Cohen G. Effect of hydroxyl radical scavengers on microsomal oxidation of alcohols and on associated microsomal reactions. Biochemistry. 1978 Jul 25;17(15):3058–3064. doi: 10.1021/bi00608a018. [DOI] [PubMed] [Google Scholar]
- Chance B., Oshino N. Analysis of the catalase--hydrogen peroxide intermediate in coupled oxidations. Biochem J. 1973 Mar;131(3):564–567. doi: 10.1042/bj1310564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G., Cederbaum A. I. Microsomal metabolism of hydroxyl radical scavenging agents: relationship to the microsomal oxidation of alcohols. Arch Biochem Biophys. 1980 Feb;199(2):438–447. doi: 10.1016/0003-9861(80)90300-8. [DOI] [PubMed] [Google Scholar]
- Cohen G. The generation of hydroxyl radicals in biologic systems: toxicological aspects. Photochem Photobiol. 1978 Oct-Nov;28(4-5):669–675. doi: 10.1111/j.1751-1097.1978.tb06993.x. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Tang R. H. Ethylene formation from methional. Biochem Biophys Res Commun. 1978 Mar 30;81(2):498–503. doi: 10.1016/0006-291x(78)91562-0. [DOI] [PubMed] [Google Scholar]
- Rahimtula A. D., O'Brien P. J. The role of cytochrome P-450 in the hydroperoxide-catalyzed oxidation of alcohols by rat-liver microsomes. Eur J Biochem. 1977 Jul 1;77(1):201–208. doi: 10.1111/j.1432-1033.1977.tb11658.x. [DOI] [PubMed] [Google Scholar]
- Sagone A. L., Jr, Decker M. A., Wells R. M., Democko C. A new method for the detection of hydroxyl radical production by phagocytic cells. Biochim Biophys Acta. 1980 Feb 21;628(1):90–97. doi: 10.1016/0304-4165(80)90354-2. [DOI] [PubMed] [Google Scholar]
- Winston G. W., Cederbaum A. I. NADPH-dependent production of oxy radicals by purified components of the rat liver mixed function oxidase system. I. Oxidation of hydroxyl radical scavenging agents. J Biol Chem. 1983 Feb 10;258(3):1508–1513. [PubMed] [Google Scholar]
- Winston G. W., Cederbaum A. I. Oxidative decarboxylation of benzoate to carbon dioxide by rat liver microsomes: a probe for oxygen radical production during microsomal electron transfer. Biochemistry. 1982 Aug 31;21(18):4265–4270. doi: 10.1021/bi00261a013. [DOI] [PubMed] [Google Scholar]


