Highlights
-
•
Graft versus host disease (GVHD), host versus graft response (HVGR), and immunotoxicity are the main hurdles faced by universal chimeric antigen receptor (UCAR) cell therapy.
-
•
Novel editing techniques (epigenetic editing and RNA writer systems), the incorporation of diverse cell types (T cell subtypes, NK cells, macrophages), and the implementation of suicide genes and safety switches are cutting-edge solutions to the challenges.
-
•
In situ CAR cell therapy holds great promise.
Keywords: Chimeric antigen receptor, Universal cell therapy, Off-the-shelf, Cancer, Immunotherapy
Abstract
Chimeric Antigen Receptor (CAR) T cell therapy has gained success in adoptive cell therapy for hematological malignancies. Although most CAR cell therapies in clinical trials or markets remain autologous, their acceptance has been limited due to issues like lengthy manufacturing, poor cell quality, and demanding cost. Consequently, “Off-the-shelf”, universal CAR (UCAR) cell therapy has emerged. Current concerns with UCAR therapies revolve around side effects such as graft versus host disease (GVHD) and host versus graft response (HVGR). Preclinical research on UCAR cell therapies aims to enhance efficacy and minimize these side effects. Common approaches involve gene editing techniques to knock out T cell receptor (TCR), human leukocyte antigen (HLA), and CD52 expression to mitigate GVHD and HVGR risks. However, these methods carry drawbacks including potential genotoxicity of the edited cells. Most recently, novel editing techniques, such as epigenetic editing and RNA writer systems, have been developed to reduce the risk of GVHD and HVGR, allowing for multiplex editing at different sites. Additionally, incorporating more cell types into UCAR cell therapies, like T-cell subtypes (DNT, γδT, virus-specific T cells) and NK cells, can efficiently target tumors without triggering side effects. In addition, the limited efficacy of T cells and NK cells against solid tumors is being addressed through CAR-Macrophages. In summary, CAR cell therapy has evolved to accommodate multiple cell types while expanding applications to various diseases, including hematologic malignancies and solid tumors, which holds tremendous growth potential and is promised to improve the lives of more patients in the future.
Graphical abstract
Introduction
Immunotherapy has emerged as a transformative innovation in cancer treatment, positioning itself as the fourth pillar alongside surgery, radiotherapy, and chemotherapy [1]. In comparison to conventional anti-cancer modalities, immunotherapy demonstrates superior precision in targeting antigens and extends disease control over a longer timeframe. Particularly, chimeric antigen receptor (CAR) cell therapy is a frontrunner in this field. Following advancements in second-generation CAR-T cell therapies, it has shown notable anti-tumor effects, especially against hematological malignancies [2]. CAR-T cell therapies against CD19+ B cell malignancies and BCMA+ multiple myeloma have been successfully introduced to the market, displaying significant clinical potential [3]. Existing commercially available CAR-T therapies rely on autologous treatments, utilizing cells derived from patients themselves. Currently, most data on long-term outcomes of autologous CAR T-cell therapy originate from patients with B-cell lymphoma or chronic lymphocytic leukemia (CLL), with overall response rates (ORRs) achieving 44–91 %, complete response (CR) rates of 28–68 % [3]. Despite their high efficacy, autologous CAR cell therapy faces limited application due to its high cost, intricate manufacturing process, severe side effects, and restricted cell availability [2]. Consequently, the concept of “Off-the-shelf”, third-party, healthy donor-derived universal CAR therapy has gained incremental attention [4].
Conventional autologous CAR-T therapy involves several steps, such as patient plasmapheresis, cell sorting, CAR virus transfection, CAR-T cell expansion, and transfusion back to the patient post-quality check [5]. The universal CAR-T cell therapy shares a similar process (Fig. 1). This process is intricate and time-consuming, demanding stringent laboratory conditions and operations. The prolonged manufacturing process poses challenges for autologous CAR therapy, where a lot of patients may not survive until the product is ready. In contrast, “off-the-shelf” CAR T-cell products offer the opportunity for the treatment of patients with rapidly progressing diseases. Moreover, patients undergoing CAR-T cell therapy after chemotherapy often exhibit reduced T-cell quantity and quality, rendering autologous CAR-cell therapy less effective [3]. On the contrary, healthy donor cells provide a more homogeneous starting material, ensuring a more predictable product quality. In short, allogeneic CARs offer numerous advantages over autologous CAR therapies (Table 1).
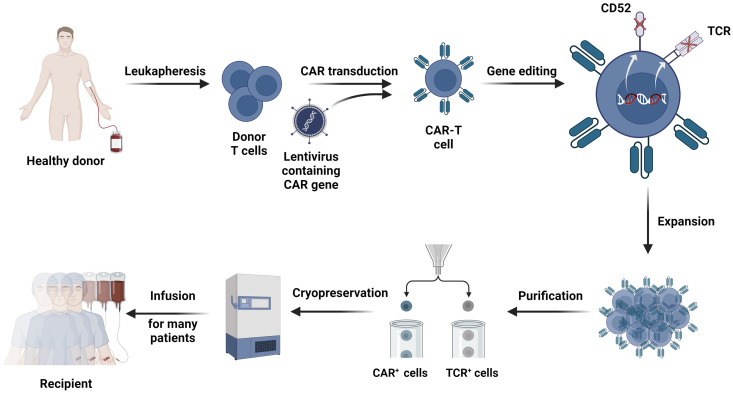
Fig. 1.
The conventional production route of the UCAR-T therapy. The first step in the manufacturing of universal CAR-T cells is the collection of donor T cells from peripheral blood mononuclear cells (PBMC), which are extracted from the donor through leukapheresis. The T cells are then isolated via magnetic bead technology. The CAR gene is then transduced into the collected T cells by lentivirus. To limit the occurrence of GVHD and HVGR, gene editing techniques are employed to knock down the expression of alloreactive genes (e.g., TCR and CD52). After acquiring stable CAR-T cells, large-scale in vitro expansion is required to obtain the desired dose. The expanded CAR-T cells are further purified to ensure safety. Eventually, the CAR-T cells are cryopreserved and readily available for infusion into multiple patients.
Table 1.
The comparison between allogenic CAR-T cell therapy and autologous CAR-T cell therapy.
| Allogeneic CAR-T therapy | Autologous CAR-T therapy | |
|---|---|---|
| Manufacturing process | ||
| Cell Source | Healthy donor | Patient (recipient) |
| Expansion capacity | High | Limited |
| Off-the-Shelf Availability | Standardized product | Customized for each patient, limited off-the-shelf availability |
| Possibility of T-cell phenotype selection | High | Limited |
| Quality of original T cells | High | Low |
| Time of accessibility | Readily available | Time-consuming, possible for manufacturing failure |
| Treatment | ||
| Requirement for lymphodepletion | Enhanced lymphodepletion regimen, or standard lymphodepletion regimen (with additional HLA depletion) | Standard lymphodepletion regimen |
| Flexibility in dosing | Flexible for redosing or changes of target | Limited flexibility as the dose is dependent on the patient's ability to yield sufficient T cells |
| In vivo persistence | Short (days to months) | Long (months to years) |
| Side effects | ||
| GVHD | High risk | Low risk |
| Immunogenicity (HVGR) | Possible | Lower risk |
| CRS and ICANS | Yes | Yes |
| Cost | Lower | Expensive, upwards to US$350,000 |
Although numerous UCAR therapies targeting hematologic malignancies have entered clinical trials, no market approvals for UCAR therapies have been obtained, potentially due to safety and efficacy concerns. Consequently, recent studies have been devoted to ameliorating these issues. This review first provides an overview of the current stage of UCAR cell therapy and then encapsulates the latest advancements while exploring the future trajectory for CAR-based therapies.
Current challenges and conventional solutions for UCAR cell therapy
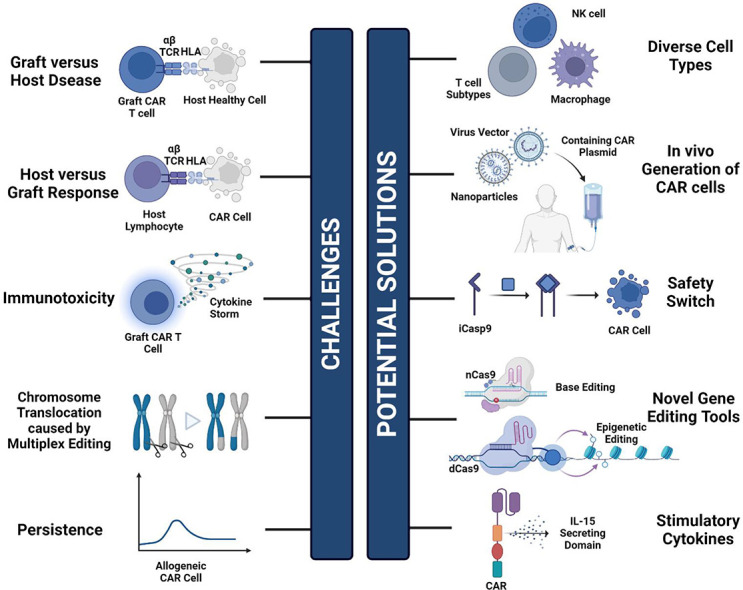
In recent years, UCAR therapy has demonstrated promising success in hematological malignancies, including aggressive B cell lymphomas, follicular lymphomas, mantle cell lymphomas, and chronic lymphocytic leukemia [3]. However, complications such as graft-versus-host disease (GVHD), host versus graft response (HVGR), and treatment regimen-related immunotoxicity remain the major obstacles [[6], [7], [8]]. A variety of solutions have been developed and become widely adopted as conventional strategies to enhance the safety and efficacy of UCAR cell therapy.
GVHD
GVHD arises from grafts attacking immunocompromised recipient tissues due to histocompatibility differences [2]. Its pathophysiology primarily involves donor T cells recognizing host tissue as foreign, leading to severe organ damage. To address this challenge, a multitude of strategies are rapidly under investigation, encompassing the application of gene editing techniques and the selection of more suitable cell sources to mitigate GVHD risk.
αβ-type T cell receptor (αβTCR) is instrumental in mediating the occurrence of GVHD. The TCR protein complex on the surface of αβ-type T cells consists of α and β chains, with only one gene encoding the constant regions of the α chain [9]. Therefore, disrupting the gene encoding the T cell receptor α-chain constant region (TRAC) emerges as a direct and effective approach to block αβ-type TCR expression. Researchers have developed several methods to prevent TCR expression on the surface of CAR-T cells, with gene editing techniques being one of the most rapidly advancing methods. Frequently employed gene editing tools include Zinc Finger Nucleases (ZFN), Transcription Activator-Like Effector Nucleases (TALEN), and Clustered Regularly Interspaced Short Palindromic Repeat Correlation 9 (CRISPR-Cas9) [10]. Additionally, the use of short hairpin RNA (shRNA) to degrade mRNA proves to be effective in reducing the adverse effects of GVHD [11] (Fig. 2). Another approach leverages the homologous recombination mechanism of cells to introduce CAR-expressing transgenes directly into the TRAC gene locus, allowing CAR transgene expression while disrupting natural TCR expression [9,12]. Another intriguing strategy to disable the TCR involves adopting an anti-TCR ScFv in combination with the KDEL endoplasmic reticulum (ER) retention domain, resulting in intracellular retention of the TCR [13,14].
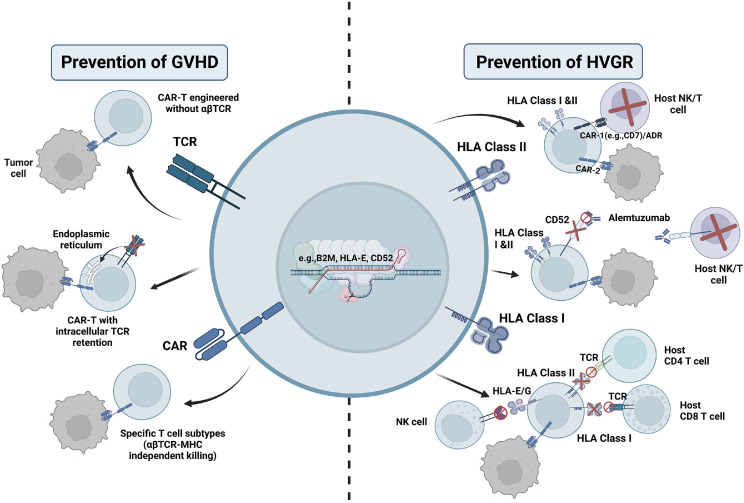
Fig. 2.
Currently developed strategies to prevent GVHD and HVGR. In terms of preventing GVHD, most strategies aim to target the αβTCR by knocking out the relevant gene or inactivating the αβTCR protein. In addition, certain specific T-cell subsets can exert a killing effect independent of αβTCR and HLA, which could also be used to prevent GVHD. For the prevention of HVG, available approaches include bispecific CAR, ADR, or monoclonal antibodies to eliminate allo-rejecting host NK and T cells. Knocking out the HLA gene while adopting NK cell inhibiting receptors can also suppress the alloreactive response. CRISPR-Cas9 and shRNA techniques are the most common instruments applied to manipulate the expression of these genes.
Furthermore, the utilization of other cells that do not perform anti-tumor function through αβTCR is under hot research to reduce the risk of GVHD (Figs. 2 and 3). These include natural killer cells (NK cells), γδ T cells, virus-specific T cells, and NKT cells, which will be discussed in detail later.
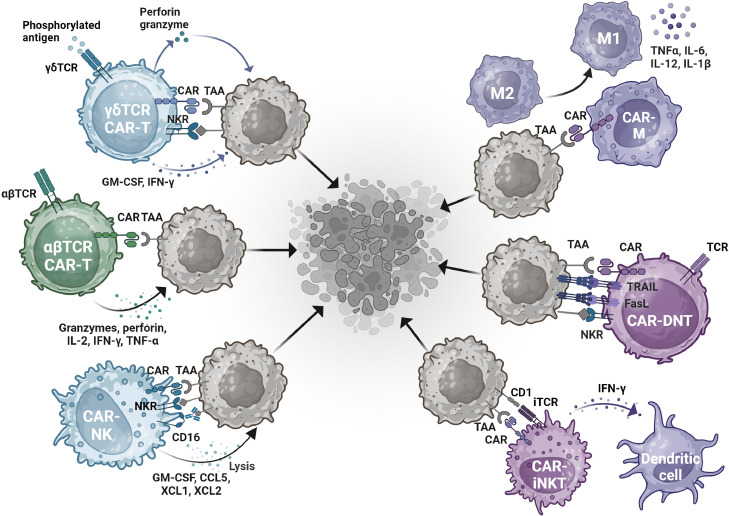
Fig. 3.
A summary of tumor cell killing mechanisms by CAR cells originating from different cell types. In conventional CAR T therapy, tumor cell apoptosis is primarily triggered by CAR recognition of tumor-associated antigens (TAA) and the release of granzymes, perforins, and other cytokines. Since this therapy is prone to side effects like GVHD, other cell types such as NK cells and certain T cell subtypes are being investigated. These cell types exert tumor-killing effects independent of the αβTCR, mainly through receptors like Fas, NKR, and CD16. Moreover, macrophages compensate for the ineffectiveness of other cells in targeting solid tumors. In addition to CAR-based recognition, CAR-Macrophages can reeducate the M2 subtype of macrophage into the M1 subtype, releasing proinflammatory cytokines to trigger further immune response against the tumor.
HVGR
Following UCAR-T treatment, the host's immune system may recognize and eliminate allogeneic CAR-T cells, thereby limiting their therapeutic effect [15]. Therefore, overcoming the challenge of host rejection is a significant hurdle in allogeneic CAR-T therapies. Typically, before the allogeneic CAR therapy, patients will receive an enhanced lymphodepletion regimen, as host T cells may swiftly attack “foreign” CAR-T cells. Commonly used lymphodepletion agents include conventional chemotherapy drugs and monoclonal antibodies such as alemtuzumab [13]. In order to protect allogeneic CAR cells from lymphodepletion, gene editing techniques are often employed to knock out CD52 on these cells, preventing recognition by the alemtuzumab [15] (Fig. 2). Combining alemtuzumab with chemotherapy allows for more effective clearance of the host T cells, albeit with an increased risk of infection [[16], [17], [18]]. Therefore, specific targeting of the alloimmune components may be preferable. For example, bispecific-CAR-T cells (biCAR-T) have been designed to prevent HVGR, with one CAR targeting tumor antigens and the other aiming at the host immune cells [13] (Fig. 2). One clinical study has adopted CD19/CD7 dual-targeted CAR for tumor killing and elimination of the alloreactive T and NK cells [19]. Moreover, a chimeric receptor, alloimmune defense receptor (ADR), has been developed to target the co-stimulatory receptor 4–1BB (CD137), which is transiently upregulated in the activated lymphocytes but not resting and non-alloreactive immune cells [17,20]. In this way, the CAR-T cells loaded with ADR can be resistant to host rejection while reducing the risk of infection.
Furthermore, gene editing techniques could also be used to eliminate HLA proteins on the surface of allogeneic CAR-T cells. Deletion of the β2-microglobulin gene, which is required for normal expression of HLA proteins, is a strategy to reduce the immunogenicity of CAR-T cells [9,21]. However, decreased HLA expression may be recognized by NK cells, leading to NK-mediated CAR-T cell elimination. To address this, countless NK cell inhibiting strategies have been introduced. For instance, targeted insertion of universal MHC I constructs (e.g., monomorphic HLA-E loaded with decoy peptides) into the B2 M gene prevents NK cells from eliminating the allogeneic cells [22] (Fig. 2). Introducing other inhibitory ligands for NK cells, such as siglec 7/9 ligands and E-cadherin, can also reduce the risk of HVGR [13]. Moreover, recent research has shown that overexpressing CD47 in allogeneic CAR T cells can further inhibit host innate immune attacks (e.g., NK cells) while suppressing adaptive immunity responses (i.e., T cells) [23].
Immunotoxicity
As the effector capacity of CAR-T cells increases, the side effect rate also rises. CAR-T therapy can trigger the overactivation of T cells, releasing high levels of cytokines such as IL-6, IL-10, and interferon-γ (IFN-γ) [10]. These pro-inflammatory cytokines may elevate blood vessel permeability, causing life-threatening diseases like vascular leakage syndrome and disseminated intravascular coagulation [24]. Approximately 77–93 % of patients treated with FDA-approved autologous CAR cell therapies result in cytokine release syndrome (CRS), with grade 3 or greater occurring in 7–46 % of patients [25]. Some patients may further develop immune effector cell-associated neurotoxicity syndrome (ICANS) [1]. To address the immunotoxicity issue, several solutions employing the concept of switchable CAR have been developed to control T cell activity.
The ON-switch CAR aims to reduce immunotoxicity by regulating T-cell activation. The signaling domain of the ON-Switch CAR is usually separate from the co-stimulation domain. Only upon the addition of heterodimerized small molecules can the two CAR domains assemble. As a consequence, the activation state of the T cells can be controlled by the dosage of the heterodimerizing small molecules [26]. On the other hand, the OFF-switch CAR primarily mitigates immunotoxicity by deactivating the CAR cells. Several strategies to degrade CARs, including suicide genes, are now under investigation. Suicide genes such as herpes-simplex-thymidine-kinase (HSV-TK) and inducible-caspase-9 (iCasp9) are intended to selectively destroy transferred cells [26]. These genes activate upon the administration of corresponding molecules, resulting in irreversible T-cell termination. Likewise, a suicide receptor is introduced, which functions by inducing the expression of a common antigen CD20 on the CAR-T cells [26]. Infusion of anti-CD20 antibodies further triggers the elimination of CAR-T cells.
Other challenges
Another challenge faced in UCAR cell therapy is the low persistence [7]. The determinants of CAR-T persistence in vivo are unknown, possibly related to intrinsic T-cell mass, the ratio of CD4+ to CD8+ T-cells, or specific co-stimulatory domains in individual CAR constructs [27]. Currently, researchers have focused on increasing the persistence by adding proinflammatory cytokines or altering co-stimulatory structural domains of the CAR structure. For example, IL-7 and IL-15 secreting domains are effective in inducing the memory phenotype of T cells, which increases the durability of UCAR cells [28,29].
Moreover, while there is now plenty of research into the gene-edited UCAR cells to reduce side effects, this might lead to another potential safety issue, the genotoxicity of gene-edited cells, which will be discussed later.
Recent advances in universal CAR-T therapy
In the last few years, with the development of gene editing techniques and the understanding of basic cell biology, several new solutions to the above challenges are constantly being investigated. Some of these novel discoveries have already progressed to the clinical stage, as outlined in Table 2.
Table 2.
List of clinical trials of UCAR-T therapy with available results. Updated till 07/04/2024.
| Title | Safety assessment population (N) | CRSN (%) | ICANSN (%) | InfectionsN (%) | GVHDN (%) | Efficacy assessment population (N) | ORRN (%) | CRN (%) | Sponsor | Strategies | Target | Indication | Phase of study | Location |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UCAR-T | ||||||||||||||
| ALLO-501[30] | 46 | Gr I–II: 10 (21.7); ≥Gr III: 1 (2.2) | ≥Gr II: absent | ≥Gr III: 11 (23.9) | absent | 32 | 24(75) | 16(50) | Allogene Therapeutics | Disruption of TRAC and CD52 genes by TALENs | CD19 | relapsed/refractory (r/r) B-Lymphoma | Phase 1 | US |
| TRUUCAR™ GC502[31] | 4 | Gr I–II: 1(33.3); ≥Gr III: 2(66.7) | ≥Gr II: absent | absent | absent | 4 | 4(100) | 3(75) | Gracell Biotechnologies | TRAC and CD7 loci were disrupted | CD19/CD7 | relapsed/refractory B-cell acute lymphoblastic leukemia (r/r B-ALL) | Phase 1 | China |
| ALLO-715[32] | 52 | Total: 27(52);≥Gr III: 1 (1.9) | Gr I–II: 5(1)] | Total: 29(56); ≥Gr III:(29) | absent | 36 | 27(75) | 18(50) | Allogene Therapeutics | Lymphodepletion with an anti-CD52 antibody (ALLO-647)-containing regimen | BCMA | r/r multiple myeloma | Phase 1 | US |
| UCART22[33] | 3 | Gr I–II: 2(67) | absent | – | – | 3 | 2 (66.7) | 1 (33.3) | Cellectis | Disruption of TRAC and CD52 genes by TALENs | CD22 | B-ALL | Phase 1 process 2 | US |
| CYAD-211[34] | 9 | Gr I: 1(11) | absent | Gr I–II: 2(22) | absent | 9 | 2 (12.6) | – | Celyad Oncology | Use of shRNA to silence mRNA coding for the CD3ζ component of the TCR | BCMA | r/r Multiple Myeloma | Phase 1 | US |
| CTX110[35] | 32 | Total: 18 (56.3); ≥Gr III: absent | Total: 3 (9.4); ≥Gr III: 2 (6.2) | ≥Gr III: 4 (12.5) | absent | 32 | 18 (56.3) | 11 (34.4) | CRISPR Therapeutics | Disruption of TRAC gene by CRISPR/Cas9 | CD19 | B cell malignancies | Phase 1 | US |
| FT819[36] | 12 | ≤Gr II: 3(25) | absent | – | absent | – | – | – | Fate Therapeutics | iPSc derived T cells, disruption of TRAC gene by CRISPR/Cas9 | CD19 | B cell malignancies | Phase 1 | US |
| ThisCART19A [37] | 8 | Gr2: 6(75) ≥Gr III: 2(25) | Total: 3 (37.5) | Total: 1 (12.5) | absent | 7 | – | 7(100) | Fundamenta Therapeutics | Intracellular retention of TCR/HLA-I | CD19 | r/r B-ALL and relapsed non-Hodgkin Lymphoma (NHL) | Phase 1 | China |
| UCART19[38] | 21 | 19(91); ≥Gr III: 3(14) | Gr I–II: 8(38); ≥Gr III: absent | 13(62) | Gr I: 2(10); ≥Gr II: absent | 21 | – | 14(67) | Servier | Disruption of TRAC and CD52 genes by TALENs technology | CD19 | r/r B-ALL | Phase 1 | US |
| P-BCMA-ALLO1[39] | 24 | Gr I: 3(14) | Gr I: 1(4) | – | absent | – | – | – | Poseida Therapeutics | Disruption of TRBC and B2 M genes by the Cas-CLOVER™ Gene Editing tool | BCMA | Multiple Myeloma | Phase 1 | US |
| PBCAR0191[40] | 15 | Total: 9(60); ≥Gr III: absent | Total: 4 (26.6); ≥Gr III: 1 (6.6) | Total: 6(40) | absent | 15 | – | 9(60) | Precision BioSciences | Disruption of TRAC gene by the versatile genome-editing platform ARCUS | CD19 | B-ALL | Phase 1 - 2 | US |
| TT52CAR19[41] | 6 | Gr II: 2 (33.3) | Gr IV: 1 (16.6) | – | Gr I: 1 (16.6) | 6 | – | – | University College, London | Disruption of TRAC and CD52 genes by CRISPR/Cas9 | CD19 | B-ALL | Phase 1 | UK |
| WU-CART-007[42] | 18 | Gr I–II: 13(72) Gr III: 1(5.56) | Gr I: 1 (5.56) | Total: 2 (11.1) | absent | 12 | – | 7 (58.3) | Washington University School of Medicine | Disruption of CD7 & TRAC genes by CRISPR/Cas9 | CD7 | r/r T-Cell Acute Lymphoblastic Leukemia/ Lymphoblastic Lymphoma (T-ALL/LBL) | Phase 1 | US |
| P. MUC1C-ALLO1[43] | 7 | absent | absent | – | absent | – | – | – | Poseida Therapeutics | Disruption of TRBC and B2 M genes by the Cas-CLOVER™ Gene Editing tool; iCasp9-based safety switch gene | Mucin1 cell surface associated C-Terminal (MUC1-C) antigen | Advanced or Metastatic Solid Tumors | Phase 1 | US |
| CTA101[44] | 6 | Total: 6(100), Gr I:3(50), GrII:2 (33.3), GrIII:1 (16.6) | absent | Total: 6(100), GrI:1 (16.6), GrII:2 (33.3), GrIII:3(50) | absent | 6 | 6(100) | 2 (33.3) | Zhejiang University | TRAC and CD52 knockout by Cas9 | CD19/CD22 | r/r B-ALL | Phase 1 | China |
| RD13–01[45] | 12 | Gr I-II: 10 (83.3), ≥Gr III: absent | absent | 12(100) | absent | 11 | 9 (88.8) | 7 (63.6) | Zhejiang University | CD7, TCR/CD3 knockout by CRISPR/Cas9 | CD7 | r/r T-ALL/LBL | Phase 1 | China |
| CARCIK-CD19[46] | 13 | GrI–II: 3(23), ≥Gr III: absent | absent | 4(30) | absent | 13 | – | 8 (61.5) | Fondazione Matilde Tettamanti Menotti De Marchi Onlus | Sleeping beauty transposon induction of CIK cells | CD19 | r/r B-ALL | Phase 1 | Italy |
| SC291[47] | 1 | absent | absent | – | – | – | – | – | MD Anderson Cancer Center | CD3 and HLA class I/II gene knoout&CD47 overexpression | CD19 | NHL and Chronic Lymphocytic Leukemia (CLL) | Phase 1 | US |
| AVC-101 (UniCAR-T-CD123)[48] | 19 | Gr I-II: 12(63.2); Gr III: 3(15.8) | Gr II: 1 (5.3) | – | – | 15 | 8(53) | – | – | Adapter CAR-T consists of a universal CAR-T cell (UniCAR-T) and a CD123 targeting module ™ | CD123 | r/r AML | Phase 1 | Germany |
| CD33CART [49] | 19 | Total: 13(68); ≥Gr III: 4(21) | Total: 1(5) | – | – | 19 | – | 2(11) | Center for International Blood and Marrow Transplant Research | – | CD33 | r/r AML | Phase 1 | US |
| Nathali-01[50] | 3 | 3(100) | absent | – | absent | 3 | 3(100) | 2 (66.7) | Cellectis | Inactivation of TRAC and CD52 by TALENs | CD20, CD22 | r/r NHL | Phase 1 | US |
| BE-CAR7[51] | 3 | 3(100) | 2 (66.7) | 1 (33.3) | 1 (33.3) | – | – | – | Great Ormond Street Hospital for Children NHS Foundation Trust | CRISPR/nCas9 base editing to inactivate three genes encoding CD52 and CD7 receptors and the β chain of the αβ T-cell receptor | CD7 | relapsed T-ALL | Phase 1 | UK |
Advances in gene editing tools
Gene editing techniques offer a powerful tool for modifying allogeneic CAR T cells, addressing concerns related to GvHD and HVGR while enhancing the therapeutic potential. In recent years, CRISPR/Cas9 has been one of the most widely adopted gene editing tools [52]. For example, disrupting the immune checkpoint PD1 via CRISPR techniques has shown promise in increasing the potency of CAR-T therapy, surpassing traditional allogeneic CAR-T approaches [53]. CRISPR techniques have also been employed in genome-wide screening, identifying genes such as IL-23 and KLF4 that can facilitate the differentiation of T cell subtypes with superior proliferative potential and memory-like phenotypes [54]. They can also be adopted to screen novel antigen targets for unknown diseases via single-guide RNA (sgRNA) libraries, reducing the likelihood of immune escape as well as improving therapeutic efficacy [55].
However, gene editing is not devoid of challenges. Conventional gene editing techniques (including CRISPR/Cas9 and TALENs) perform editing by inducing double-strand breaks (DSB) in DNA, with risks of occurring at off-target sites [56]. Besides, universal CAR cell therapy often requires simultaneous multiplexed editing, which may trigger chromosomal translocations and other aberrations [57]. Fortunately, new solutions to these challenges are now emerging. Several Cas9 enzyme variants have been developed to overcome the disadvantages of DSB. For example, the Nickase Cas9 (nCas9) contains mutations in the RuvC or HNH domains that allow Cas9 to cleave only targeted or non-targeted single-strand DNA. The combination usage of two pairs of nCas9/gRNA complexes can reduce the generation of off-target DSBs [58]. Moreover, nCas9 can be conjugated to cytosine and adenosine deaminases for single-base pair editing. Recently, nCas9-mediated base editing inducing premature stop codons for gene knockout has progressed into the clinical stage, exhibiting molecular remission in patients [56]. On the other hand, enzymatically dead Cas9 (dCas9) can be ligated to other functional epigenetic enzymes or transcriptional regulators to induce gene expression alterations independent of direct DNA editing [58].
Furthermore, the delivery system for conventional CAR-T therapies is often lentiviral, posing risks on random genomic integration, which increases the chance for insertional mutagenesis and functional gene disruption [2]. As a result, recent advancements in gene-edited CAR-T cells have been focused on employing virus-free transfer systems. For example, one group reported adopting the CRISPR-Cas9 system and virus-free gene-transfer strategies with Sleeping Beauty transposons to deplete HLA-1 and TCR in CAR-T cells [59]. These edited cells demonstrate comparable in vivo and in vitro anti-tumor abilities compared to conventional CAR-T cells, with highly specific editing of HLA-I and TCR and no significant off-target effects [59].
In addition to CRISPR-like techniques that directly alter DNA material, several other innovative and safer options have been proposed—for example, epigenetic editing. Unlike conventional gene editing techniques that rely on DNA breakage, epigenetic editing can durably regulate the expression of multiple genes at once without introducing genomic changes [60]. Utilizing a dCas9-based epigenetic editor, one group successfully silenced the expression of TCR and major histocompatibility complex (MHC) class I and II without causing translocations or truncations, as observed with Cas9-based multiple DNA editing [60]. Epigenetic drugs have also been proven potent in enhancing the anti-tumor function of CAR-T cells, maintaining the memory phenotype, and reducing T cell exhaustion [61]. Another approach involves an RNA-based gene writer system that catalyzes various editing reactions employing target-stimulated reverse transcription (TPRT) biochemistry [62]. This technique allows simultaneous multiplex editing without inducing translocations, which is crucial since UCAR cells often require the deletion of multiple genes to avoid side effects.
Alternative T cell subtypes
To date, the majority of the CAR-T cells are derived from T cells in peripheral blood mononuclear cells (PBMC), resulting in a highly heterogeneous pool of cells. Nevertheless, research has indicated that certain T cell subsets may exhibit greater effectiveness than a mixture of different T cell subtypes [2]. Another focus of innovation in CAR-T therapy has therefore been the selection of preferred T cell types (Table 3).
Table 3.
List of clinical trial results for UCAR therapies based on other cell types. Updated till 07/04/2024.
| Title | Safety assessment population (N) | CRSN (%) | ICANSN (%) | InfectionsN (%) | GVHDN (%) | Efficacy assessment population (N) | ORRN (%) | CRN (%) | Sponsor | Strategies | Target | Indication | phase of study | Location |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Other T cell subtypes | ||||||||||||||
| ADI-001[63] | 9 | Gr I- II: 2(22) | Gr I: 1(11) | ≥Gr III: 1(11) | absent | 9 | 7(78) | 7(78) | Adicet Bio | γδ T | CD20 | r/r B-cell NHL | Phase 1 | US |
| KUR-502[64] | NHL (n = 5) and ALL (n = 2) | Gr I: 2/2(100) in ALL group | absent | – | absent | NHL (n = 5) and ALL (n = 2) | 4(57) | 3(43) | Athenex | iNKT cell, IL15, and short hairpin RNA expression (downregulates HLA I, II) | CD19 | r/r B-Cell Malignancies | Phase 1 | US |
| CAR EBV-CTL [65] | 16 | absent | absent | – | 3 | – | – | – | Memorial Sloan Kettering Cancer Center | EBV virus specific T cell | CD19 | r/r B-Cell Malignancies | Phase 1 | US |
| CD30. CAR-EBVST cells [66] | 14 | Gr I: 4 (28.5) | – | – | absent | 14 | 9 (69.2) | 5 (38.5) | Baylor College of Medicine | EBV virus specific T cell | CD30 | CD30-Positive Lymphoma | Phase I/II trial | US |
| CD19CAR/virus specific T cells [67] | 6 | absent | absent | – | absent | 6 | 2 (33.3) | 2 (33.3) | Baylor College of Medicine | Virus specific T cells | CD19 | CD19+B cell malignancies | Phase 1 | US |
| RJMty19[68] | 12 | Gr I /II: 4 (33.3) | absent | 1 (8.3) | absent | 5 | 3(25) | 1 (8.3) | Zhejiang University | DNT cells | CD19 | r/r B-cell NHL | Phase 1 | China |
| UCAR-NK | ||||||||||||||
| iC9/CAR.19/IL15-Transduced CB-NK Cells [69] | 11 | absent | absent | GrI: 2 (18.1) GrIII:1 (9.1) | absent | 11 | 8(73) | 7(64) | M.D. Anderson Cancer Center | NK cells; iCasp9-based safety switch gene; IL-15 | CD19 | r/r B-cell lymphoma or leukemia | Phase 1 | US |
| NKX101[70] | 6 | absent | absent | 3(50) | absent | 6 | – | 3(50) | Nkarta | NK cells | NKG2D | Acute Myeloid Leukemia (AML) | Phase 1 | US |
| AFM13-NK [71] | 42 | absent | absent | – | absent | 42 | 39 (92.8) | 28 (66.7) | M.D. Anderson Cancer Center | NK cells; combined with AFM13 (a CD30/CD16A bispecific antibody) | CD30 | Refractory CD30-Positive Lymphomas | Phase 1 | US |
| Cnty-101[28] | 1 | absent | absent | – | – | 1 | – | 1(100) | Century Therapeutics | iPSC-derived NK cells, IL-15, EGFR safety switch, and Alloevasion ™ edits (B2 M and CIITA knockouts, and HLA-E knock-in) | CD19 | r/r NHL | Phase 1 | US |
| CAR19/IL-15[72] | 37 | Gr I: 1 (2.7) | absent | Gr I-II: 7 (18.9); Gr III:8 (21.6) | absent | 37 | 18 (48.6) | 14 (37.8) | M.D. Anderson Cancer Center | Cord blood-derived NK cells, IL-15, iCasp9-based safety switch gene | CD19 | B Lymphoid Malignancies | Phase 1/2 | US |
γδ T cell
According to the peptide chain structure of the TCR, T cells can be classified into αβ T cells, which consist of α and β chains, and γδ T cells, characterized by γ and δ chains [2]. γδ T cells typically represent 1–5 % of the total circulating T cells [4]. These γδ T cells play a crucial role in the innate immune response, whose killing is independent of HLA and antigen-presenting cells (Fig. 3). This intrinsic feature provides γδ T cells an inherent advantage in targeting solid tumors that lack specific tumor-associated antigens [2]. Since its killing mechanism is independent of TCR and HLA, concerns related to GVHD are alleviated [73]. One group has reported that over 98 % of CAR γδ T cells express IFN-γ under basal conditions, indicating high efficacy killing [73]. Furthermore, a pioneering allogeneic CD20-targeted CAR γδ T cell therapy has already entered clinical trials and demonstrated promising results in patients [74].
invariant Natural Killer T (iNKT) cell
Invariant Natural Killer T cells (iNKT) are rare, CD1d-restricted glycolipid-reactive T cells that link adaptive and innate immunity [75]. iNKT cells possess the ability to kill CD1d+ tumor cells through direct cytotoxicity (Fig. 3). Additionally, they can modulate the immune responses of NK cells and dendritic cells through cytokine secretion, thereby enhancing the anti-tumor response in collaboration with endogenous T cells [4]. Notably, allogeneic iNKT cells do not cause GVHD, making them an ideal candidate for “off-the-shelf” immunotherapy [75].
Previous studies have demonstrated that iNKT cells engineered with CAR exhibit comparable or superior cytotoxicity and a better safety profile than conventional CAR-T cells, particularly in the context of solid tumors [2]. Currently, there is an ongoing investigation into bispecific CAR-iNKT cell therapy. Experiments in mice have indicated that CD19/CD133 CAR-iNKT cell therapy, in an NKG2D-dependent manner, surpasses CAR-T cell immunotherapy and holds potential in preventing immune escape, leptomeningeal disease, and lineage switching without significant hematological toxicity [75].
Moreover, novel bispecific engagers have been developed to enhance the anti-cancer activity of iNKT cells. Researchers have designed a bispecific iNKT cell engager (biNTe) wherein one arm engages the invariant TCR (iTCR), and the other engages the tumor antigen of interest [76]. Data indicates that biNTe is highly effective in killing and virtually eliminating patient-derived bone marrow myeloma plasma cells [76]. If this biomaterial can be used in conjunction with CAR to enhance the efficacy of iNKT cells, it could serve as an “off-the-shelf” platform for treating myeloma and other hematological or solid tumors.
Double-negative T (DNT) cell
Double-negative T cells (DNT) represent another rare subtype of mature T cells, expressing CD3 but lacking CD4 and CD8[77]. DNTs possess both anti-tumor and immunomodulatory properties. Research has demonstrated the strong inhibitory capacity of DNTs against CD8 T cells, CD4 T cells, B cells, and NK cells, leading to tolerance of allografts and potent prevention of GVHD [78]. Additionally, DNTs exhibit robust anti-tumor properties through HLA-independent killing, primarily mediated by FasL, NKG2D, DNAM-1, TRAIL, and NKp 30[77,78] (Fig. 3).
Currently, “Off-the-shelf” CAR-DNT cell therapy targeting C-type lectin-like molecule-1 (CLL1) has been developed for the treatment of Relapsed/Refractory Acute Myeloid Leukemia and has demonstrated promising results [79]. A first-in-class, open-label, single-dose, phase 1 study of CD19 CAR-DNT cell therapy RJMty19 has also been initiated [80]. Remarkably, there were no cases of GvHD or ICANS observed after a single infusion of RJMty19, highlighting the safety profile of CAR-DNT cell therapy.
Virus-specific T cells
In addition to gene editing approaches, another strategy for mitigating GvHD involves the usage of CAR-T cells based on virus-specific T cells, such as Epstein-Barr virus (EBV) T cells. EBV T cells can maintain the expression of endogenous TCR with inherently low allosteric reactivity due to the fixed recognition of defined viral antigens [81,82]. Consequently, the risk of GVHD is significantly diminished. Recent studies have illustrated that CD20/CD19 bispecific CAR EBV T cells generated from healthy donors exhibit stable CAR expression, a high proportion of T cells with memory phenotypes, and efficient HLA-independent killing [81]. Furthermore, animals treated with these bispecific CAR EBV T cells showed superior tumor growth inhibition without treatment-related toxicity compared to those treated with autologous CD20/CD19 bispecific CAR T cells.
T memory stem cell (TSCM)
T memory stem cells (TSCM) are a rare subpopulation of memory lymphocytes with self-renewal and pluripotent capacity. As previously mentioned, one of the challenges in universal CAR T cell therapy is the low persistence in vivo. Results from the clinic have demonstrated that CAR T cells originating from less differentiated TSCMs exhibit higher self-renewal ability, increased homing to tumor sites, and higher in vivo persistence compared to the effector T cells [83]. Common approaches in favoring TSCM over effector T cells include the application of cytokines such as IL-7 and IL-15. In addition, some pharmacological methods have been discovered recently to increase T cell stemness. For example, metabolic reprogramming inhibitors targeting the PI3K/AKT/mTOR pathway can restrict the differentiation of T cells [84]. Agonists targeting the Wnt/β-catenin pathway could also restrict T cell differentiation by upregulating transcription factors such as Tcf1 and Lef1 or epigenetic regulator PRMT1[[85], [86], [87]]. Furthermore, regulating genes in CAR T cells, such as the transcription factor FOXO1, might provide a more prolonged increase in T-cell stemness and CAR T cell persistence compared to the treatment of inhibitors [88,89].
Recent advances in universal CAR-NK therapy
In addition to T cells, NK cells offer a promising alternative for allogeneic cellular immunotherapy. NK cells, which fall within the innate immune system, are cytotoxic lymphocytes that differentiate between healthy and abnormal cells primarily through the balance between inhibitory and activating receptors [90]. If the activating signal (e.g., NKG2D, NKG2C, CD16) delivered is stronger than the inhibitory signal (e.g., KIR, NKG2A), it will result in the activation of NK cells [91]. This ability allows NK cells to recognize tumors with down-regulated MHC-I expression, making them effective against cancer cells [92]. Upon activation, NK cells may perform killing mechanisms such as cytolysis via granzymes and perforins, and antibody-dependent cytotoxicity (ADCC) which function through the binding of CD16 receptors to the Fc region of IgG (Fig. 3). All of these mechanisms are independent of the αβ TCR, addressing the susceptibility of GVHD.
Compared to CAR-T therapy, CAR-NK therapy offers several advantages. First, NK cells have various modalities of tumor killing. CAR NK cells can eliminate cancer cells expressing the target through CAR-mediated killing and those lacking the target through CAR-independent innate NK cytotoxicity [91]. This HLA-independent property prevents NK cells from causing GVHD. Second, compared to CAR-T therapy, the incidence of CRS in patients treated with CAR NK cells is very low [91]. CAR T cell activation releases proinflammatory cytokines, while NK cells primarily secrete chemokines and granulocyte-macrophage colony-stimulating factor (GM-CSF)[1,91]. Third, CAR-NK cells can be generated from various allogeneic cell sources since NK cell activation does not necessitate an MHC route. Current clinical trials on CAR-NK cells are primarily based on the NK92 cell line and PBMC, with umbilical cord blood and embryonic stem cells as alternative sources [2,93,94]. Finally, CAR-NK therapy is safer for treating T-cell-derived malignancies, as the risk of fratricide attack is significantly reduced due to the absence of shared antigens between malignant T cells and CAR-NK cells [95,96].
Recently, there has been a trend in the immunotherapy field to adopt induced pluripotent stem cells (iPSCs) as a cell source for CAR-NK cell therapy [97]. iPSC-derived NK cells are homogeneous, display long-term expansion potential, and allow large-scale production of standardized products that can be administered in multiple doses. In preclinical studies, one cryopreserved human iPSC-derived CAR-NK cell therapy has been found to maintain considerable antigen-specific cytotoxicity in vitro [98]. Moreover, a case study has shown promising results with multiple doses of Cnty-101, which is an iPSC-derived allogeneic CD19 targeting CAR-NK product [28].
Furthermore, previously mentioned CAR-NK cell therapies have been focused on tilting the balance of activating and inhibiting receptors towards the side of activating receptors. Now, CAR-NK cell therapy is also being developed for ADCC-mediated killing. Engineering an Fc-receptor that combines a high-affinity Fc-binding domain with an SLNK12 CAR signaling domain can lead to ADCC-mediated killing at a lower antibody concentration compared to conventional NK cells [99].
At present, a recognized drawback regarding CAR-NK cell therapy is the short survival cycle of NK cells, with a half-life of <10 days [1]. As a result, various strategies are now being explored to enhance their durability. IL-7 and IL-15 have proven effective in increasing the longevity of CAR-NK cells [28,29]. Reprogramming CAR NK cells for memory phenotypes and prolonged in vivo survival is another area of active exploration. Recently, one study showed that DAP10 co-stimulation-induced epigenetic reprogramming of CD5 CAR-NK cells can lead to enhanced cellular adaptation and memory formation, resulting in better anti-tumor potential [100]. It is noteworthy, however, that the durability requirements for CAR-cell therapy may vary between diseases, requiring a tailored approach [101].
Recent advances in universal CAR-Macrophage therapy
CAR-T cell therapy has achieved remarkable success in hematological malignancies, but it has yet to yield outcomes in solid tumors. Its limited effect on solid tumors may be due to impediments in the trafficking, infiltration, recognition, and killing of cancer cells by T cells [1]. Solid tumors often have barriers such as the extracellular matrix (ECM) and an immunosuppressive tumor microenvironment (TME), hindering the efficacy of CAR-T and CAR-NK cells. To address these challenges, researchers are exploring new avenues. CAR-macrophages have recently emerged as a potential solution for targeted therapies in solid tumors.
CAR-macrophages offer several advantages in targeting solid tumors. Macrophages, with the highest infiltration rate among innate immune cells in the TME, play a crucial role in modulating immune responses [102]. In addition to direct tumor eradication through phagocytic mechanisms, they also act as antigen presenters, immune stimulators, and modulators of the TME. Tumor-associated macrophages (TAMs) are usually activated as M1 (classically activated) or M2 (selectively activated) phenotypes [103]. M1 macrophages demonstrate anti-tumor properties through phagocytosis and the release of reactive oxygen and nitrogen species (ROS/iNOS) [102]. They can also initiate immune responses by stimulating NK cells and cytotoxic T cells via pro-inflammatory cytokines [104]. On the other hand, M2 macrophages have pro-tumor effects and contribute to an immunosuppressive TME. CAR-macrophages can counteract the immunosuppressive microenvironment by re-educating M2 macrophages to the M1 phenotype (Fig. 3). Furthermore, CAR-macrophages can enhance the expression of matrix metalloproteinases (MMP), contributing to the remodeling of the ECM and potentially aiding the infiltration of T cells and NK cells [103].
To date, CAR-Macrophage therapy is largely in the preclinical phase, with a Phase I trial targeting HER2 overexpressing solid tumors. However, CAR macrophage therapies face multiple challenges in the manufacture and treatment process. Unlike other immune cells, macrophage has a low desire to circulate in the bloodstream and has limited proliferation capacity [103]. As a result, patients can only receive a limited number of macrophages, highly reducing the effectiveness of the treatment. Fortunately, similar to CAR-NK cells, CAR-macrophages can be derived from a variety of sources, including iPSCs, which have shown potent phagocytosis and immune activity [102]. In terms of CAR design, a second generation of CAR-macrophages has been reported, featuring a CD3ζ-TIR dual-signaling CAR [105]. This design demonstrates targeted phagocytosis, antigen-dependent M1 polarization, and resistance to M2 polarization in a nuclear factor kappa B (NF-κB)-dependent manner. While CAR-macrophage is a promising immunotherapy for solid tumors, further studies are needed to improve their efficacy and safety, especially in the clinical setting.
In situ CAR cell therapy
Despite the considerable success achieved by adoptive cell therapy over the last decade, their manufacture still relies on manual or semi-manual ex vivo production, which is of high complexity and bench-to-bench product variability [106]. Moreover, although allogeneic CAR cell therapy has many advantages over autologous therapies, the additional gene editing and cell purification steps complicate the manufacturing protocol, which not only delays production and increases costs but also reduces lymphocyte viability [107].
At present, a novel type of “Off-the-shelf” CAR therapy has emerged in the field, focusing on the possibility of generating CAR cells in vivo (Table 4). For instance, the VivoVec™ platform is a CAR engineering platform comprising lentiviral particles and CAR transgene payloads. The surface of the lentiviral particles includes a multidomain fusion protein with T-cell activating ligands and agonistic ligands. With a single injection of VivoVec™ particles, CAR T cells are efficiently generated in vivo and expanded against specific antigens without the need for lymphodepletion chemotherapy or exogenous supportive cytokines, while removing cells expressing target antigens and forming a memory population of CAR T cells [108]. Besides, dual-targeting lentiviral vectors utilizing bio-orthogonal chemistry and click chemistry have demonstrated effective anti-tumor effects in humanized mouse models [109].
Table 4.
List of recent findings on in situ CAR cell therapy. Updated till 07/04/2024.
| Reference | State | Target Antigen | Vehicle | Target Cell |
|---|---|---|---|---|
| Magee et al. [62]. | Preclinical | – | Lipid nanoparticle | T cell |
| Parker et al. [108]. | Preclinical | CD20 | Lentiviral vector | T cell |
| Mei et al. [109]. | Preclinical | CD3 | Lentiviral vector | T cell |
| Krotova et al. [110]. | Preclinical | CD19 | Lentiviral vector | T cell |
| Andorko et al. [111]. | Preclinical | CD20 | Lentiviral vector | T cell and NK cell |
| Beltran-Garcia et al. [112]. | Preclinical | – | Lipid nanoparticles and virus-like particles | T cell, NK cell and macrophage |
| Green et al. [113]. | Preclinical | CD19 | Fusosomes (viral vectors pseudotyped with modified paramyxovirus envelopes targeting specific cell types) | T cell |
| Rurik et al. [114]. | Preclinical | CD5 | Lipid nanoparticle | T cell |
| Smith et al. [115]. | Preclinical | CD3 | PBAE polymers with PGA coating | T cell |
| Parayath et al. [116]. | Preclinical | CD8 | PBAE polymers with PGA coating | T cell |
In recent years, biomaterials have gained attention in tumor immunotherapy due to their biocompatibility, targeting capability, and controllable release. Biomaterials also offer cost-effectiveness and versatility, enabling industrial production and commercialization. Therefore, the concept of generating CAR-modified cells via biomaterials has been proposed [10,117]. Previously described RNA-writer system is delivered through lipid nanoparticles, which achieved significant reporter gene expression in humanized mouse and non-human primate models [62]. Feeding the RNA writer system with LNP into primary human T cells resulted in a substantial population of CAR+ T cells without comprising cell viability and proliferative capacity. Studies have also explored mRNA LNPs and cell-targeted mRNA virus-like particles (VLPs), both of which enhanced the killing function of transduced T cells, NK cells, and macrophages [112]. While the use of biomaterials is promising, concerns exist regarding the interaction of nanomaterials with the immune system [5]. Overall, further efforts are needed to improve the safety and utility of biomaterials in CAR-T therapy.
Discussion
Universal CAR cell therapy, often referred to as “Off-the-shelf” CAR cell therapy, holds significant promise for cancer treatment and market viability. “Off-the-shelf” CAR cell therapies have four main advantages, 1) Sufficient cell stock. Current UCAR cell therapies can be derived from PBMCs, cell lines, and iPSCs. 2) Ready-to-use. UCAR cells are pre-treated and cryopreserved for immediate use, eliminating the time delay in manufacturing autologous CAR cell therapy. 3) Multiple doses are permitted. UCAR immune cell therapy allows for the redosing and switching of target antigens to counteract patient resistance. 4) Inexpensive. Through large-scale production, universal CAR cell therapies are expected to reduce production costs.
Nevertheless, UCAR cell therapies face challenges such as GVHD, HVGR, and CRS. For GVHD and HVGR, current research has focused on eliminating the TCR and HLA on CAR-T cells via gene editing techniques. To address immunotoxicity side effects, the latest research has been focused on developing suicide genes and safety switches to artificially control the CAR-engineered cells. In addition, alternative cell types such as NK cells, macrophages, and certain T cell subtypes can perform killing in an HLA-independent manner, offering a potential solution. In the future, with advances in the field of gene therapy such as more specific delivery to target cell types and tissues, in situ CAR-T might present unlimited potential.
Both CAR-T and CAR-NK therapies exhibit limited efficacy in solid tumors, prompting the exploration of combination therapies. Combining CAR therapy with chemotherapy, immune checkpoint inhibitors, oncolytic viruses, and radiotherapy may enhance CAR cell infiltration and remodel the tumor microenvironment. Low-dose chemotherapy has been found to promote antigen presentation to CAR-T cells, increasing persistence [104]. Additionally, oncolytic viruses can convert “cold” tumors into “hot” tumors, improving the recruitment and effector function of T cells and NK cells [118].
Looking forward, the concept of CAR-EVERYTHING is proposed. The application of CAR cell therapy may extend beyond cancer, but to treat other diseases, such as cardiac fibrosis [114,119]. In addition, the use of CAR T-cell therapy in autoimmune diseases is of great interest. Preclinical studies have strongly supported the use of B-cell-targeted CAR T cells in autoimmune diseases, with CD19 CAR T cells abrogating disease-specific B-cell autoimmunity and organ inflammation in a mouse model of systemic lupus erythematosus [120]. Recently, the first application of allogeneic CAR T cell therapy has been reported in patients with systemic autoimmune diseases [121]. Still, target antigen selection, modification of CAR structures, and adjuvant therapeutic regimens for these cells require further optimization. In conclusion, continued research and optimization are expected to lead the development of advanced CAR-based cell therapies, benefiting a broader range of patients in the future.
CRediT authorship contribution statement
Ziyu Wu: Writing – review & editing, Writing – original draft, Investigation, Data curation. Yifan Wang: Writing – review & editing, Writing – original draft, Supervision, Investigation, Funding acquisition, Data curation, Conceptualization. Xin Jin: Writing – review & editing, Writing – original draft, Supervision, Investigation, Funding acquisition, Data curation, Conceptualization. Luqiao Wang: Writing – review & editing, Writing – original draft, Supervision, Investigation, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Yifan Wang, Email: anwyf@zju.edu.cn.
Xin Jin, Email: xin_jin@zju.edu.cn.
Luqiao Wang, Email: wangluqiao@zju.edu.cn.
References
- 1.Pan K., Farrukh H., Chittepu V.C.S.R., Xu H., xian Pan C, Zhu Z. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J. Exp. Clin. Cancer Res. 2022;41:119. doi: 10.1186/s13046-022-02327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin H., Cheng J., Mu W., Zhou J., Zhu L. Advances in Universal CAR-T Cell Therapy. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.744823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappell K.M., Kochenderfer J.N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol. 2023;20(6):359–371. doi: 10.1038/s41571-023-00754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez C., Gruber I., Arber C. Off-the-shelf allogeneic T cell therapies for cancer: opportunities and challenges using naturally occurring “Universal” donor T cells. Front. Immunol. 2020;11:583716. doi: 10.3389/fimmu.2020.583716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin Y.T., Li Y.P., He X.W., Wang X., Li W.Y., Zhang Y.K. Biomaterials promote in vivo generation and immunotherapy of CAR-T cells. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1165576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naeem M., Hazafa A., Bano N., Ali R., Farooq M., Razak S.I.A., et al. Explorations of CRISPR/Cas9 for improving the long-term efficacy of universal CAR-T cells in tumor immunotherapy. Life Sci. 2023;316 doi: 10.1016/j.lfs.2023.121409. [DOI] [PubMed] [Google Scholar]
- 7.Sterner R.C., Sterner R.M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):1–11. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young R.M., Engel N.W., Uslu U., Wellhausen N., June C.H. Next-generation CAR T-cell therapies. Cancer Discov. 2022;12(7):1625–1633. doi: 10.1158/2159-8290.CD-21-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martínez Bedoya D., Dutoit V., Migliorini D. Allogeneic CAR T cells: an alternative to overcome challenges of CAR T cell therapy in glioblastoma. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.640082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xin T., Cheng L., Zhou C., Zhao Y., Hu Z., Wu X. In-Vivo induced CAR-T cell for the potential breakthrough to overcome the barriers of current CAR-T cell therapy. Front. Oncol. 2022;12:809754. doi: 10.3389/fonc.2022.809754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossi M., Steklov M., Huberty F., Nguyen T., Marijsse J., Jacques-Hespel C., et al. Efficient shRNA-based knockdown of multiple target genes for cell therapy using a chimeric miRNA cluster platform. Mol. Ther. Nucleic. Acids. 2023;34 doi: 10.1016/j.omtn.2023.102038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling X., Chang L., Chen H., Liu T. Efficient generation of locus-specific human CAR-T cells with CRISPR/cCas12a. STAR Protocols. 2022;3(2) doi: 10.1016/j.xpro.2022.101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moradi V., Omidkhoda A., Ahmadbeigi N. The paths and challenges of “off-the-shelf” CAR-T cell therapy: an overview of clinical trials. Biomed. Pharmacother. 2023;169 doi: 10.1016/j.biopha.2023.115888. [DOI] [PubMed] [Google Scholar]
- 14.Maciocia P., Wawrzyniecka P., Kassimatis L., Pule M. A protein-based method to develop allogeneic chimeric antigen receptor T-Cells. Blood. 2018;132:700. [Google Scholar]
- 15.An Y., Jin X., Zhang H., Zhang M., Mahara S., Lu W., et al. Off-the-Shelf” allogeneic CAR cell therapy-neglected HvG effect. Curr. Treat Options Oncol. 2023;24(5):409–441. doi: 10.1007/s11864-023-01061-8. [DOI] [PubMed] [Google Scholar]
- 16.Guarnera C., Bramanti P., Mazzon E. Alemtuzumab: a review of efficacy and risks in the treatment of relapsing remitting multiple sclerosis. Ther Clin Risk Manag. 2017;13:871–879. doi: 10.2147/TCRM.S134398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo F., Mamonkin M., Brenner M.K., Heslop H.E. Taking T-Cell Oncotherapy Off-the-Shelf. Trends Immunol. 2021;42(3):261–272. doi: 10.1016/j.it.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wulf G.G., Altmann B., Ziepert M., D'Amore F., Held G., Greil R., et al. Alemtuzumab plus CHOP versus CHOP in elderly patients with peripheral T-cell lymphoma: the DSHNHL2006-1B/ACT-2 trial. Leukemia. 2021;35(1):143–155. doi: 10.1038/s41375-020-0838-5. [DOI] [PubMed] [Google Scholar]
- 19.Li S., Wang X., Yuan Z., Liu L., Li Y., Liu J., et al. Abstract CT196: early results of a safety and efficacy study of allogeneic TruUCARTM GC502 in patients with relapsed/refractory B-cell acute lymphoblastic leukemia (r/r B-ALL) Cancer Res. 2022;82(12_Supplement):CT196. [Google Scholar]
- 20.Mo F., Watanabe N., McKenna M.K., Hicks M.J., Srinivasan M., Gomes-Silva D., et al. Engineered off-the-shelf therapeutic T cells resist host immune rejection. Nat. Biotechnol. 2021;39(1):56–63. doi: 10.1038/s41587-020-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D., Quan Y., Yan Q., Morales J.E., Wetsel R.A. Targeted disruption of the β2-microglobulin gene minimizes the immunogenicity of human embryonic stem cells. Stem Cells Transl Med. 2015;4(10):1234–1245. doi: 10.5966/sctm.2015-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner D.L., Fritsche E., Pulsipher M.A., Ahmed N., Hamieh M., Hegde M., et al. Immunogenicity of CAR T cells in cancer therapy. Nat. Rev. Clin. Oncol. 2021;18(6):379–393. doi: 10.1038/s41571-021-00476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neelapu S.S., Budde L.E., McGuirk J.P., Dahiya S., Deol A., Thompson P.A., et al. Phase 1 Study of SC291, a Hypoimmune, Allogeneic CD19-Directed CAR T Cell Therapy for Relapsed/Refractory B-Cell Malignancies (ARDENT) - Initial Clinical Data. Blood. 2023;142:6852. [Google Scholar]
- 24.Tvedt T.H.A., Vo A.K., Bruserud Ø., Reikvam H. Cytokine release syndrome in the immunotherapy of hematological malignancies: the biology behind and possible clinical consequences. J. Clin. Med. 2021;10(21):5190. doi: 10.3390/jcm10215190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chohan K.L., Siegler E.L., Kenderian S.S. CAR-T Cell therapy: the efficacy and toxicity balance. Curr Hematol Malig Rep. 2023;18(2):9–18. doi: 10.1007/s11899-023-00687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y.J., Abila B., Mostafa Kamel Y. CAR-T: what Is Next? Cancers (Basel) 2023;15(3):663. doi: 10.3390/cancers15030663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L. Clinical determinants of relapse following CAR-T therapy for hematologic malignancies: coupling active strategies to overcome therapeutic limitations. Current Res. Translat. Med. 2022;70(1) doi: 10.1016/j.retram.2021.103320. [DOI] [PubMed] [Google Scholar]
- 28.Ramachandran I., Rothman S., Clausi M., Mcfadden K., Salantes B., Jih G., et al. Multiple doses of Cnty-101, an iPSC-Derived Allogeneic CD19 Targeting CAR-NK product, are safe and result in tumor microenvironment changes associated with response: a case study. Blood. 2023;142:1654. [Google Scholar]
- 29.Xiang J., Devenport J., Carter A.J., Staser K., Rettig M.P., Kim M.Y., et al. An “Off-the-Shelf” CD2 Universal CAR-T therapy combined with a long-acting IL-7 for T-Cell malignancies. Blood. 2023;142:764. doi: 10.1038/s41375-023-02039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neelapu S.S., Nath R., Munoz J., Tees M., Miklos D.B., Frank M.J., et al. ALPHA Study: ALLO-501 produced deep and durable responses in patients with relapsed/refractory non-hodgkin's lymphoma comparable to autologous CAR T. Blood. 2021;138(Supplement 1):3878. [Google Scholar]
- 31.Li S., Wang X., Yuan Z., Liu L., Li Y., Liu J., et al. Abstract CT196: early results of a safety and efficacy study of allogeneic TruUCARTM GC502 in patients with relapsed/refractory B-cell acute lymphoblastic leukemia (r/r B-ALL) Cancer Res. 2022;82(12_Supplement):CT196. [Google Scholar]
- 32.Mailankody S., Matous J.V., Liedtke M., Sidana S., Oluwole O.O., Mohan M., et al. Universal updated phase 1 data highlights role of allogeneic anti-BCMA ALLO-715 Therapy for Relapsed/Refractory multiple myeloma. Blood. 2022;140(Supplement 1):4620–4622. [Google Scholar]
- 33.Jain N., Chevallier P., Liu H., Schiller G.J., Méar J.B., DeAngelo D.J., et al. Updated results of the phase I BALLI-01 Trial of UCART22 Process 2 (P2), an Anti-CD22 Allogeneic CAR-T cell product manufactured by cellectis biologics, in patients with relapsed or refractory (R/R) CD22+ B-Cell Acute Lymphoblastic Leukemia (B-ALL) Blood. 2023;142:4847. [Google Scholar]
- 34.Al-Homsi A.S., Anguille S., Deeren D., Nishihori T., Meuleman N., Abdul-Hay M., et al. Immunicy-1: targeting BCMA with Cyad-211 to establish proof of concept of an shRNA-based allogeneic CAR T Cell therapy platform. Blood. 2021;138(Supplement 1):2817. [Google Scholar]
- 35.McGuirk J.P., Tam C.S., Kröger N., Riedell P.A., Murthy H.S., Ho P.J., et al. CTX110 Allogeneic CRISPR-Cas9-Engineered CAR T Cells in Patients (Pts) with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL): results from the Phase 1 Dose Escalation Carbon Study. Blood. 2022;140(Supplement 1):10303–10306. [Google Scholar]
- 36.Mehta A., Farooq U., Chen A., McGuirk J.P., Ly T., Wong L., et al. Interim Phase I Clinical Data of FT819-101, a Study of the First-Ever, Off-the-Shelf, iPSC-Derived TCR-Less CD19 CAR T-Cell Therapy for Patients with Relapsed/Refractory B-Cell Malignancies. Blood. 2022;140(Supplement 1):4577–4578. [Google Scholar]
- 37.Hu Y., Wei G., Fu S., Xiao P., Feng J., Zhang M., et al. Intracellular Retention of Tcrαβ/CD3 to Generate Novel Allogeneic CAR-T Cells (ThisCART19A) with Enhanced Antitumor Potency for Treating B-ALL. Blood. 2023;142:2111. [Google Scholar]
- 38.Benjamin R., Graham C., Yallop D., Jozwik A., Ciocarlie O., Jain N., et al. Preliminary data on safety, cellular kinetics and anti-leukemic activity of UCART19, an Allogeneic Anti-CD19 CAR T-Cell product, in a pool of adult and pediatric patients with high-risk CD19+ Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia. Blood. 2018;132(Supplement 1):896. [Google Scholar]
- 39.Dholaria B., Kocoglu M.H., Kin A., Asch A.S., Ramakrishnan A., Bachier C., et al. Early Safety Results of P-BCMA-ALLO1, a Fully Allogeneic Chimeric Antigen Receptor T-Cell (CAR-T), in Patients with Relapsed /Refractory Multiple Myeloma (RRMM) Blood. 2023;142:3479. [Google Scholar]
- 40.Jain N., Kantarjian H., Solomon S.R., He F., Sauter C.S., Heery C.R., et al. Preliminary safety and efficacy of PBCAR0191, an Allogeneic “Off-the-Shelf” CD19-Directed CAR-T for Patients with Relapsed/Refractory (R/R) CD19+ B-ALL. Blood. 2021;138(Supplement 1):650. [Google Scholar]
- 41.Ottaviano G., Georgiadis C., Gkazi S.A., Syed F., Zhan H., Etuk A., et al. Phase 1 clinical trial of CRISPR-engineered CAR19 universal T cells for treatment of children with refractory B cell leukemia. Sci. Transl. Med. 2022;14(668):eabq3010. doi: 10.1126/scitranslmed.abq3010. [DOI] [PubMed] [Google Scholar]
- 42.Ghobadi A., Aldoss I., Maude S.L., Bhojwani D., Wayne A.S., Bajel A., et al. Phase 1/2 Dose-Escalation/Dose-Expansion Study of Anti-CD7 Allogeneic CAR-T Cells (WU-CART-007) in Relapsed or Refractory (R/R) T-Cell Acute Lymphoblastic Leukemia/Lymphoblastic Lymphoma (T-ALL/LBL) Blood. 2023;142(Supplement 1):770. [Google Scholar]
- 43.Henry J., Oh D., Eskew J., Baranda J., Rivera I.I.R., Dumbrava E., et al. 728 Phase 1 study of P-MUC1C-ALLO1 allogeneic CAR-T cells in patients with epithelial-derived cancers. J. Immunother. Cancer. 2022;10 [Google Scholar]
- 44.Hu Y., Zhou Y., Zhang M., Ge W., Li Y., Yang L., et al. The Safety and Efficacy of a CRISPR/Cas9-Engineered Universal CAR-T Cell Product (CTA101) in Patients with Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia. Blood. 2020;136(Supplement 1):52. [Google Scholar]
- 45.Hu Y., Zhou Y., Zhang M., Zhao H., Wei G., Ge W., et al. Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological malignancies: a phase I clinical study. Cell Res. 2022;32(11):995–1007. doi: 10.1038/s41422-022-00721-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magnani C.F., Gaipa G., Lussana F., Belotti D., Gritti G., Napolitano S., et al. Sleeping Beauty–engineered CAR T cells achieve antileukemic activity without severe toxicities. J. Clin. Invest. 2020;130(11):6021–6033. doi: 10.1172/JCI138473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neelapu S.S., Budde L.E., McGuirk J.P., Dahiya S., Deol A., Thompson P.A., et al. Phase 1 Study of SC291, a Hypoimmune, Allogeneic CD19-Directed CAR T Cell Therapy for Relapsed/Refractory B-Cell Malignancies (ARDENT) - Initial Clinical Data. Blood. 2023;142:6852. [Google Scholar]
- 48.Wermke M., Metzelder S., Kraus S., Sala E., Vucinic V., Fiedler W., et al. Updated Results from a Phase I Dose escalation study of the rapidly-switchable universal CAR-T Therapy UniCAR-T-CD123 in Relapsed/Refractory AML. Blood. 2023;142:3465. [Google Scholar]
- 49.Shah N.N., Tasian S.K., Kohler M.E., Hsieh E.M., Baumeister S.H.C., Summers C., et al. CD33 CAR T-Cells (CD33CART) for Children and Young Adults with Relapsed/Refractory AML: dose-Escalation Results from a Phase I/II Multicenter Trial. Blood. 2023;142:771. [Google Scholar]
- 50.Abramson J.S., Ramakrishnan A., Pierola A.A., Braunschweig I., Cartron G., Thieblemont C., et al. Preliminary results of Nathali-01: a First-in-Human Phase I/IIa Study of UCART20x22, a dual allogeneic CAR-T Cell Product Targeting CD20 and CD22, in relapsed or refractory (R/R) non-hodgkin lymphoma (NHL) Blood. 2023;142:2110. Supplement 1. [Google Scholar]
- 51.Chiesa R., Georgiadis C., Syed F., Zhan H., Etuk A., Gkazi S.A., et al. Base-Edited CAR7 T cells for relapsed T-Cell acute lymphoblastic leukemia. N. Engl. J. Med. 2023;389(10):899–910. doi: 10.1056/NEJMoa2300709. [DOI] [PubMed] [Google Scholar]
- 52.Wei W., Chen Z.N., Wang K. CRISPR/Cas9: a powerful strategy to improve CAR-T cell persistence. Int. J. Mol. Sci. 2023;24(15):12317. doi: 10.3390/ijms241512317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verma M., Obergfell K., Topp S., Panier V., Wu J. The next-generation CAR-T therapy landscape. Nat. Rev. Drug Discovery. 2023;22(10):776–777. doi: 10.1038/d41573-023-00140-7. [DOI] [PubMed] [Google Scholar]
- 54.Zhu W., Kelly C., Dagur P., Dunbar C.E., Cordes S. CRISPR activation screen to optimize chimeric antigen receptor (CAR) T Cell immunophenotype. Blood. 2023;142:4820. [Google Scholar]
- 55.Xiang M., Li H., Zhan Y., Ma D., Gao Q., Fang Y. Functional CRISPR screens in T cells reveal new opportunities for cancer immunotherapies. Mol. Cancer. 2024;23(1):73. doi: 10.1186/s12943-024-01987-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robert Chiesa, Christos Georgiadis, Farhatullah Syed, Hong Zhan, Annie Etuk, Soragia Athina Gkazi, et al. Base-Edited CAR7 T Cells for relapsed T-Cell Acute lymphoblastic leukemia. N. Engl. J. Med. 2023;389(10):899–910. doi: 10.1056/NEJMoa2300709. [DOI] [PubMed] [Google Scholar]
- 57.Georgiadis C., Rasaiyaah J., Gkazi S.A., Preece R., Etuk A., Christi A., et al. Base-edited CAR T cells for combinational therapy against T cell malignancies. Leukemia. 2021;35(12):3466–3481. doi: 10.1038/s41375-021-01282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dimitri A., Herbst F., Fraietta J.A. Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Mol. Cancer. 2022;21(1):78. doi: 10.1186/s12943-022-01559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ceballos Bolaños C., Calviño C., Pierola A.A., Jauregui P., Calleja-Cervantes M.E., San-Martin P., et al. Optimization of a universal allogeneic CAR-T Cells combining CRISPR and transposon-based technologies for treatment of acute myeloid leukemia. Blood. 2023;142:3457. doi: 10.3389/fimmu.2023.1270843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schafer J., Trombley J., Hallisey B., Ahmad K., Clarkson S., Udo T., et al. Durable multiplex epigenetic editing for generation of allogeneic CAR T without chromosomal rearrangements. Blood. 2023;142:3446. [Google Scholar]
- 61.Gu T., Zhu M., Han Y., Wang R., Qian P., Huang H. Epigenetic reprogramming by histone deacetylase inhibitors improves CAR-T Cell functions. Blood. 2023;142:2080. [Google Scholar]
- 62.Magee M.S., De Iaco A., Rodriguez J., Rottman J.B., Liu D., Schiroli G., et al. Deploying an RNA-based gene writer system and Lipid Nanoparticle (LNP) delivery to generate functional chimeric antigen receptor (CAR) T Cells with in vitro and in vivo anti-tumor activity. Blood. 2023;142:2073. [Google Scholar]
- 63.Neelapu S.S., Stevens D.A., Hamadani M., Frank M.J., Holmes H., Jacobovits A., et al. A Phase 1 Study of ADI-001: anti-CD20 CAR-engineered allogeneic Gamma Delta1 (γδ) T Cells in Adults with B-Cell Malignancies. Blood. 2022;140(Supplement 1):4617–4619. [Google Scholar]
- 64.Ramos C.A., Courtney A.N., Robinson S.N., et al. Allogeneic NKT Cells Expressing a CD19-Specific CAR in Patients with Relapsed or Refractory B-Cell Malignancies. Presented at: Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR; Salt Lake City, UT: 2022. [Google Scholar]
- 65.Shahid S., Flynn G.C., Mauguen A., Bieler J., Prockop S.E., Scaradavou A., et al. Long Term Follow-up after treatment with allogeneic Off-the-Shelf CAR T Cell therapy for relapsed or refractory B-Cell malignancies. Blood. 2023;142:3476. [Google Scholar]
- 66.Quach D.H., Ramos C.A., Lulla P.D., Sharma S., Ganesh H.R., Nouraee N., et al. CD30.CAR-Modified Epstein-Barr Virus-Specific T Cells (CD30.CAR EBVSTs) Provide a Safe and Effective Off-the-Shelf therapy for patients with CD30-Positive lymphoma. Blood. 2022;140(Supplement 1):412–414. [Google Scholar]
- 67.Cruz C.R.Y., Micklethwaite K.P., Savoldo B., Ramos C.A., Lam S., Ku S., et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122(17):2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiao X., Liu H., Qiu X., Chen P., Li X., Wang D., et al. CD19-CAR-DNT cells (RJMty19) in patients with relapsed or refractory large B-cell lymphoma: a phase 1, first-in-human study. eClinicalMedicine. 2024;70 doi: 10.1016/j.eclinm.2024.102516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu E., Marin D., Banerjee P., Macapinlac H.A., Thompson P., Basar R., et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020;382(6):545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sauter C.S., Borthakur G., Mountjoy L., Rotta M., Liu H., Murthy H.S., et al. A Phase 1 Study of NKX101, a chimeric antigen receptor natural Killer (CAR-NK) Cell therapy, with fludarabine and cytarabine in patients with acute myeloid leukemia. Blood. 2023;142:2097. [Google Scholar]
- 71.Nieto Y., Banerjee P., Kaur I., Griffin L., Barnett M., Ganesh C., et al. Innate Cell Engager (ICE®) AFM13 combined with preactivated and expanded (P+E) cord blood (CB)-Derived Natural Killer (NK) Cells for Patients with Refractory CD30-Positive Lymphomas: final Results. Blood. 2023;142:774. [Google Scholar]
- 72.Marin D., Li Y., Basar R., Rafei H., Daher M., Dou J., et al. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial. Nat. Med. 2024;30(3):772–784. doi: 10.1038/s41591-023-02785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dwivedi A., Fu L., Chien C.D., Pouzolles M., Shah N.N., Taylor N. Engineering Off-the-Shelf Gamma Delta CAR T Cells for the Treatment of Acute Myeloid Leukemia. Blood. 2023;142:4827. [Google Scholar]
- 74.Moreno M.A., Kennedy-Wilde J., Wrong A.D., Jahchan N., Galimi F., Ye Y., et al. Expansion, persistence and pharmacodynamic profile of ADI-001, a First-in-Class Allogeneic CD20-Targeted CAR Gamma Delta T Cell Therapy, in Patients with Relapsed/Refractory Aggressive B-Cell Non-Hodgkin's Lymphoma. Blood. 2023;142:3478. [Google Scholar]
- 75.Ren H., Elliott N., Lye B., Cross J., Field L., Ponnusamy K., et al. Bispecific CAR-iNKT Immunotherapy for High Risk MLL-Rearranged Acute Lymphoblastic Leukemia. Blood. 2023;142:766. [Google Scholar]
- 76.Karaxhuku K., Ponnusamy K., Ren H., Lye B., Leontari I., Zaidi M., et al. Development of Novel Invariant TCR-Anchored Bispecific Engagers to Enhance Anti-Cancer Activity of iNKT Cells. Blood. 2023;142:4828. [Google Scholar]
- 77.Vasic D., Lee J.B., Leung Y., Khatri I., Na Y., Abate-Daga D., et al. Allogeneic double-negative CAR-T cells inhibit tumor growth without off-tumor toxicities. Sci. Immunol. 2022;7(70):eabl3642. doi: 10.1126/sciimmunol.abl3642. [DOI] [PubMed] [Google Scholar]
- 78.Chen X., Wang D., Zhu X. Application of double-negative T cells in haematological malignancies: recent progress and future directions. Biomark. Res. 2022;10(1):11. doi: 10.1186/s40364-022-00360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beilei X., Zhang C., Gao J., Li Z., Ding S., Liu L., et al. Developing “Off-the-Shelf” CLL1 CAR-DNT Therapeutics for the R/R Acute Myeloid Leukemia. Blood. 2023;142:3442. [Google Scholar]
- 80.Xiao X., Lei W., Jiang H., Qiu X., Li X., Chen P., et al. A Phase 1 Study of RJMty19: anti-CD19 Humanized CAR-Engineered Allogeneic Double Negative T Cells in Adults with B-Cell Non-Hodgkin's Lymphoma. Blood. 2023;142(Supplement 1):2094. [Google Scholar]
- 81.Cha S., Charbonneau M., Brito A., Habibi A., Pham C., Nguyen C. ATA3431: allogeneic CD19/CD20 Bispecific CAR EBV T Cells for the Treatment of B-Cell Malignancies. Blood. 2023;142:4800. [Google Scholar]
- 82.Keller M.D., Bollard C.M. Virus-specific T-cell therapies for patients with primary immune deficiency. Blood. 2020;135(9):620–628. doi: 10.1182/blood.2019000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.López-Cantillo G., Urueña C., Camacho B.A., Ramírez-Segura C. CAR-T Cell Performance: how to Improve Their Persistence? Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.878209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Glaviano A., Foo A.S.C., Lam H.Y., Yap K.C.H., Jacot W., Jones R.H., et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer. 2023;22:138. doi: 10.1186/s12943-023-01827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wittling M.C., Cole A.C., Brammer B., Diatikar K.G., Schmitt N.C., Paulos C.M. Strategies for Improving CAR T Cell Persistence in Solid Tumors. Cancers (Basel) 2024;16(16):2858. doi: 10.3390/cancers16162858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pavlovic K., Carmona-Luque Md., Corsi G.I., Maldonado-Pérez N., Molina-Estevez F.J., Peralbo-Santaella E., et al. Generating universal anti-CD19 CAR T cells with a defined memory phenotype by CRISPR/Cas9 editing and safety evaluation of the transcriptome. Front. Immunol. 2024;15:1401683. doi: 10.3389/fimmu.2024.1401683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sung B.Y., Lin Y.H., Kong Q., Shah P.D., Glick Bieler J., Palmer S., et al. Wnt activation promotes memory T cell polyfunctionality via epigenetic regulator PRMT1. J. Clin. Invest. 2022;132(2) doi: 10.1172/JCI140508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chan J.D., Scheffler C.M., Munoz I., Sek K., Lee J.N., Huang Y.K., et al. FOXO1 enhances CAR T cell stemness, metabolic fitness and efficacy. Nature. 2024;629(8010):201–210. doi: 10.1038/s41586-024-07242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doan A.E., Mueller K.P., Chen A.Y., Rouin G.T., Chen Y., Daniel B., et al. FOXO1 is a master regulator of memory programming in CAR T cells. Nature. 2024;629(8010):211–218. doi: 10.1038/s41586-024-07300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin X., Sun Y., Dong X., Liu Z., Sugimura R., Xie G. IPSC-derived CAR-NK cells for cancer immunotherapy. Biomed. Pharmacother. 2023;165 doi: 10.1016/j.biopha.2023.115123. [DOI] [PubMed] [Google Scholar]
- 91.Dagher O.K., Posey A.D. Forks in the road for CAR T and CAR NK cell cancer therapies. Nat. Immunol. 2023;24(12):1994–2007. doi: 10.1038/s41590-023-01659-y. [DOI] [PubMed] [Google Scholar]
- 92.Titov A., Zmievskaya E., Ganeeva I., Valiullina A., Petukhov A., Rakhmatullina A., et al. Adoptive Immunotherapy beyond CAR T-Cells. Cancers (Basel) 2021;13(4):743. doi: 10.3390/cancers13040743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yilmaz A., Cui H., Caligiuri M.A., Yu J. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J. Hematol. Oncol. 2020;13(1):168. doi: 10.1186/s13045-020-00998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heipertz E.L., Zynda E.R., Stav-Noraas T.E., Hungler A.D., Boucher S.E., Kaur N., et al. Current Perspectives on “Off-The-Shelf” Allogeneic NK and CAR-NK Cell Therapies. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.732135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rafei H., Basar R., Acharya S., Zhang P., Liu P., Moseley S.M., et al. Targeting T-Cell Lymphoma Using CD70-Directed Cord Blood-Derived CAR-NK Cells. Blood. 2023;142:4811. [Google Scholar]
- 96.Włodarczyk M., Pyrzynska B. CAR-NK as a rapidly developed and efficient immunotherapeutic strategy against cancer. Cancers (Basel) 2022;15(1):117. doi: 10.3390/cancers15010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xue D., Lu S., Zhang H., Zhang L., Dai Z., Kaufman D.S., et al. Induced pluripotent stem cell-derived engineered T cells, natural killer cells, macrophages, and dendritic cells in immunotherapy. Trends Biotechnol. 2023;41(7):907–922. doi: 10.1016/j.tibtech.2023.02.003. [DOI] [PubMed] [Google Scholar]
- 98.Fu J., Jiang L., Zhu Z., Yan Y., Wu G., Wei M., et al. Efficacy of Human iPSC-Derived CAR-NK cells targeting multiple myeloma cells. Blood. 2023;142:4802. [Google Scholar]
- 99.Goodman S., Lyon K., Hartwig K., Peng S., Li Q., Murray D., et al. Engineering superaffinity antibody dependent cellular cytotoxcity receptors into iPSC-Derived NK Cells As Next-generation immunotherapies for cancer. Blood. 2023;142:2060. [Google Scholar]
- 100.Basar R., May Daher M, Uprety N., Ensley E., Nunez Cortes A.K., Acharya S., et al. DAP10 Co-stimulation imparts memory-like features to CD5 targeting cord blood derived CAR-NK Cells. Blood. 2023;142:2089. [Google Scholar]
- 101.Kilgour M.K., Bastin D.J., Lee S.H., Ardolino M., McComb S., Visram A. Advancements in CAR-NK therapy: lessons to be learned from CAR-T therapy. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1166038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen Y., Yu Z., Tan X., Jiang H., Xu Z., Fang Y., et al. CAR-macrophage: a new immunotherapy candidate against solid tumors. Biomed. Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111605. [DOI] [PubMed] [Google Scholar]
- 103.Hadiloo K., Taremi S., Heidari M., Esmaeilzadeh A. The CAR macrophage cells, a novel generation of chimeric antigen-based approach against solid tumors. Biomark. Res. 2023;11:103. doi: 10.1186/s40364-023-00537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maalej K.M., Merhi M., Inchakalody V.P., Mestiri S., Alam M., Maccalli C., et al. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol. Cancer. 2023;22:20. doi: 10.1186/s12943-023-01723-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lei A., Yu H., Lu S., Lu H., Ding X., Tan T., et al. A second-generation M1-polarized CAR macrophage with antitumor efficacy. Nat. Immunol. 2023:1–15. doi: 10.1038/s41590-023-01687-8. [DOI] [PubMed] [Google Scholar]
- 106.Nie S., Qin Y., Ou L., Chen X., Li L. In situ reprogramming of immune cells using synthetic nanomaterials. Adv. Mater. 2024;36(15) doi: 10.1002/adma.202310168. [DOI] [PubMed] [Google Scholar]
- 107.Parayath N.N., Stephan M.T. In Situ Programming of CAR T Cells. Annu Rev Biomed Eng. 2021;23:385–405. doi: 10.1146/annurev-bioeng-070620-033348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parker M., Ulrich-Lewis J., Tang W., Nicolai C., Michels K., Hernandez S., et al. VivovecTM surface-engineered lentiviral particles mediate in vivo CAR T generation with potent and highly durable activity in non-human primates and tumor-bearing humanized mice. Blood. 2023;142:765. [Google Scholar]
- 109.Mei H., Chen Z., Hu Y., Mei H. Bio-Orthogonally Redirected Bispecific Lentiviral Vectors for In Vivo CAR-T Cell Generation. Blood. 2023;142:2062. [Google Scholar]
- 110.Krotova K., Nenavath G.N., Packiriswamy N., Vandergaast R., Moy M., Ziegler C., et al. Vivo generation of functional CD19 CAR-T cells using a serum-stable CD3-targeted lentiviral vector. Blood. 2023;142:767. [Google Scholar]
- 111.Andorko J.I., Russell R.M., Schnepp B.C., Singh R., Boral D., O'Malley T., et al. Targeted In Vivo Generation of CAR T and NK cells utilizing an engineered lentiviral vector platform. Blood. 2023;142:763. [Google Scholar]
- 112.Beltran-Garcia J., Han S., Perez B.S., Gunn J., Kwon E., Kaufman D. Development of novel lipid nanoparticles and virus-like particles for in vivo engineering of immune cells for targeted cancer therapy. Blood. 2023;142:3632. [Google Scholar]
- 113.Green J., Tucker A., Vakil A., Scott-Skandera M., Vagin V., Dingar D., et al. Feasibility of extracorporeal delivery of fusosomes to generate CAR T Cells In Vivo. Blood. 2023;142:3631. [Google Scholar]
- 114.Rurik J.G., Tombácz I., Yadegari A., Méndez Fernández P.O., Shewale S.V., Li L., et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375(6576):91–96. doi: 10.1126/science.abm0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith T.T., Stephan S.B., Moffett H.F., McKnight L.E., Ji W., Reiman D., et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nature Nanotech. 2017;12(8):813–820. doi: 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parayath N.N., Stephan S.B., Koehne A.L., Nelson P.S., Stephan M.T. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 2020;11(1):6080. doi: 10.1038/s41467-020-19486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Niu H., Zhao P., Sun W. Biomaterials for chimeric antigen receptor T cell engineering. Acta Biomater. 2023;166:1–13. doi: 10.1016/j.actbio.2023.04.043. [DOI] [PubMed] [Google Scholar]
- 118.Ma R., Li Z., Chiocca E.A., Caligiuri M.A., Yu J. The emerging field of oncolytic virus-based cancer immunotherapy. Trends Cancer. 2023;9(2):122–139. doi: 10.1016/j.trecan.2022.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aghajanian H., Rurik J.G., Epstein J.A. CAR-based therapies: opportunities for immuno-medicine beyond cancer. Nat Metab. 2022;4(2):163–169. doi: 10.1038/s42255-022-00537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schett G., Mackensen A., Mougiakakos D. CAR T-cell therapy in autoimmune diseases. Lancet North Am. Ed. 2023;402(10416):2034–2044. doi: 10.1016/S0140-6736(23)01126-1. [DOI] [PubMed] [Google Scholar]
- 121.Baker D.J., June C.H. Off-the-shelf CAR-T cells could prove paradigm shifting for autoimmune diseases. Cell. 2024;187(18):4826–4828. doi: 10.1016/j.cell.2024.07.056. [DOI] [PubMed] [Google Scholar]