Abstract
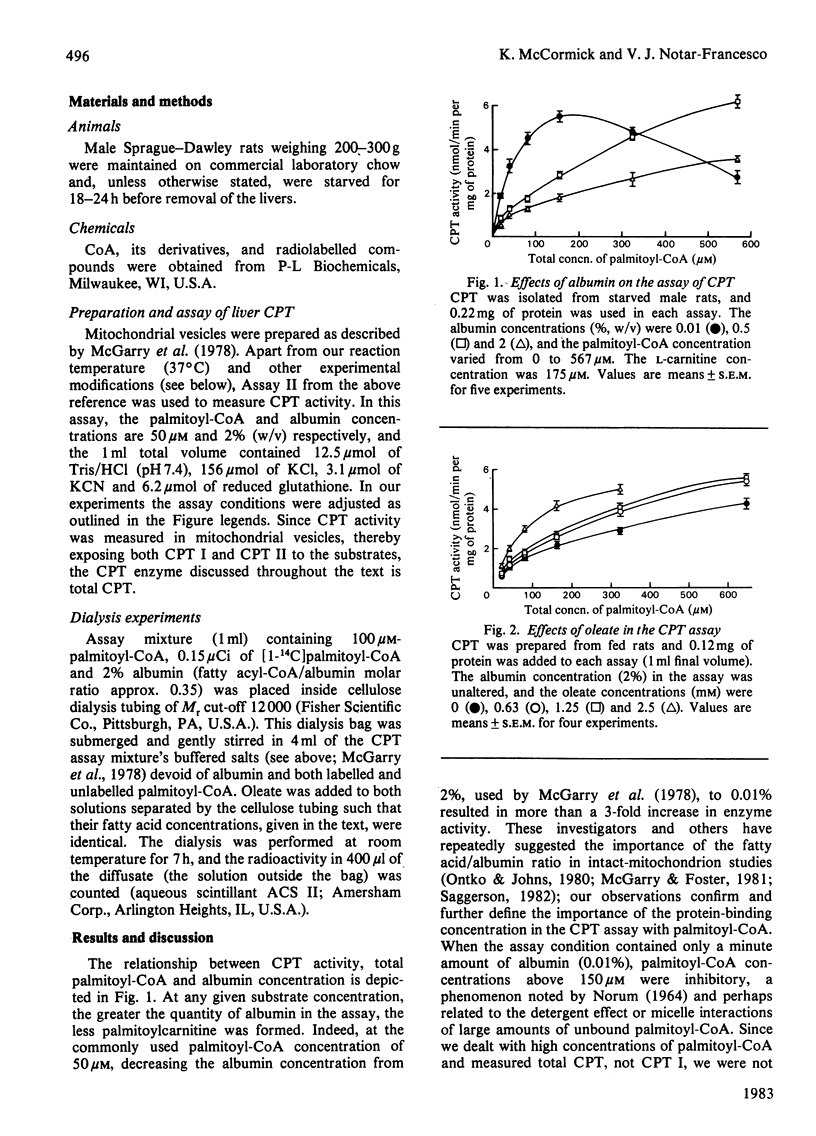
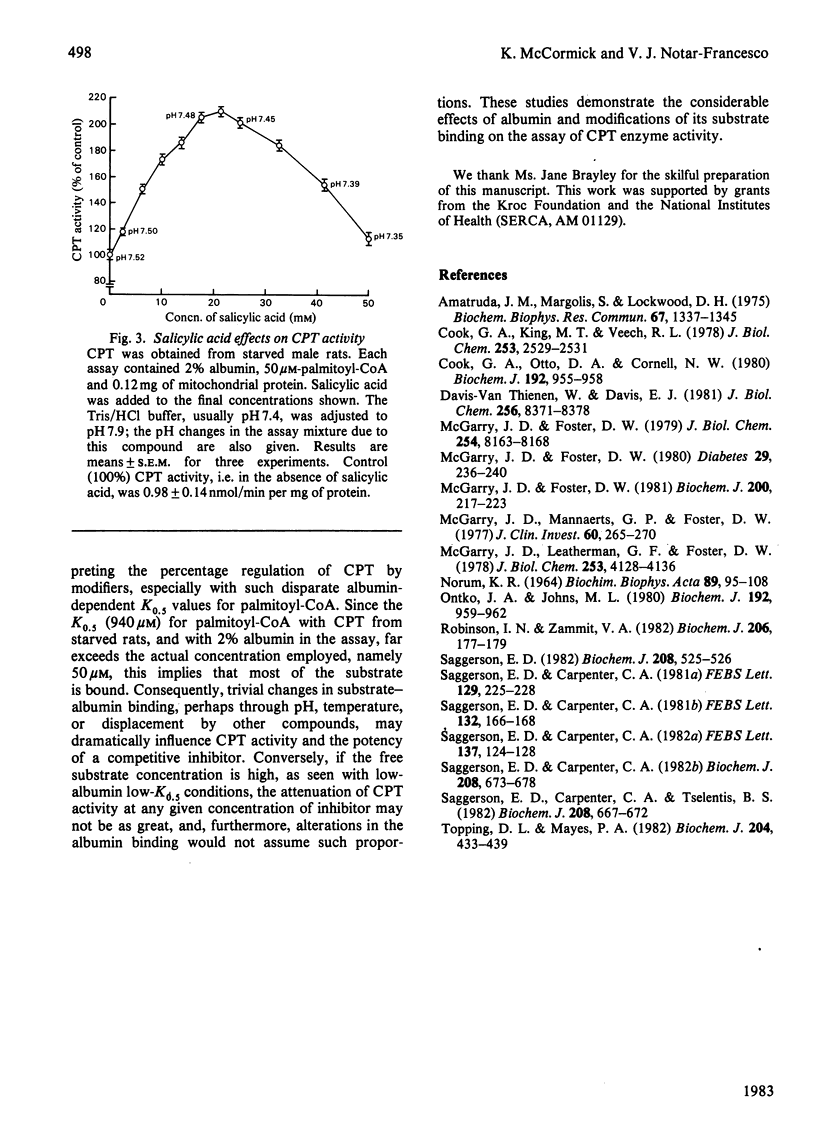
Alterations in the long-chain acyl-CoA binding to albumin in the carnitine palmitoyltransferase (CPT) assay appreciably affect the reaction at commonly used substrate concentrations. Since in the CPT assay the latter are typically well below saturation or Vmax. values, the measured enzyme activity depends on both the absolute quantity of albumin in the CPT assay and any biochemical modification of its binding. The present study verifies the striking dependence of the K0.5 for palmitoyl-CoA on albumin and the misleading 'activation' of the enzyme by compounds that also avidly bind to albumin. In assessing the intracellular physiological relevance of any modifier of CPT, the effects of protein binding in the assay assume particular importance. Indeed, any compound that alters CPT activity may do so, not directly, but as an assay artifact changing the free or unbound substrate concentrations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amatruda J. M., Margolis S., Lockwood D. H. Regulation of ketone body production from (14C)palmitate in rat liver mitochondria: effects of cyclic nucleotides and unlabeled fatty acids. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1337–1345. doi: 10.1016/0006-291x(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Cook G. A., King M. T., Veech R. L. Ketogenesis and malonyl coenzyme A content of isolated rat hepatocytes. J Biol Chem. 1978 Apr 25;253(8):2529–2531. [PubMed] [Google Scholar]
- Cook G. A., Otto D. A., Cornell N. W. Differential inhibition of ketogenesis by malonyl-CoA in mitochondria from fed and starved rats. Biochem J. 1980 Dec 15;192(3):955–958. doi: 10.1042/bj1920955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-van Thienen W., Davis E. J. The effects of energetic steady state, pyruvate concentration, and octanoyl-(--)-carnitine on the relative rates of carboxylation and decarboxylation of pyruvate by rat liver mitochondria. J Biol Chem. 1981 Aug 25;256(16):8371–8378. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Effects of exogenous fatty acid concentration on glucagon-induced changes in hepatic fatty acid metabolism. Diabetes. 1980 Mar;29(3):236–240. doi: 10.2337/diab.29.3.236. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Importance of experimental conditions in evaluating the malonyl-CoA sensitivity of liver carnitine acyltransferase. Studies with fed and starved rats. Biochem J. 1981 Nov 15;200(2):217–223. doi: 10.1042/bj2000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. In support of the roles of malonyl-CoA and carnitine acyltransferase I in the regulation of hepatic fatty acid oxidation and ketogenesis. J Biol Chem. 1979 Sep 10;254(17):8163–8168. [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORUM K. R. PALMITYL-COA:CARNITINE PALMITYLTRANSFERASE. PURIFICATION FROM CALF-LIVER MITOCHONDRIA AND SOME PROPERTIES OF THE ENZYME. Biochim Biophys Acta. 1964 Jul 8;89:95–108. [PubMed] [Google Scholar]
- Ontko J. A., Johns M. L. Evaluation of malonyl-CoA in the regulation of long-chain fatty acid oxidation in the liver. Evidence for an unidentified regulatory component of the system. Biochem J. 1980 Dec 15;192(3):959–962. doi: 10.1042/bj1920959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson I. N., Zammit V. A. Sensitivity of carnitine acyltransferase I to malonly-CoA inhibition in isolated rat liver mitochondria is quantitatively related to hepatic malonyl-CoA concentration in vivo. Biochem J. 1982 Jul 15;206(1):177–179. doi: 10.1042/bj2060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Malonyl CoA inhibition of carnitine acyltransferase activities: effects of thiol-group reagents. FEBS Lett. 1982 Jan 11;137(1):124–128. doi: 10.1016/0014-5793(82)80329-3. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Response to starvation of hepatic carnitine palmitoyltransferase activity and its regulation by malonyl-CoA. Sex differences and effects of pregnancy. Biochem J. 1982 Dec 15;208(3):673–678. doi: 10.1042/bj2080673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A., Tselentis B. S. Effects of thyroidectomy and starvation on the activity and properties of hepatic carnitine palmitoyltransferase. Biochem J. 1982 Dec 15;208(3):667–672. doi: 10.1042/bj2080667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. Does fasting decrease the inhibitory effect of malonyl-CoA on hepatic beta-oxidation? Biochem J. 1982 Nov 15;208(2):525–528. doi: 10.1042/bj2080525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. Insulin and non-esterified fatty acids. Acute regulators of lipogenesis in perfused rat liver. Biochem J. 1982 May 15;204(2):433–439. doi: 10.1042/bj2040433. [DOI] [PMC free article] [PubMed] [Google Scholar]


