Abstract
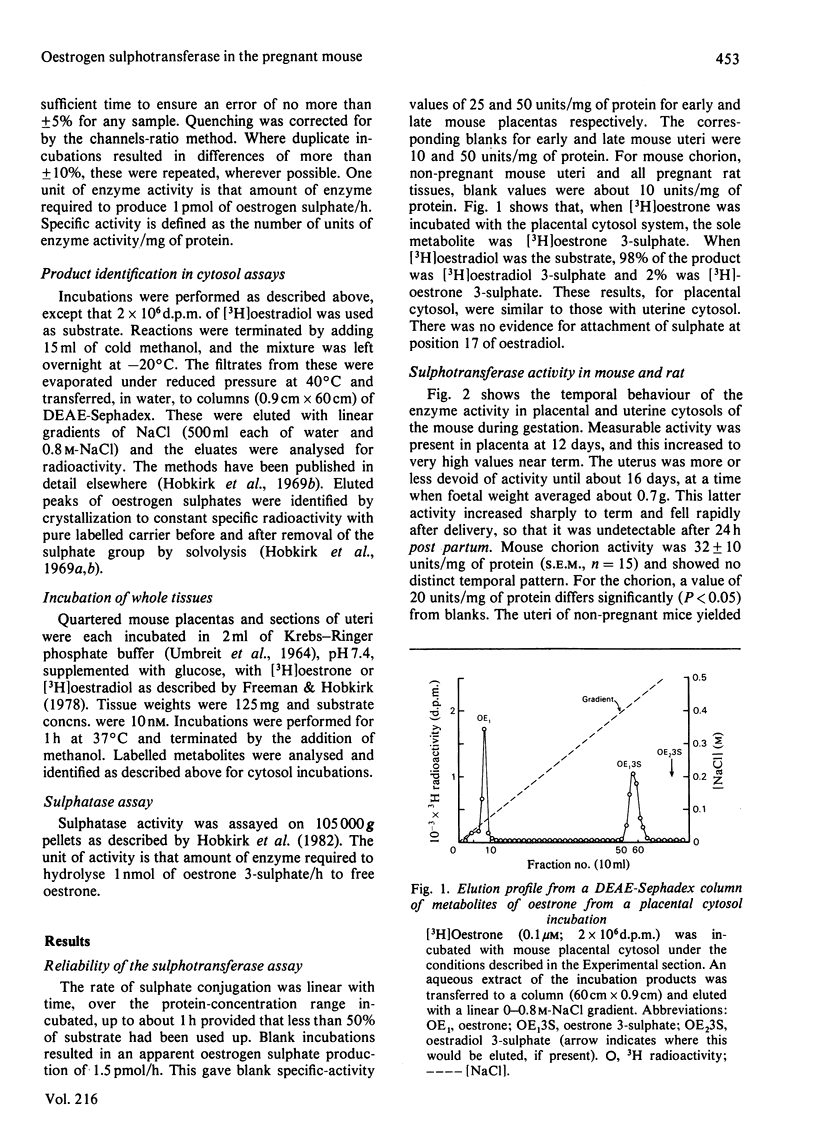
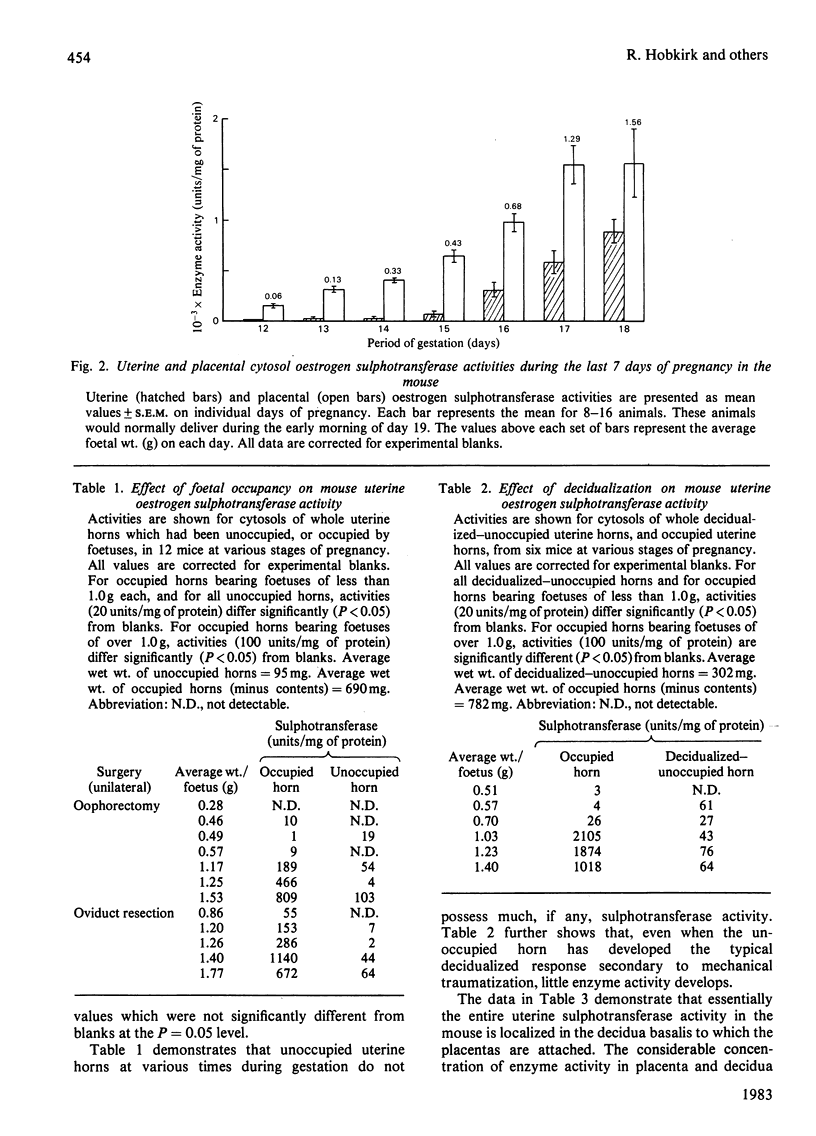
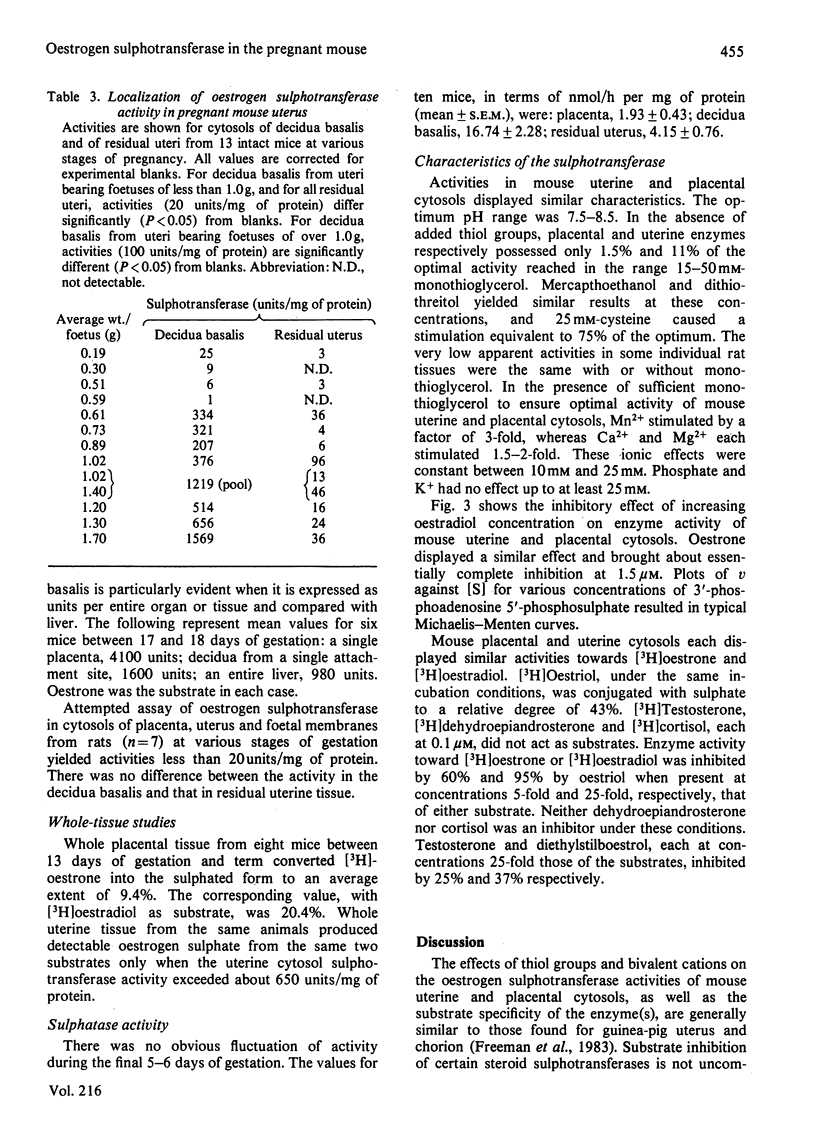
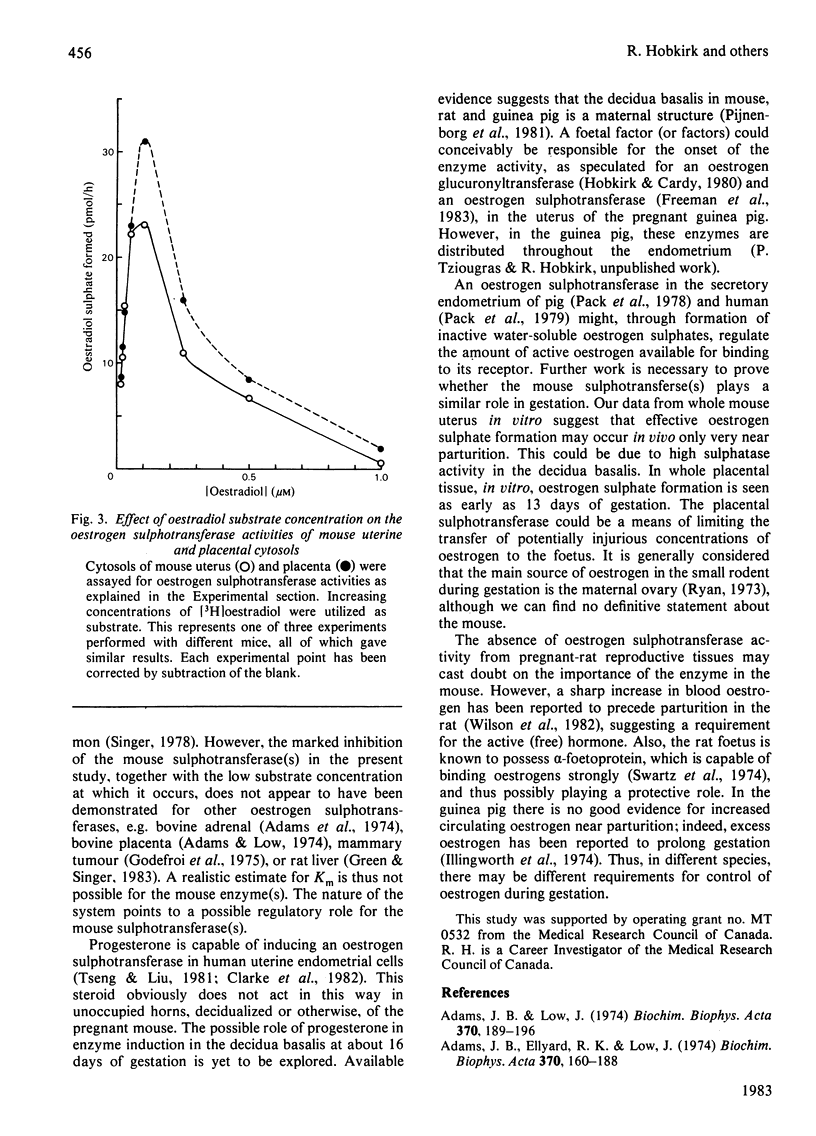
The mouse placenta possesses a soluble oestrogen sulphotransferase activity which increases markedly from at least 12 days of gestation until term. At about 16 days of gestation, a similar activity is found in the uterus. This activity also increases until term and disappears rapidly post partum. The uterine enzyme activity appears to require the presence of the foetal unit for its onset, since unoccupied horns, whether their endometrial stromal cells are differentiated to decidual cells or not, are essentially devoid of it. Uterine cytosols from non-pregnant mice are also inactive in this respect. In late gestation, the uterine sulphotransferase is confined to the decidua basalis, the areas to which the placentas are attached. The sulphotransferase(s) of placenta and uterus has an absolute requirement for 3'-phosphoadenosine 5'-phosphosulphate, and possesses little activity in the absence of exogenous thiol groups. Stimulation is also seen in the presence of Mn2+, Mg2+ or Ca2+. Oestrone and oestradiol, and to a lesser degree oestriol, are substrates for the enzyme(s), whereas testosterone, cortisol and dehydroepiandrosterone are not. Oestrone and oestradiol at higher concentrations (1.0-1.5 microM) completely inhibit the enzyme(s). These enzymes could play a role in altering tissue concentrations of active oestrogens during gestation in the mouse. Oestrogen sulphotransferase activity is low or absent in reproductive tissues of the pregnant rat.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. B., Ellyard R. K., Low J. Enzymic synthesis of steroid sulphates. IX. Physical and chemical properties of purified oestrogen sulphotransferase from bovine adrenal glands, the nature of its isoenzymic forms and a proposed model to explain its wave-like kinetics. Biochim Biophys Acta. 1974 Nov 25;370(1):160–188. doi: 10.1016/0005-2744(74)90042-4. [DOI] [PubMed] [Google Scholar]
- Adams J. B., Low J. Enzymic synthesis of steroid sulphates. X. Isolation of oestrogen sulphotransferase from bovine placenta and comparison of its properties with adrenal oestrogen sulphotransferase. Biochim Biophys Acta. 1974 Nov 25;370(1):189–196. doi: 10.1016/0005-2744(74)90043-6. [DOI] [PubMed] [Google Scholar]
- Buirchell B. J., Hähnel R. Metabolism of estradiol-17beta in human endometrium during the menstrual cycle. J Steroid Biochem. 1975 Nov-Dec;6(11-12):1489–1494. doi: 10.1016/0022-4731(75)90202-2. [DOI] [PubMed] [Google Scholar]
- Clarke C. L., Adams J. B., Wren B. G. Induction of estrogen sulfotransferase in the human endometrium by progesterone in organ culture. J Clin Endocrinol Metab. 1982 Jul;55(1):70–75. doi: 10.1210/jcem-55-1-70. [DOI] [PubMed] [Google Scholar]
- Dwyer R. J., Robertson H. A. Oestrogen sulphatase and sulphotransferase activities in the endometrium of the sow and ewe during pregnancy. J Reprod Fertil. 1980 Sep;60(1):187–191. doi: 10.1530/jrf.0.0600187. [DOI] [PubMed] [Google Scholar]
- Freeman D. J., Saidi F., Hobkirk R. Estrogen sulfotransferase activity in guinea pig uterus and chorion. J Steroid Biochem. 1983 Jan;18(1):23–27. doi: 10.1016/0022-4731(83)90325-4. [DOI] [PubMed] [Google Scholar]
- Godefroi V. C., Locke E. R., Singh D. V., Brooks S. C. The steroid alcohol and estrogen sulfotransferases in rodent and human mammary tumors. Cancer Res. 1975 Jul;35(7):1791–1798. [PubMed] [Google Scholar]
- Green J. M., Singer S. S. Enzymatic sulfation of steroids. XVIII. study of the specific estradiol-17 beta sulfotransferase of rat liver cytosol, that converts the estrogen to its 3-sulfate, and some elements of the endocrine control of its production. Can J Biochem Cell Biol. 1983 Jan;61(1):15–22. doi: 10.1139/o83-003. [DOI] [PubMed] [Google Scholar]
- Hobkirk R., Cardy C., Nilsen M., Saidi F. Estrone sulfatase activity in guinea pig tissues. J Steroid Biochem. 1982 Jul;17(1):71–76. doi: 10.1016/0022-4731(82)90594-5. [DOI] [PubMed] [Google Scholar]
- Hobkirk R., Cardy C. UDPGA-dependent estrogen glucuronyltransferase of guinea-pig uterus: assay, temporal relationships in pregnancy and some characteristics. J Steroid Biochem. 1980 Sep;13(9):1039–1045. doi: 10.1016/0022-4731(80)90135-1. [DOI] [PubMed] [Google Scholar]
- Hobkirk R., Musey P., Nilsen M. Chromatographic separation of estrone and 17 beta-estradiol conjugates on DEAE-Sephadex. Steroids. 1969 Aug;14(2):191–206. doi: 10.1016/0039-128x(69)90033-6. [DOI] [PubMed] [Google Scholar]
- Hobkirk R., Nilsen M., Blahey P. R. Conjugation of urinary phenolic steroids in the nonpregnant human female with particular reference to estrone sulfate. J Clin Endocrinol Metab. 1969 Mar;29(3):328–337. doi: 10.1210/jcem-29-3-328. [DOI] [PubMed] [Google Scholar]
- Illingworth D. V., Challis J. R., Ackland N., Burton A. M., Heap R. B., Perry J. S. Parturition in the guinea-pig; plasma levels of steroid hormones, steroid-binding proteins, and oxytocin, and the effect of corticosteroids, prostaglandins and adrenocorticotrophin. J Endocrinol. 1974 Dec;63(3):557–570. doi: 10.1677/joe.0.0630557. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pack B. A., Brooks S. C. Cyclic activity of estrogen sulfotransferase in the gilt uterus. Endocrinology. 1974 Dec;95(6):1680–1690. doi: 10.1210/endo-95-6-1680. [DOI] [PubMed] [Google Scholar]
- Pack B. A., Christensen C., Douraghy M., Brooks S. C. Nuclear and cytosolic estrogen receptor in gilt endometrium throughout the estrous cycle. Endocrinology. 1978 Dec;103(6):2129–2136. doi: 10.1210/endo-103-6-2129. [DOI] [PubMed] [Google Scholar]
- Pack B. A., Tovar R., Booth E., Brooks S. C. The cyclic relationship of estrogen sulfurylation to the nuclear receptor level in human endometrial curettings. J Clin Endocrinol Metab. 1979 Mar;48(3):420–424. doi: 10.1210/jcem-48-3-420. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R., Robertson W. B., Brosens I., Dixon G. Review article: trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta. 1981 Jan-Mar;2(1):71–91. doi: 10.1016/s0143-4004(81)80042-2. [DOI] [PubMed] [Google Scholar]
- Singer S. S. Enzymatic sulfation of steroids. II. The sulfation of corticosterone by the glucocorticoid sulfotransferases of rat liver cytosol. Biochim Biophys Acta. 1978 Feb 13;539(1):19–30. doi: 10.1016/0304-4165(78)90117-4. [DOI] [PubMed] [Google Scholar]
- Singer S. S. Enzymatic sulfation of steroids. VI. A simple, rapid method for routine enzymatic preparation of 3'-phosphoadenosine-5'-phosphosulfate. Anal Biochem. 1979 Jul 1;96(1):34–38. doi: 10.1016/0003-2697(79)90550-5. [DOI] [PubMed] [Google Scholar]
- Tseng L., Liu H. C. Stimulation of arylsulfotransferase activity by progestins in human endometrium in vitro. J Clin Endocrinol Metab. 1981 Aug;53(2):418–421. doi: 10.1210/jcem-53-2-418. [DOI] [PubMed] [Google Scholar]
- Wilson L., Jr, Stanisc D., Khan-Dawood F., Dawood M. Y. Alterations in reproductive tissue prostaglandins E and F, 6-keto-prostaglandin F1 alpha and thromboxane B2 with gestational age in the rat. Biol Reprod. 1982 Dec;27(5):1207–1215. doi: 10.1095/biolreprod27.5.1207. [DOI] [PubMed] [Google Scholar]


