Abstract
Oxygen-radical production stimulated from rat polymorphonuclear leucocytes by either unopsonized latex particles (diameter = 1.01 microM) or chemotactic peptide (N-formyl-Met-Leu-Phe) was monitored by using luminol-dependent chemiluminescence. Azide inhibited by more than 80% the luminescence response induced by chemotactic peptide whether added before or after stimulation. However, the luminescence response to latex particles was progressively less susceptible to azide inhibition if the azide was added after the stimulus. Cytochalasin B, which was shown to abolish phagocytosis of the latex beads, also abolished the chemiluminescence response. However, the same cells showed a greatly enhanced response to chemotactic peptide. Cytochalasin B-treated cells secreted approx. 45% of total cellular myeloperoxidase in response to chemotactic peptide, but there was no detectable secretion in response to unopsonized latex particles. Microperoxidase equivalent to 20% of cellular peroxidase activity added to the cells before addition of the stimulus had no effect on the response to latex particles but increased approx. 2-fold the peak rate of chemiluminescence induced by chemotactic peptide. It was concluded that the unopsonized latex particles stimulated oxygen-radical production by the mechanism that involved endocytosis, whereas chemotactic peptide stimulated production by a mechanism that involved exocytosis of myeloperoxidase, the latter mechanism requiring an increase in intracellular free [Ca2+].
Full text
PDF

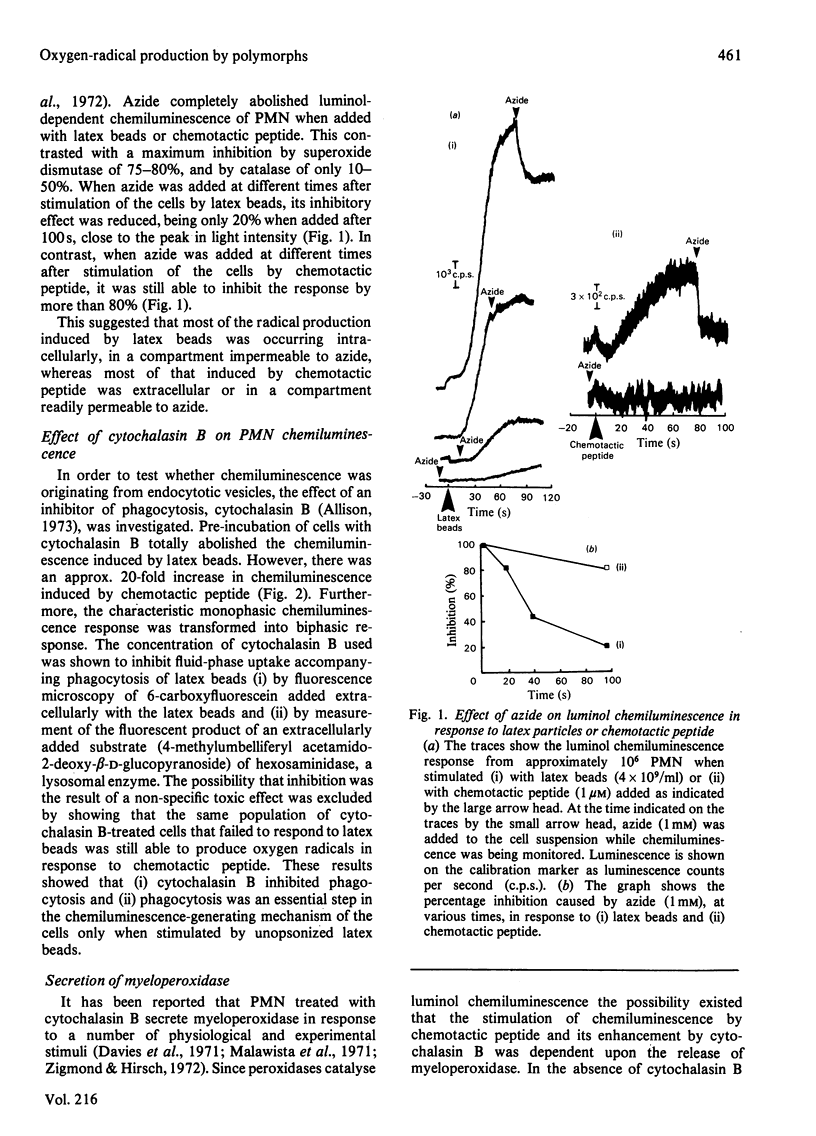
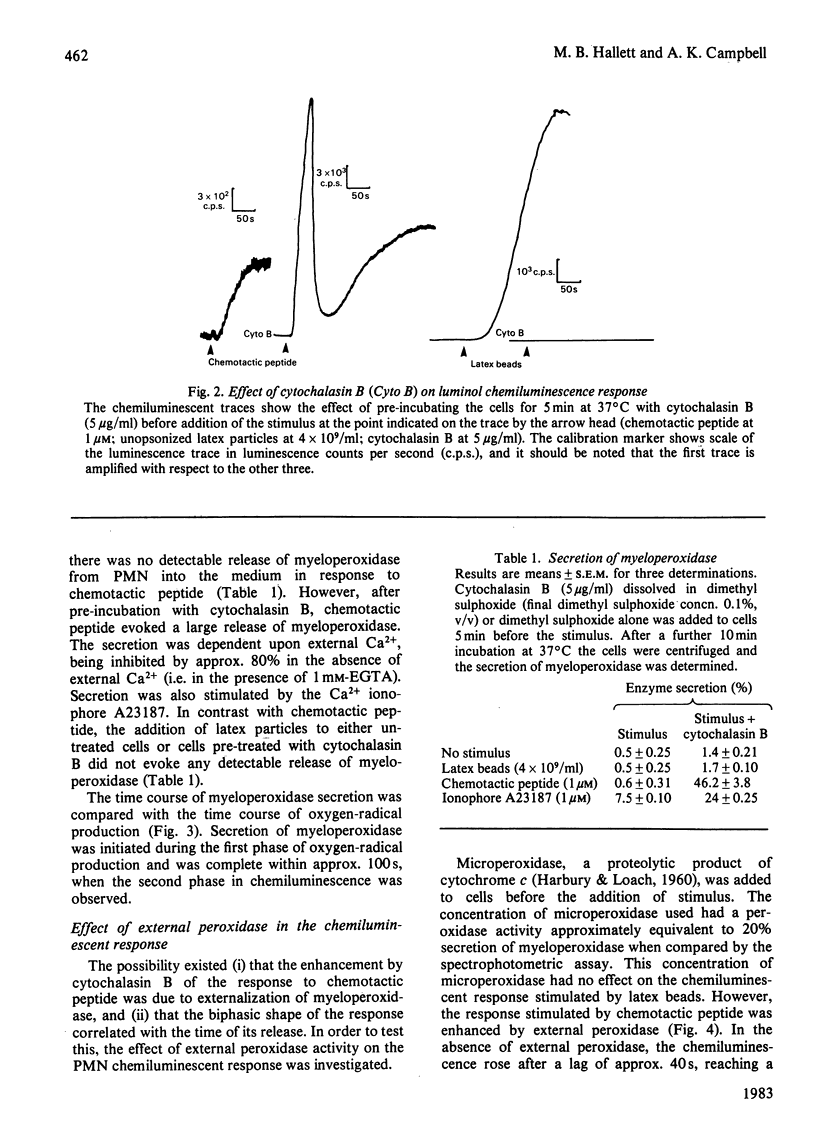
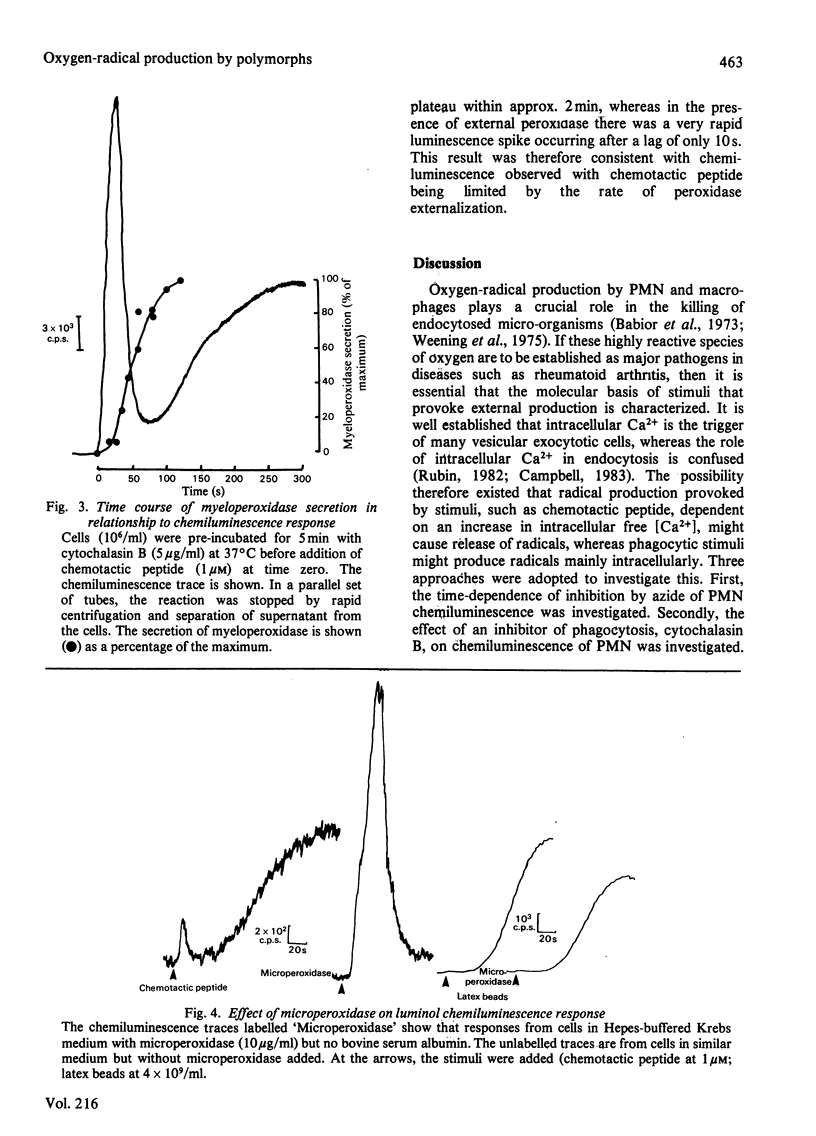


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. The role of microfilaments and microtubules in cell movement, endocytosis and exocytosis. Ciba Found Symp. 1973;14:109–148. doi: 10.1002/9780470719978.ch6. [DOI] [PubMed] [Google Scholar]
- BECKER H., MUNDER G., FISCHER H. Uber den Leukocytenstoffwechsel bei der Phagocytose. Hoppe Seylers Z Physiol Chem. 1958;313:266–275. doi: 10.1515/bchm2.1958.313.1.266. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. K., Hallett M. B. Measurement of intracellular calcium ions and oxygen radicals in polymorphonuclear leucocyte-erythrocyte 'ghost' hybrids. J Physiol. 1983 May;338:537–550. doi: 10.1113/jphysiol.1983.sp014688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. T., Estensen R., Quie P. G. Cytochalasin B. 3. Inhibition of human polymorphonuclear leukocyte phagocytosis. Proc Soc Exp Biol Med. 1971 May;137(1):161–164. doi: 10.3181/00379727-137-35535. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARBURY H. A., LOACH P. A. Oxidation-linked proton functions in heme octa- and undecapeptides from mammalian cytochrome c. J Biol Chem. 1960 Dec;235:3640–3645. [PubMed] [Google Scholar]
- Hallett M. B., Campbell A. K. Measurement of changes in cytoplasmic free CA2+ in fused cell hybrids. Nature. 1982 Jan 14;295(5845):155–158. doi: 10.1038/295155a0. [DOI] [PubMed] [Google Scholar]
- Hallett M. B., Luzio J. P., Campbell A. K. Stimulation of Ca2+-dependent chemiluminescence in rat polymorphonuclear leucocytes by polystyrene beads and the non-lytic action of complement. Immunology. 1981 Nov;44(3):569–576. [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970 Sep 11;169(3950):1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., Gee J. B., Bensch K. G. Cytochalasin B reversibly inhibits phagocytosis: functional, metabolic, and ultrastructural effects in human blood leukocytes and rabbit alveolar macrophages. Yale J Biol Med. 1971 Dec;44(3):286–300. [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. The subcellular distribution and some properties of the cytochrome b component of the microbicidal oxidase system of human neutrophils. Biochem J. 1979 Jul 15;182(1):181–188. doi: 10.1042/bj1820181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]
- Williams A. J., Cole P. J. Polymorphonuclear leucocyte membrane-stimulated oxidative metabolic activity---the effect of divalent cations and cytochalasins. Immunology. 1981 Dec;44(4):847–858. [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Effects of cytochalasin B on polymorphonuclear leucocyte locomotion, phagocytosis and glycolysis. Exp Cell Res. 1972 Aug;73(2):383–393. doi: 10.1016/0014-4827(72)90062-6. [DOI] [PubMed] [Google Scholar]


