Abstract
The right ventricle and its stress response is perhaps the most important arbiter of survival in patients with pulmonary hypertension of many causes. The physiology of the cardiopulmonary unit and definition of right heart failure proposed in the 2018 World Symposium on Pulmonary Hypertension have proven useful constructs in subsequent years. Here, we review updated knowledge of basic mechanisms that drive right ventricular function in health and disease, and which may be useful for therapeutic intervention in the future. We further contextualise new knowledge on assessment of right ventricular function with a focus on metrics readily available to clinicians and updated understanding of the roles of the right atrium and tricuspid regurgitation. Typical right ventricular phenotypes in relevant forms of pulmonary vascular disease are reviewed and recent studies of pharmacological interventions on chronic right ventricular failure are discussed. Finally, unanswered questions and future directions are proposed.
Shareable abstract
A summary of recent understanding of the pathobiology and pathophysiology of the right ventricle and its interaction with the pulmonary vasculature https://bit.ly/3SdvBz9
Introduction
The adaptation of the right heart, and the right ventricle (RV) specifically, to the stress of pulmonary hypertension (PH) is the central determinant of outcomes in these highly mortal pulmonary vascular diseases. The field has made substantial progress in understanding basic mechanisms of RV stress responses, imaging of the right heart in health and disease and even some recent attempts at treating right heart failure (RHF) specifically, rather than ameliorating pulmonary vascular disease alone. Recent advances in these topics since the 6th World Symposium on Pulmonary Hypertension (WSPH) in 2018 are covered in this article.
Definition of right heart failure
It is well documented that survival in patients with PH is closely related to right heart function. This is discussed extensively in recent reviews [1–6] and in previous reports from task forces at the 2013 [7] and 2018 [8] WSPH. Indeed, this was shown in the national prospective registry for primary PH, now called pulmonary arterial hypertension (PAH), where the majority of deaths were attributable to RHF or cardiac arrest, and a prediction equation was derived incorporating right atrial (RA) pressure, pulmonary artery (PA) pressure and cardiac output measurements [9]. RHF in PH was defined in the previous WSPH document as “a clinical syndrome characterised by decreased RV function that leads to insufficient blood flow and/or elevated filling pressures at rest or during physiologically demanding conditions, such as exercise, developmental growth or pregnancy. The cardinal symptoms of RHF include dyspnoea, fatigue, and congestion” [8].
In this definition, RHF encompasses the RV, the RA, tricuspid valve, coupling to the arterial and/or venous systems and interactions with the left ventricle and pericardium. RHF is a dynamic notion, which may progress to circulatory failure, but also has the potential for “reverse remodelling”, for example in the context of pulmonary endarterectomy for chronic thromboembolic PH (CTEPH) [10].
Here, we discuss the previous definitions of RHF and do not propose new updates to the definition provided in the 6th WSPH document [8].
New understanding of RV pathology mechanisms
At the time of the 2018 WSPH, our task force described expansion of understanding of genetic contributions to RV failure, identified unanswered questions in the role of sex hormones in RV failure and highlighted the then relatively nascent understanding of metabolism in RV dysfunction. Since that meeting, major advances have been made in many aspects of these pathways. This section focuses on new knowledge since the 6th WSPH. Figure 1 summarises key pathophysiological mechanisms presently understood to impact RV function, with a time frame for their role. With increasing interest and publications in this topic, we are unable to discuss all recent publications and have focused on several key areas of research. Other ongoing areas of interest reviewed elsewhere [11, 12] include, but are not limited to, the role of the RV capillaries as the contribution of vascular disease or dysfunction versus perfusion/demand mismatch is unanswered [13, 14], neurohormonal activation, multi-omics approaches and the role of the autonomic nervous system.
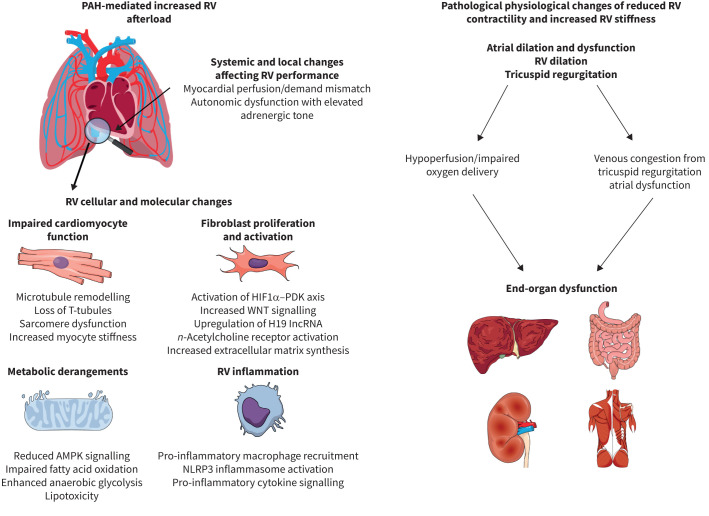
FIGURE 1.
The systemic, organ, cellular and molecular causes and subsequent physiological consequences of right ventricular (RV) dysfunction in pulmonary arterial hypertension (PAH). Increased afterload paired with derangements in autonomic tone and myocardial perfusion/demand mismatch lead to pathogenic changes in cardiomyocyte structure and function driven in part by mitochondrial metabolic dysfunction, heightened fibrosis due to fibroblast proliferation and activation and pathological inflammation. These cellular and organ level alterations increase RV stiffness and impair contractility that leads to right atrial dilation/dysfunction, RV dilation and tricuspid regurgitation. This ultimately results in end-organ hypoperfusion and venous congestion causing hepatic and renal dysfunction. HIF: hypoxia-inducible factor; PDK: pyruvate dehydrogenase kinase; WNT: wingless/int; lncRNA: long noncoding RNA; n-acetylcholine: nicotinic acetylcholine; AMPK: AMP kinase; NLRP: NACHT-, LRR-, and pyrine domain-containing protein.
RV inflammation and fibrosis
Both pre-clinical and clinical data suggest that chronic inflammation exacerbates RV dysfunction, and thus combatting inflammation may have RV-enhancing effects. First, an RNA-sequencing study evaluated the divergent RV phenotypes in Fischer and Sprague Dawley rats exposed to Sugen 5416-hypoxia (SuHx) and found that the more severe RV phenotype in Fischer rats is characterised by a pro-inflammatory milieu [15]. Another transcriptomics-based investigation of monocrotaline (MCT) and SuHx rats identified that multiple inflammatory pathways are activated [16]. Importantly, interventions aimed at reducing RV inflammation demonstrate RV-enhancing effects in pre-clinical studies. Small-molecule antagonism of glycoprotein-130, the master receptor for the interleukin (IL)-6 superfamily of cytokines, augments RV function by suppressing microtubule remodelling and reprograming mitochondrial metabolism in MCT rats [17]. In addition, the pathogenic role of macrophage NACHT-, LRR- and pyrine domain-containing protein (NLRP)3 activation in macrophages is evident in both the MCT and SuHx rodents, and suppression of the NLRP3 inflammasome combats RV dysfunction and reduces RV fibrosis in MCT rats. In RV samples from PAH patients, IL-1β levels are higher compared with controls, highlighting the potential translational value of antagonising NLRP3 [18].
Detrimental RV fibrosis results from a complex interplay between inflammatory cytokines, immune cells, cardiomyocytes and fibroblasts. Recent advances in fibroblast biology have defined targetable pathways to suppress fibroblast activation and pathological RV fibrosis. The importance of RV cardiomyocyte–fibroblast crosstalk is now evident as cardiomyocyte-derived acetylcholine activates RV fibroblasts via the α7 nicotinic acetylcholine receptor. Treatment of SuHx rats with mecamylamine, a nicotinic acetylcholine receptor blocker, suppresses RV fibrosis and restores RV diastolic function [19]. In addition, RV fibroblasts from MCT animals have higher levels of DNA methyltransferases-1 and -4, which stimulate hypoxia-inducible factor-1α activation. Hypoxia-inducible factor-1α depresses mitochondrial function via pyruvate dehydrogenase kinase (PDK)-1 and -3 expression [20]. This epigenetic axis ultimately promotes excess collagen type-III synthesis and RV fibrosis, and suppression of this axis with dichloroacetate combats RV fibrosis/dysfunction in MCT rats. Importantly, RV fibroblasts from PAH patients have elevated PDK-1 levels, which highlights an important link to human biology [20]. Another epigenetic regulator of RV fibroblasts is the long noncoding RNA H19. Antagonism of H19 prevents the differentiation of fibroblasts into myofibroblasts as it reduces collagen I and III transcript levels in vitro, and augments RV function in vivo [21]. Finally, the wingless/int (Wnt) signalling pathway is induced in RV samples from PAH compared with normal RV controls, and its activity correlates with RV fibrosis. In RV fibroblasts, Wnt signalling promotes myofibroblast transdifferentiation, and small-molecule antagonism of Wnt blunts RV fibrosis and restores RV function in rodents [22]. Interestingly, the aforementioned fibroblast pathways may not be terminally activated as RV fibrosis and dysfunction regress, once RV afterload normalises. Progressive debanding of the PA in mice normalises RV function and RV fibrosis recedes, which may be mediated via suppression of growth factor genes [23].
Importantly, human-level data also support the hypothesis that the inflammation/fibrosis axis negatively impacts RV function in PAH. Serum levels of IL-6 are elevated in PAH patients with RV dysfunction [24]. Patients with higher IL-6 levels display heightened sensitivity to increasing afterload, and they have higher N-terminal pro-brain natriuretic peptide (NT-proBNP) levels [17]. A transcriptomics analysis of human RV tissue shows that IL-6 transcript abundance is elevated in PAH patients with a decompensated RV phenotype, and the Janus kinase/signal transducers and activators of transcription signalling pathway, the pathway activated by IL-6, is one of the most enriched pathways when comparing patients with decompensated RV to controls [25].
In advanced stages of RV dysfunction determined by imaging and haemodynamics, multiple pathways associated with inflammation and extracellular matrix remodelling are upregulated in both rodents and humans [26]. Moreover, a panel of five extracellular matrix proteins in the serum provide prognostic value in both German and UK cohorts [26]. Boucherat et al. [25] also show that serum fibrotic markers predict RV dysfunction and identify the pro-fibrotic protein latent transforming growth factor-β binding protein (LTBP)-2 as an important biomarker of RV failure, as its levels increase with the severity of RV dysfunction. In addition, LTBP-2 levels add predictive value to current risk stratification models, in two independent PAH cohorts. Whether these markers of disease have a mechanistic role in RV dysfunction and/or failure is less well understood.
Recent work demonstrates the relevance of RV stiffness to impaired cardiac reserve and exercise capacity, showing the physiological relevance of RV stiffness in human PAH [27]. PAH patients with low cardiac reserve exhibit higher exercise-induced right-sided pressures, RV stiffness and decreased left ventricular filling and stroke work using pressure–volume loop analysis, compared to PAH patients with high cardiac reserve. Therefore, exercise unmasks significant RV stiffness with consequent pathological right–left ventricular interaction. Finally, imaging markers of fibrosis are observed in RV dysfunction; cardiac magnetic resonance imaging (MRI) studies show that elevation in RV free wall T1 signal, a marker of fibrosis, is strongly associated with reduced RV ejection fraction (RVEF) and higher PA stiffness [28]. Thus, human data demonstrate that an inflammation–fibrosis axis is associated with RV dysfunction.
Metabolic dysfunction and intervention
Metabolic dysregulation in RV dysfunction is well described, as several metabolic pathways are impaired or pathologically induced in failing RV cardiomyocytes. Multiple studies show interventions that restore or enhance fatty acid oxidation have favourable RV effects in rodents. This finding appears to have direct human relevance, as disrupted lipid metabolism is evident in human PAH, where there are lower levels of RV acylcarnitines, the lipid species metabolised by mitochondria to generate ATP [29]. In two rodent models of RVF, carnitine supplementation restores the RV acylcarnitine/free carnitine ratio and improves RV function [30]. Additionally, alternate-day fasting heightens AMP kinase (AMPK) activation, which prevents dysregulation of peroxisomal and mitochondrial fatty acid oxidation proteins in both MCT and SuHx rats. These molecular changes are associated with enhanced RV function, despite minimal changes in PAH severity [31]. Moreover, small molecule inhibition of With No Lysine kinase enhances AMPK signalling, combats downregulation of fatty acid oxidation proteins, restores RV acylcarnitine levels, prevents the accumulation of toxic ceramide lipids and augments RV–PA coupling in MCT rats [32]. Thus, multiple lines of evidence suggest that restoring RV fatty acid/lipid metabolism and the resultant suppression of lipotoxicity may augment RV function. Enhanced glycolysis may also contribute to RV dysfunction and recent work implicates overactive poly (ADP-ribose) polymerase 1 in the promotion of ectopic RV glycolytic gene expression [33]. The later section on “Update on management of chronic RV failure” presents further discussion of metabolic interventions for the RV.
Sex differences
Although PAH is a female-predominant disease, females exhibit superior RV function and adaption when compared to males [34, 35], and thus sex differences are an active area of investigation. Sex hormones are proposed to play a key role in these findings, and not surprisingly, there are divergent relationships between RV function and sex hormones in males and females. While both males and females with PAH have lower dehydroepiandrosterone-sulfate (DHEA-S) levels than controls, serum DHEA-S levels are only associated with RVEF in females. PAH females have lower total and free testosterone as compared to controls, but males with PAH do not have divergent testosterone levels. However, higher testosterone in males is associated with a lower RVEF [36].
In pre-clinical studies, the importance of oestrogen signalling via its intracellular signalling molecule oestrogen receptor-α is now documented. In human RV failure, oestrogen receptor-α, but not oestrogen receptor-β is downregulated, and oestrogen receptor-α protein levels are negatively correlated with RV function [37]. In rodents, 17β-oestradiol treatment activates oestrogen receptor-α, which stimulates bone morphogenetic protein receptor (BMPR)2 signalling, and improves RV function. However, rats that harbour a mutation in oestrogen receptor-α that reduces its levels by >90% do not increase cardiac output with 17β-oestradiol treatment to the same degree as wild-type animals [37], suggesting that oestrogen receptor-α drives a large portion of oestrogen's RV-protective effects. Using the same oestrogen receptor-α mutant rats, divergent effects of PA banding are observed in males and females. In particular, oestrogen receptor-α mutant females display RV–PA uncoupling and RV fibrosis, a finding that is absent in males [38].
Right atrial adaptation in PH
The RA in PAH patients faces an increased workload due to pressure overload because of RV diastolic stiffness [39] and volume overload due to tricuspid regurgitation and vena cava backflow [40]. In a recent study, the function of the RA in PAH was investigated in detail by combining pressure–volume, single cardiomyocyte function and histopathological analyses [41]. This study revealed that despite an increase in RA hypertrophy and contractility, atrial cardiomyocyte function was unaltered. However, there was increased fibrosis and capillary rarefaction. The authors identified the RA–RV coupling index (dividing RA minimal volume by RV end-diastolic volume) as a potential tool to quantify RA–RV coupling in clinics. One possible caveat of an RA volume to RV end-diastolic volume is that both parameters increase with maladaptation and could potentially pseudonormalise. However, future studies should reveal whether RA–RV coupling indeed has additive value and whether improving RA adaptation would be of clinical benefit.
In addition, it is recognised that the RA is the location of storage of several important proteins, such as bone morphogenetic protein (BMP)10, atrial natriuretic peptide and brain natriuretic peptides [42, 43]. Important information on triggers of secretion and impact on right heart adaptation are currently lacking. It is currently unclear whether atrial natriuretic peptide or BMP10 may provide more specific insights in RHF than the currently used NT-proBNP. This is especially of potential interest, as one of the main targets of sotatercept is BMP10. Therefore, a better understanding of RA adaptation and the RA secretome is essential for understanding the complete picture of right heart adaptation in PAH patients.
Adaptation versus maladaptation
The physiology describing adaptive to maladaptive hypertrophy is well described [44]; however, the underlying molecular changes that accompany or cause this transition remain elusive. Cartilage intermediate layer protein 1 has increased expression in the pressure-overloaded RV in rodents and in the plasma in PH patients with maladaptive RV function [45]. In a different vein of inquiry, Zelt et al. [46] compared RV remodelling in the SuHx model in two rat strains, one with maladaptive remodelling (Fischer) and one without (Sprague Dawley). They found increased oxidative metabolism in the Fischer strain and higher expression of adenylate kinase 1 in the failing RVs, which translates into reduced ATP shuttling from the mitochondria to the contractile fibres. This remains an area of active investigation and future study of the mechanistic role of these findings is required.
Effect of sotatercept on the RV
Sotatercept is a novel activin receptor IIa-Fc fusion protein. It inhibits activation of Smad2/3 mediated transforming growth factor (TGF)-β-signalling and thereby restores the BMP/TGF-β balance in PAH [47]. Although initial clinical results are promising and show large improvements in pulmonary vascular resistance (PVR) and RV remodelling [48], the effect on long-term RV function and adaptation is not clear. A recent phase 2b study (Sotatercept Phase 2 Exploratory Clinical Trial in PAH) by Waxman et al. [49], on exercise haemodynamics, provides more insights on the topic. The authors show that sotatercept significantly increases peak oxygen uptake with exercise with a systemic extraction ratio and workload. As discussed by the authors, if oxygen delivery increases with arterial oxygen content, cardiac output does not need to increase to meet metabolic demands. From a basic science perspective, the unchanged cardiac output could also suggest that BMP/TGF-β signalling may play a different role in RV adaptation. In addition, pre-clinical evidence exists that especially Smad3-mediated signalling in the pressure-overloaded heart is crucial for proper adaptation [50]. Inhibition of Smad3-mediated signalling in left ventricular pressure overload results in disturbed fibroblast–macrophage signalling, causing systolic maladaptation and accelerated progression to heart failure. Furthermore, prior work shows a potential role for BMPR2 mutation in promoting dysfunctional metabolism in RV cardiomyocytes [51], suggesting that pharmacological alteration of this and related TGF-β-signalling pathways may either favourably or unfavourably alter RV metabolism. Therefore, the effect of sotatercept on RV adaptation deserves deeper understanding. The absence of increase in cardiac output may be less clinically relevant in patients responding to sotatercept with a RV afterload reduction >40% and/or increase in peak oxygen consumption [52]. However, understanding the long-term effects of sotatercept on the RV, independent of the increase haemoglobin, in patients not responding to sotatercept is important from a basic science and clinical perspective.
Bridging translation: the emerging use of large animal models of RV dysfunction
Most pre-clinical mechanistic data for PAH-mediated RV dysfunction are gathered from rodent studies, but now bovine, ovine and porcine models of RV dysfunction are reported in the literature, with molecular phenotyping in two species. Physiological studies show there is impaired RV reserve in a porcine model of CTEPH [53], and sheep subjected to progressive PA banding exhibit a reduction in cardiac output and progressive RV fibrosis [54]. A magnetic resonance hyperpolarisation imaging study shows that PA banding induces disruptions in pyruvate metabolism prior to overt echocardiographic changes in RV function [55], highlighting the potential importance of disrupted metabolism as an early marker of RV dysfunction. In a calf model of hypoxia-mediated PH that causes severe PH, RV–PA uncoupling occurs, but cardiac output is preserved. Both transcriptomic and proteomic analyses demonstrate changes in the cellular hypertrophy, the cytoskeleton, inflammation and fibrosis pathways in the RV [56]. Finally, a combined transcriptomic and proteomics analysis comparing MCT rats, PA-banding pigs with reduced RVEF on cardiac MRI, and humans suggests that pigs exhibit a metabolic molecular signature that is comparable to human RV failure, but the severity of RV dysfunction and its relationship to the observed molecular changes needs to be examined further. However, there are also similarly dysregulated pathways in all three species, which highlights potential therapeutic targets that need further evaluation [57]. While there is hope that large-animal models may serve as important transitional models between rodents and humans for studying interventions against RV failure, this hypothesis is untested. We anticipate further integration of large-animal models in pre-clinical studies and testing of therapeutics in large-animal models to prioritise the most promising targets for RV-enhancing treatments in humans.
To summarise, there have been the following major advances in understanding the pathobiology of RV failure since the 6th WSPH:
1) Enhanced understanding of chronic inflammation in promoting RV dysfunction and the pathways that promote fibroblast activation and pathologic RV fibrosis.
2) Greater detail in the specific features of altered RV metabolism in RV dysfunction and failure; how these alterations may be targeted by metabolic interventions; and the molecular consequences of metabolic interventions.
3) Further exploration of the role of sex hormones including DHEA-S and testosterone in the RV in human PAH and understanding of the specific oestrogen receptors that mediate RV function.
4) Physiology and pathology studies have expanded knowledge of RA function in PAH and the role of tricuspid regurgitation.
5) There has been increasing interest in large animal models of RV dysfunction as these may serve as an intermediate step to bridge from rodent studies to human disease.
Evaluation of RV function with a focus on meaningful and reproducible metrics
Since the 6th WSPH [8], several advances have been made. These led to 1) novel insights on right heart physiology; 2) better delineation of reference values; 3) outcome-based grading systems for right heart adaptation; 4) establishing minimally important differences for imaging based end-points; and 5) early experience with deep learning initiatives for the right heart.
Insights on right heart physiology from coupling to stiffness and regional patterns of contraction
At the 6th WSPH [8], we made the distinction between “intrinsic” and coupled parameters. Intrinsic parameters describe RV function using less load-dependent measures, i.e. end-systolic or maximal ventricular elastance measured using pressure–volume loops. In contrast, the parameters of RV function commonly used in clinical practice (such as stroke volume (SV), RVEF, RV free-wall strain (RVFWS) and tricuspid annular plane systolic excursion (TAPSE) or its ratio with RV systolic pressure) measure RV coupling rather than intrinsic RV contractile status. Three important caveats need to be highlighted when assessing right heart parameters. First, not all metrics have the same sensitivity to increases in afterload. For example, a study by Tello et al. [58] suggested that RV myocardial strain was more closely associated with RV–PA coupling and RV end-diastolic stiffness. Second, in the context of significant tricuspid regurgitation, these coupled measures of RV systolic function overestimate effective contractile function [59, 60]. Third, following cardiac surgery, TAPSE or RVFWS do not reflect overall function in view of the disturbance of annular motion [61, 62].
Stiffening of the RV due to the mechanisms described earlier helps to ensure maintenance of the normal shape of the RV in the context of pressure overload, but can also impair inflow to the RV. This has important consequences for the RA and systemic venous pressure and congestion. Recent work shows that atrial contraction in the presence of a stiff RV is the main mechanism of reversal of flow in the vena cava [40] with adverse consequences for liver and kidney function. Furthermore, the “stiff” RV has direct structural and functional consequences for the RA which carry prognostic information [39]. It is recognised that uncoupling of the RA from the RV takes place in end-stage RV failure [63]. Of interest, in heart failure with preserved ejection fraction (HFpEF)-PH, the coupling between the RV and RA may be less compromised than in PAH, despite similar pressure-overload. Uncoupling of the RA to the RV together with RV dilatation leads to significant tricuspid regurgitation [59] with important pathophysiological consequences related to more volume but less pressure overload [63]. Volume and pressure overload also contribute to congestive hepatopathy, liver disease and renal venous congestion. This has led to a renewed interest in venous excess imaging in PH [64, 65].
Pressure–volume loop analysis has been instrumental in understanding global RV function in the presence of PH over the past 30 years. Pressure–volume loop-derived measures of ventricular elastance, end-systolic elastance (Ees)/arterial elastance (Ea) ratio, chamber stiffness and diastolic elastance are often used as the reference standards against which noninvasive parameters are compared [66, 67]. However, it is recognised that these global functional assessments are the average sum of contractility or stiffness patterns which vary largely from the apex to the tricuspid valve in PH [68]. In addition, ventricular interdependency exists as a consequence of a prolonged RV contraction time in comparison to the left ventricle [69]. As a result, the RV continues to contract while the pulmonary valve is closed, moving the septum leftward leading to the so called “post-systolic shortening” or isovolumetric post-systolic contraction. The authors anticipate that in the coming years we should arrive at a better description of RV function, taking into account the heterogeneity of regional myocardial performance in relation to volume change (example image in figure 2). Technical advances in four-dimensional flow measurements and strain imaging might provide a more complete picture of RV work.
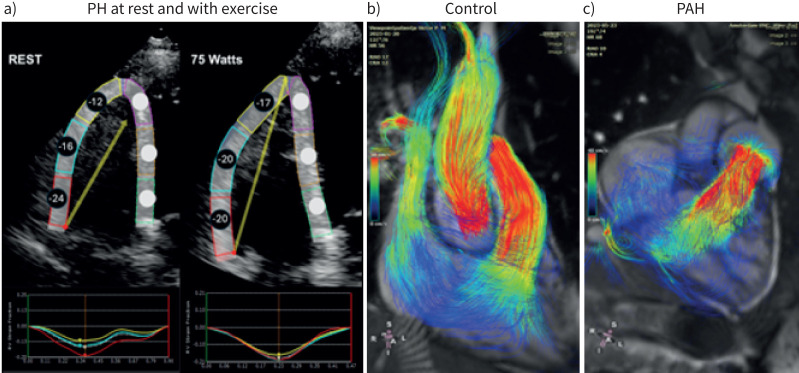
FIGURE 2.
Novel right ventricular (RV) imaging in pulmonary hypertension. a) RV longitudinal strain patters demonstrate regional heterogeneity. A 65-year-old female with scleroderma presenting with undifferentiated dyspnoea underwent supine bicycle echocardiography. Rest images demonstrate a heterogeneous pattern of strain-derived regional contractility [70], tricuspid annular plane systolic excursion (TAPSE) 2.12 cm, normal RV systolic pressure (RVSP). With 75 W of exercise, the basal segment does not augment while there is increase in regional contractility of the midventricular and apical segments, TAPSE 1.53 cm, associated with chamber dilatation (increase in RV end-diastolic area and end-systolic area, increase midventricular dimension) and RVSP 58 mmHg [71]. Echocardiography-based strain imaging on exercise is technically difficult and not at this stage recommended for standard clinical use. Magnetic resonance imaging four-dimensional flow measurements in b) a healthy control and c) a patient with pulmonary arterial hypertension (PAH). In the healthy person only forward flow and the RV serves as a bellow in which all parts of the RV contribute to the flow movement. In PAH the contribution of the apical region to forward flow is minimal, indicating the heterogeneous contribution of the RV to power output. In addition, the flow is not linear in the atrium and vortex flow can be observed in the pulmonary artery.
Defining reference values for the right heart
Over the past 5 years, there have been concerted efforts to better define reference values for the right heart. The World Alliance Societies of Echocardiography delineated reference values for RV size, systolic function and diastolic parameters using multicentre and diverse cohorts [72–74]. These new reference values will be integrated in the upcoming American Society of Echocardiography and European Association of Cardiovascular Imaging guidelines on the assessment of the right heart. Reference ranges for cardiovascular magnetic resonance imaging (CMR) in adults and children also addressing right heart and pulmnonary artery values were published in 2020 [75].
Outcome-based grading systems for right heart dysfunction in PAH
Reference values alone are not sufficient to grade RV dysfunction or adaptation as, ideally, these should be guided by outcome analysis. Using the Assessing the Spectrum of Pulmonary Hypertension Identified at a Referral Center (ASPIRE) registry, Lewis et al. [76] identified thresholds for SV, ejection fraction and RV volumes that were associated with 1-year survival. In their study, a RVEF <37%, between 37% and 54% and >54% identified high, intermediate and low risk of decreased survival, respectively, in PAH [76]. Based on this study, these thresholds are now incorporated in the 2022 European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines for PH [77]. A more recent study by Celant et al. [78] confirmed the importance of right heart MRI measures, but found different thresholds for RVEF corresponding to 45% and 20%, respectively. These different thresholds are probably attributable to differences in post-processing methodology where trabeculations are excluded from volumes in the study by Lewis et al. [76], but included in the study by Celant et al. [78]. This highlights the importance of standardising post-processing protocols or using appropriate method-specific thresholds. In parallel to the MRI studies, Celestin et al. [79] validated the ESC/ERS guideline thresholds for echocardiographic metrics and further proposed a consistent grading system for different parameters of RV and RA remodelling and function [79, 80].
The road from individual metrics to right heart adaptation profiles
The multiplicity of indices measuring RV function leads to a key question: how can clinicians best select the most informative and reproducible indices? The question is not about choosing the best single metric, but rather about finding the best combination of metrics for clinical care. Unsupervised learning and association studies have highlighted that right heart parameters are closely intertwined with NT-proBNP displaying a more central connectivity [81, 82]. Therefore, combining coupled or integrated measures of the right heart with biomarkers such as NT-proBNP may overcome limitations of individual markers, shifting the focus to adaptation profile rather than isolated parameters. Several studies in recent years have focused on identifying profiles of ventricular adaptation. For example, a study by Ghio et al. [83] found that combining information from TAPSE, tricuspid regurgitation severity and inferior vena cava size may help better define right heart adaptation profiles. Fine et al. [84] showed that RV myocardial strain and NT-proBNP levels may carry significant prognostic information in PH. More recently, the Registry to Evaluate Early and Long-Term PAH Disease Management echocardiographic risk score (REVEAL-ECHO) combined four echocardiographic markers (RV enlargement, function, tricuspid regurgitation and pericardial effusion) with aetiology to derive a prognostic score in PAH. As the authors discussed, this may help reclassify patients in low or intermediate risk to higher risk categories [85].
Defining right heart reverse remodelling in PAH
Since the last symposium, multicentre studies and registries have identified meaningful thresholds for RV reverse remodelling (RVRR) in PAH. The Right Ventricular Remodelling in Pulmonary Arterial Hypertension (REPAIR) study, which evaluated the effect of macitentan on RV and haemodynamic outcomes in patients with PAH used SV as a pre-defined primary end-point [86]. The authors found that SV increased on average by 12 mL by week 26 and was also associated with improvement of several other right heart remodelling and functional parameters.
More recently, using the ASPIRE registry, Alabed et al. [87] identified minimally important differences for cardiac MRI end-points in PAH. Using three classifiers (emPHasis-10, walking distance and mortality) and four methods (two distribution-based and two anchor-based), the authors identified a change in RVEF of 5%, and a change of RV end-diastolic and end-systolic volumes of 17 mL as indicative of improvement [87].
Similar, smaller, studies were conducted using echocardiography. In an initial study in 102 PAH patients, after 1 year of monotherapy, RVRR arbitrarily assessed by decreased two-dimensional echocardiography RV end-diastolic area, RA area and left ventricular eccentricity index was associated with a 94% survival rate at 2 years, compared to 43% in patients without RVRR [88]. In a subsequent study in 69 idiopathic (I)PAH patients treated with initial combination of drugs compared with matched monotherapy controls, when RVRR with an improved event-free survival was achieved, PVR decreased by >40% [89]. The likelihood of RVRR was related to percentage decrease in PVR in a sigmoid-shape fashion, with rapidly increasing rate of RVRR with every additional decrease in PVR by >50%, and associated improvement in risk scores [90].
In the REPAIR study, the selection of SV was guided by the fact that SV is a strong prognostic marker at baseline and follow-up [9, 91, 92], and, as it can be measured invasively and noninvasively, offers a metric that is already available in clinical practice. If SV is combined with the volumetric state of the RV (SV/end-systolic volume (ESV)), prognostic significance may further increase [39, 93–97]. If volumetric changes of the RV are related to pressure, this reflects myocardial performance or work, which is defined as pressure × volume, and reflected as the area of the pressure–volume loop. The combinations that reflect RV work include among others: TAPSE/systolic pulmonary artery pressure (sPAP); RV-ESV (% predicted) and PA pulsatility [93]; and RVFWS. In studies including diastolic parameters of RV function, it was found that RA pressure is an independent measure of prognosis [9, 97, 98].
The rise of automated analysis of the right heart
Based on recent publications and technological advances, we foresee that machine learning and deep learning will play an important role for clinical phenotyping, both segmental and nonsegmental image analysis, disease classification and prediction of outcome as well as quality assurances [93, 99–102]. These innovations will be developed in further details in other task forces [103].
Table 1 summarises some of the open questions that are likely to be addressed in future.
TABLE 1.
Selected open questions or future directions for the assessment of the right heart
| Right heart diastolic performance | What are the best metrics that define right heart diastolic performance? More recently, right atrial strain and volume measures or peak lateral tricuspid annulus systolic velocity/right atrial area index ratio have emerged as promising. In addition, a greater emphasis is placed on systemic venous volume, e.g. portal, hepatic and renal venous flow patterns. |
| Integrated right heart profiles | Can we further develop better metrics (multimodality) of right heart adaptation in PH that integrate systolic and diastolic performance? |
| Diagnostic algorithms | Which parameters of right heart structure function best inform diagnostic algorithms in PH? The focus should shift to early detection of PH and multimodality approaches. |
| Prognostic algorithms | How do imaging metrics best complement clinical prognostic scores? |
| Surrogate end-point research | Validate in different cohorts the most robust surrogate end-point for PH research. Efforts will also evolve toward defining consistent right heart response profiles in PH. |
| Indexing of right heart measures | Validate the optimal scaling metrics for right heart dimensions and function beyond body surface area. These metrics may include parameters of body mass, height, age and sex as well as cardiac time periods and physical activity levels. Moreover, indexing may require different scaling depending on the measures, i.e. volumes, areas, linear dimensions. |
| Standardisation of right heart measures | How best to define the diastolic and systolic phase to measure both right atrium and ventricle size? This may be particularly challenging in the presence of abnormal septal motion. Can we define a more informative internal reference, e.g. centre line, for the RV that takes into account the geometrical complexity of the RV? Should measurements be performed at the compacted or noncompacted region, and should volumes always be inclusive of trabecular muscle? How best to ensure reliable implementation of three-dimensional right heart echocardiography in routine clinical practice? |
| Reporting | Can we develop a consistent grading system for right heart dysfunction? Can we improve reporting for the right heart by ensuring comments on pulmonary flow profiles including pulmonary regurgitation, septal curvature and geometry of the heart? Can we standardise/integrate comments on image quality? Understand how imaging metrics require contextualisation based on individual patient characteristics. |
| Defining reference change values | How best to define meaningful changes for right heart measures considering analytic and biological variation? |
| Molecular imaging | Can we use right heart imaging profiles to gain better insights on mechanism of right heart adaptation? Imaging would benefit from molecular and tissue characterisation-based technology. This can be also useful to test specific targeted therapeutics. |
| Deep learning | How best to ensure PRIME checklist for deep learning development? How best to implement right heart-related deep learning in practice? |
| Right heart-specific targets to combat RV failure | What molecular targets are the most promising to allow for right heart specific effects? How will we stratify RV physiological (evaluating right atrial and RV systolic function, diastolic function and volume changes) or molecular (metabolic disruptions or pro-inflammatory/fibrotic changes) to enrich populations for new interventions in the future? |
RV: right ventricle; PH: pulmonary hypertension; PRIME: Proposed Requirements for Cardiovascular Imaging-Related Machine Learning Evaluation.
RV phenotypes in different PAH subtypes or different WSPH groups
There is wide variation in RV structure and function based on age, sex and race/ethnicity among healthy individuals [72, 73, 104–108], and also in the context of specific diseases within the WSPH classification. These variations may be attributable to the aetiology and onset of increased pulmonary vascular load (e.g. congenital heart disease (CHD) [109] versus development in adulthood), nutritional [108] and environmental exposures [110], pre-load versus afterload, intrinsic cardiac disease versus disease of the pulmonary vasculature, and disease-specific characteristics. Here we review updated understanding of specific typical RV phenotypes in different forms of PH with representative images for each subtype shown in figure 3, while recognising that individual patients may not fit these typical morphological or clinical phenotypes, and therefore full diagnostic evaluation is always warranted.
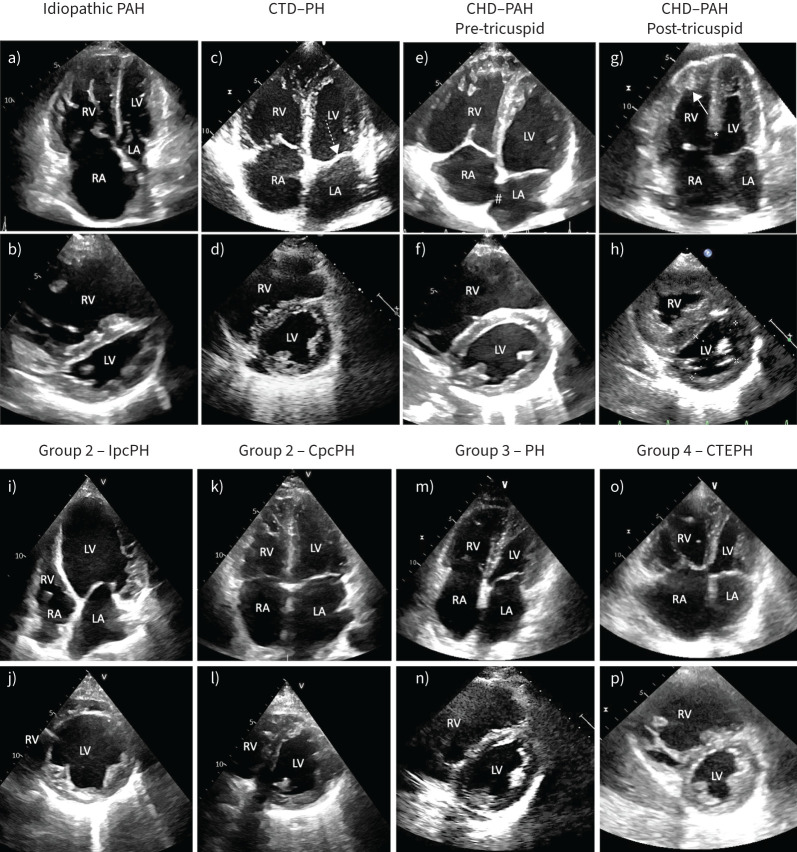
FIGURE 3.
a–h) While distinguishing types of pulmonary hypertension (PH) based on echocardiography is challenging and depends on the stage of disease, presented here are example images of echocardiographic features of PH subtypes. a) and b) Severe idiopathic pulmonary arterial hypertension (PAH) with a) marked dilation of the right chambers and b) flattening of the interventricular septum, with a squeezed left ventricle (LV) (eccentricity index >1.5). Due to the prominence of the right chambers, this may be described as “right phenotype”. c) and d) PH associated with connective tissue disease (CTD). Patients show prominence of the right chambers, but may have thickening of the mitral (dashed arrow) or the aortic valve and a certain degree of LV diastolic dysfunction. e) and f) PAH associated with an atrial septal defect (#) demonstrating enlarged right atrium (RA) and right ventricle (RV). g) and h) PAH associated with a ventricular septal defect (*) in natural history (Eisenmenger syndrome). Typically, these patients show severe right ventricular hypertrophy (arrow) with a normal or only mildly dilated right ventricle. i–p) Typical echocardiographic features of group 2, 3 and 4 PH. i) and j) Typical remodelling of a patient with heart failure and reduced ejection fraction with isolated post-capillary PH (IpcPH) (“left phenotype”). There is a marked prevalence of the left chambers (g) and a round-shaped left ventricle (eccentricity index 1). k) and l) Patients with combined post- and pre-capillary PH (CpcPH) show an intermediate phenotype (“neither right nor left”) with i) bi-atrial dilation and left ventricle prevalence, but j) initial flattening of the interventricular septum. m) and n) Patients with PH associated with lung disease have usually a prevalence of the right chambers (“right phenotype”), but they are often older than PAH patients and may have a component of left-heart involvement. o) and p) Patients with chronic thromboembolic PH (CTEPH) have a “right phenotype” indistinguishable from PAH patients. Nevertheless, some may have by chance a left-heart disease and features of left heart involvement. CHD: congenital heart disease; LA: left atrium.
RV phenotypes in CHD-related PH
In patients with CHD, the RV generally adapts to increased load early in fetal life, similar to normal individuals with significant hypertrophy of the RV and better RV–PA coupling; however, this adaptation may be maintained post-natally in many forms of CHD-PAH over several decades compared to other forms of PAH, depending on early correction (e.g. ventricular septal defect) [111]. Therefore, it is not surprising that the RV in CHD-associated PAH can differ substantially from idiopathic or other types of PAH. A broad variety of underlying congenital lesions can predispose to PH and ultimately define the resultant RV changes [112]. These include left-to-right shunts, left heart disease, post-operative complications in which pulmonary blood flow has been augmented surgically or a combination of these features.
Underlying this observation is normal physiology at the time of birth: in normal individuals, the PA pressure falls to normal levels in the 24 post-natal hours and pre-natal RV hypertrophy regresses to the “normal” phenotype. However, in some forms of CHD RV afterload remains high after birth, and the RV does not “atrophy” post-natally. For example, in transposition of the great arteries (TGA), or with valvular pulmonary stenosis, the RV remains at high pressure after birth and so never “detrains”, retaining its muscularity and systolic function, with a systemic level afterload. In fact, in congenitally corrected TGA, the RV is the subaortic ventricle and can persist without failure for eight decades or more [113]. The molecular mechanisms underpinning this phenomenon are undefined, although some key proteins have been implicated [114].
Other features of CHD can define RV phenotypes including abnormal RV volume and/or pressure, abnormal interventricular septal motion, or both coexisting [112]. Thus, in CHD, the phenotype of the RV depends predominantly on whether that ventricle is mainly volume or pressure loaded, and from what age. Where the volume load is predominant, for example in atrial septal defect, the RV dilates and may develop eccentric hypertrophy from the volume load, before developing a pressure load. However, in ventricular septal defect, where the RV volume and pressure are both much higher than normal from birth, there is simultaneous chamber dilatation and concentric hypertrophy. Thus, the RV phenotype depends exquisitely on the haemodynamic loading conditions of the RV, which may vary greatly according to the size and location of the underlying CHD.
RV in connective tissue disease associated PAH
Scleroderma (SSc)-associated PAH is the most common form of PAH with connective tissue disease (CTD) in the Western world, although systemic lupus erythematosus-associated PAH is a much more common cause of CTD-PAH in China [115]. Patients with SSc-PAH are at the opposite end of RV adaptation compared to CHD-PAH or IPAH. They have reduced intrinsic myocardial contractility (both decreased end-systolic elastance and cardiomyocyte contractility), increased RV fibrosis and depressed RV–PA coupling compared with patients with IPAH, despite having seemingly less severe PAH compared to patients with IPAH [116, 117]. In response to submaximal exercise, SSc-PAH patients fail to augment RV contractility, and, with increasing afterload, RV–PA coupling declines even further compared to baseline. In contrast, patients with IPAH can augment myocardial contractility (Ees) and improve coupling (Ees/Ea) with similar levels of exercise [118].
RV in CTEPH
The RV in CTEPH on average is marginally more dilated, stiffer and less hypertrophic than in PAH at similar load levels [119]. In addition, CTEPH patients with distal disease have slightly better RV function than patients with more proximal disease, despite having similar PVR and compliance [120]. An explanation for this observation was provided by Fukumitsu and co-workers [121, 122] who showed that the early return of reflected waves in more proximal disease created higher RV wall stress than late arrival of reflected disease as is the case in distal CTEPH or IPAH. This abnormal increased RV wall stress in CTEPH caused by proximal pulmonary vascular obstructions might also induce abnormal stiffness of the RV [119]. Of interest, it was found that even after pulmonary endarterectomy there is an incomplete recovery of the RV together with impaired peripheral oxygen uptake [123–126].
RV in group 2 PH
RV function predicts outcome in left heart failure [126]. This was previously shown by isotopic or thermodilution curve-derived ejection fraction measurements [127, 128] and more recently confirmed by the echocardiography of TAPSE or longitudinal strain to sPAP ratios [129, 130], or semi-quantitative visual assessment integrating these variables [131]. The prediction of outcome in these studies is not different in heart failure with preserved, mid-range or reduced left ventricular ejection fraction (HFpEF, HFmrEF and HFrEF, respectively) [129–131] and, interestingly, only loosely related to the level of PAP [128–131], even though a stronger impact of PAP on decreased TAPSE is observed in HFpEF and HFmrEF [130]. PH secondary to heart failure, isolated post-capillary PH or combined pre- and post-capillary PH is in itself a potent predictor of survival in heart failure [126, 132], underscoring the importance of excessive afterload as a determinant of RV failure in heart failure patients.
Another major determinant of RV failure in heart failure is ventricular interaction. The RV and the left ventricle are encircled by common fibres and share the septum within a nonacutely distensible pericardium, resulting in systolic and diastolic interdependence [133]. This may explain observations of preserved RV–PA coupling in the presence of relatively higher PAP in HFpEF or HFmrEF than in HFrEF [130, 134]. However, there is overlap. Depressed RV–PA coupling in the presence of marginally increased PAP was reported in experimental models of both HFrEF and HFpEF [135, 136]. In vitro RV sarcomere contractility in patients with HFpEF is reported to be either preserved or depressed [137, 138]. These discrepancies can be explained by HFpEF as an inhomogeneous entity with variable impact of comorbidities including ageing, obesity, hypertension, chronic lung or kidney diseases, atrial fibrillation and, importantly, associated systemic inflammatory responses as a cause of myocardial stiffening and eventual failure [139, 140].
A hallmark of the diagnosis of heart failure is pulmonary congestion, but associated RV failure may result in a predominantly systemic congestion, defining the so-called right heart phenotype [139]. Associated increase in systemic venous pressures has negative effects on renal and hepatic function, which in turn contributes to worsen survival [65, 141–143]. The mortality rate of the right heart phenotype in heart failure as assessed by an echocardiographic examination is particularly high, reaching 40% at 1 year [131].
Pulmonary congestion in heart failure may be disclosed by a fluid challenge or an exercise stress test. Exercise-induced pulmonary congestion in heart failure was recently reported to be worse in patients with combined pre- and post-capillary PH than in those with isolated post-capillary PH in proportion of altered indices of RV function, suggesting a possible contribution of impaired lung lymphatic drainage due to increased central venous pressure [144]. Similar results were reported in heart failure patients after a fluid challenge [145], confirming the apparent paradox of RHF as a cause of increase in lung water content in heart failure.
Lung ultrasound assessment of pulmonary congestion may be of added value to echocardiographic prediction scores for the differential diagnosis between PAH and combined pre- and post-capillary PH, but still with limited specificity as predominant enlargement of right heart chambers may also occur in HFpEF and some congestion detected in PAH [145]. Specificity of echocardiography combined with lung ultrasound for the diagnosis of PAH versus combined pre- and post-capillary PH is improved by repetition of the measurements after a fluid challenge [145].
Exercise stress testing may also identify latent RV failure in heart failure with either invasive measurements of the ratio of end-systolic to arterial elastances (Ees/Ea) or echocardiography to assess RV–PA coupling [146–148]. In these studies, the Ees/Ea ratio normally increases in controls, but is unchanged or decreased in heart failure patients, with a further negative impact of increased PVR, but a better preservation of RV function in female patients [146, 147]. Similar results were reported with echocardiographic measurements of fractional area change, S-wave or TAPSE to sPAP ratios, which were most decreased in the patients with a high PVR who also developed pulmonary congestion, altered gas exchange, negative ventricular interaction and worse peak oxygen uptake [147]. Multicentre registries are being reported showing the feasibility of entirely noninvasive exercise echocardiographic measurements of both the pulmonary circulation and RV function, disclosing prognostically relevant differences between healthy subjects and patients with different types of PH [148].
The RV in chronic lung diseases
The diagnosis of a right-sided phenotype of heart failure in patients with chronic lung diseases emerged along with the introduction of cardiac catheterisation in clinical practice in the 1940s. This was addressed by a consensus conference sponsored by the World Health Organization in 1963. The report heavily relied on a pathological definition of “cor pulmonale” as a RV hypertrophy resulting from diseases which affect the structure or function of the lungs [149]. This (mostly) post-mortem definition proved impractical, so cor pulmonale became better understood as RV dilatation and hypertrophy, in response to increased PAP caused by lung diseases, disordered ventilation or hypoxic exposure [150]. The term “cor pulmonale” was abandoned at the 4th WSPH in 2008 because of perceived unconvincing clinical relevance [151], but is still used in respiratory medicine [152].
Radionuclide angiographic studies in the 1970s and 1980s showed that RVEF is depressed in approximately half of COPD patients, and that this proportion increases at exercise with typical blunting of the increase in RVEF normally seen in healthy subjects [153]. Patients with COPD and similar PAP, right atrial pressure and PVR present with a lower isotopic RVEF in case of systemic congestion symptomatology [154]. In fact, echocardiographic studies confirmed that RV function may be markedly depressed with decreased indices of systolic function, dyssynchrony and increased dimensions in patients with either COPD or idiopathic pulmonary fibrosis (IPF) and only mildly increased PAP or PVR [155–157]. This disconnect between RV function and PH has been tentatively explained by the effects of systemic inflammation, hypoxaemia, hypercapnia, pulmonary hyperinflation and hormonal disturbances [155, 156].
There was a report of cardiac MRI of decreased RV volumes in proportion to the severity of airflow obstruction and emphysema in large cohorts of COPD patients, fuelling a notion of “cor pulmonale parvus” [158]. A smaller RV in early outpatient COPD could be possibly explained by increased intrathoracic pressures on dynamic hyperinflation impeding systemic venous return along with some degree of RV hypertrophy in the context of only mildly increased PAP [159]. Whether cor pulmonale parvus may be an early stage of “true” cor pulmonale or RHF remains uncertain.
A decreased isotopic RVEF is associated with a shortened life expectancy in COPD [160]. This was subsequently confirmed by echocardiographic assessments of RV structure and function in both COPD and IPF [131, 152, 161, 162]. The life expectancy of RHF is shorter in respiratory diseases than in heart failure, CTEPH or PAH [131, 162].
Update on management of chronic RV failure
Despite an abundance of basic and clinical data on the molecular and physiological changes that underlie RHF, there are no specific therapies presently available. While management of RV failure in the critical care setting is discussed elsewhere, here we discuss new knowledge on RV-targeted interventions in the setting of chronic RHF. As discussed earlier, extensive basic science data coupled with available drugs to target these molecular signalling pathways have driven interest in metabolic interventions to improve RV function in humans.
Two trials were published that highlight important ongoing controversies including whether fatty acid oxidation suppression in the RV is compensatory or a further detriment to RV function. Ranolazine, a drug with pleotropic actions including partial suppression of fatty acid oxidation, was evaluated in pre-capillary PH with RV dysfunction. In this pilot study of ranolazine (n=9) versus placebo (n=6), ranolazine increased RVEF as measured by cardiac MRI [163] and was accompanied by a borderline decrease in RV glucose uptake [164]. In contrast, metformin, which also has multiple pharmacological effects including enhanced fatty acid oxidation, increased RV fractional area change as a secondary end-point in a pilot study of 20 subjects with PAH [165]. Further study of both drugs is necessary before recommendations for their use could be confidently made. Whether glucagon-like peptide-1 agonists or sodium-glucose contrasporter-2 inhibitors, shown to be efficacious in HFpEF, modulate RV metabolism or improve RV function, is presently unknown.
Tricuspid regurgitation presents in approximately one-third of patients with PAH and recent work suggests that this is the result of altered RV structure and function and contributes to excess mortality [166, 167]. The natural question arising from these newer data on tricuspid regurgitation is whether there may be a role for tricuspid valve interventions in severe tricuspid regurgitation in pre-capillary PH [63]. While randomised trial data are lacking, there are some observational studies that suggest that individuals with global RV dysfunction compared with longitudinal RV dysfunction and those with both low TAPSE and RVEF ≤45% have worse outcomes with transcatheter tricuspid valve repair [168]. Others have begun to identify “proportional tricuspid regurgitation” to the degree of PH. In this framework, in subjects undergoing transcatheter tricuspid valve interventions, the presence of tricuspid regurgitation proportionate to the degree of elevation in PA pressure was a significant predictor of 2-year mortality. None of these studies specifically assessed pre-capillary PH, thus their extrapolation to this population is limited; however, these data do suggest potential phenotypes may benefit from or be at risk for harm from tricuspid regurgitation interventions.
Translating new knowledge to the care of patients
Current insights show that the RV can recover to a great extent if the loading conditions of the RV are reduced by >40–50% [3]. However, even in the current treatment era such a reduction in load may not be achievable in all patients suffering from PH. In addition, different treatments might have different physiological consequences. Whereas the “classical” PAH drugs might act as volume responders by increasing SV, upcoming treatments like sotatercept might act more as a pressure responder, by substantially reducing RV load [48]. This different RV response on a reduction of pulmonary vascular load might be attributed to the effect that sotatercept does not seem to alter systemic vascular resistance and does not induce a reflex increase in SV mediated by the baroreceptor, but direct effects of the drug on the RV cannot be excluded yet. For this we propose the following.
The impact of potential drugs in PAH should be appropriately tested on the RV in translational research including adequate animal models and in isolated cell models.
Imaging of the RV/right heart is considered highly desirable in all phase 2 and 3 trials.
Monitoring of patients during their treatment should include RV metrics as outlined herein and improvement of RV function should be considered as one of the primary treatment goals in PH to achieve long-term survival.
Multimodal imaging platforms should be initiated/enhanced to come up with optimised strategies to monitor our patients and treatment effects.
Shareable PDF
Footnotes
Conflict of interest: A.R. Hemnes reports grants from NIH/NHLBI, consultancy fees from Bayer, Gossamer Bio, Merck, Janssen, United Therapeutics and Tenax, participation on a data safety monitoring board or advisory board with NIH/NHLBI, leadership roles with Nashville Ballet and Pulmonary Vascular Research Institute, and stock (or stock options) with Tenax Therapeutics. D.S. Celermajer has no potential conflicts of interest to disclose. M. D'Alto reports consultancy fees and payment or honoraria for lectures, presentations, manuscript writing or educational events from Merck Sharp and Dhome, Dompé, AOP and Janssen, and support for attending meetings from Dompé, AOP and Janssen. F. Haddad reports grants from Johnson & Johnson, and consultancy fees from Merck. P.M. Hassoun reports grants from NIH/NHLBI (R01 R01HL114910), and participation on a data safety monitoring board or advisory board with MSD and ARIA-CV. K.W. Prins reports grants from NHLBI (R01 HL158795 and 162927) and Bayer (PHAB grant), and consultancy fees from Edwards. R. Naeije reports consultancy fees from AOP Orphan Pharma, Johnson & Johnson, United Therapeutics and Lung Biotechnology, payment or honoraria for lectures, presentations, manuscript writing or educational events from AOP Orphan Pharma, support for attending meetings from AOP Orphan Pharma, and participation on a data safety monitoring board or advisory board with Johnson & Johnson, AOP Orphan Pharma and United Therapeutics. A. Vonk Noordegraaf reports payment or honoraria for lectures, presentations, manuscript writing or educational events from Actelion and Johnson & Johnson.
This article has an editorial commentary: https://doi.org/10.1183/13993003.01222-2024
References
- 1.Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol 2017; 69: 236–243. doi: 10.1016/j.jacc.2016.10.047 [DOI] [PubMed] [Google Scholar]
- 2.Lahm T, Douglas IS, Archer SL, et al. Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med 2018; 198: e15–e43. doi: 10.1164/rccm.201806-1160ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanz J, Sánchez-Quintana D, Bossone E, et al. Anatomy, function, and dysfunction of the right ventricle: JACC state-of-the-art review. J Am Coll Cardiol 2019; 73: 1463–1482. doi: 10.1016/j.jacc.2018.12.076 [DOI] [PubMed] [Google Scholar]
- 4.Leopold JA, Kawut SM, Aldred MA, et al. Diagnosis and treatment of right heart failure in pulmonary vascular diseases: a National Heart, Lung, and Blood Institute Workshop. Circ Heart Fail 2021; 14: e007975. doi: 10.1161/CIRCHEARTFAILURE.120.007975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naeije R, Richter MJ, Rubin LJ. The physiological basis of pulmonary arterial hypertension. Eur Respir J 2022; 59: 2102334. doi: 10.1183/13993003.02334-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tello K, Naeije R, de Man F, et al. Pathophysiology of the right ventricle in health and disease: an update. Cardiovasc Res 2023; 119: 1891–1904. doi: 10.1093/cvr/cvad108 [DOI] [PubMed] [Google Scholar]
- 7.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013; 62: Suppl. 25, D22–D33. doi: 10.1016/j.jacc.2013.10.027 [DOI] [PubMed] [Google Scholar]
- 8.Vonk Noordegraaf A, Chin KM, Haddad F, et al. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J 2019; 53: 1801900. doi: 10.1183/13993003.01900-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Annal Intern Med 1991; 115: 343–349. doi: 10.7326/0003-4819-115-5-343 [DOI] [PubMed] [Google Scholar]
- 10.D'Armini AM, Zanotti G, Ghio S, et al. Reverse right ventricular remodeling after pulmonary endarterectomy. J Thorac Cardiovasc Surg 2007; 133: 162–168. doi: 10.1016/j.jtcvs.2006.08.059 [DOI] [PubMed] [Google Scholar]
- 11.Hassoun PM. Pulmonary arterial hypertension. N Engl J Med 2021; 385: 2361–2376. doi: 10.1056/NEJMra2000348 [DOI] [PubMed] [Google Scholar]
- 12.Houston BA, Brittain EL, Tedford RJ. Right ventricular failure. N Engl J Med 2023; 388: 1111–1125. doi: 10.1056/NEJMra2207410 [DOI] [PubMed] [Google Scholar]
- 13.Ichimura K, Boehm M, Andruska AM, et al. 3D imaging reveals complex microvascular remodeling in the right ventricle in pulmonary hypertension. Circ Res 2024; 135: 60–75. doi: 10.1161/CIRCRESAHA.123.323546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowler ED, Hauton D, Boyle J, et al. Energy metabolism in the failing right ventricle: limitations of oxygen delivery and the creatine kinase system. Int J Mol Sci 2019; 20: 1805. doi: 10.3390/ijms20081805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suen CM, Chaudhary KR, Deng Y, et al. Fischer rats exhibit maladaptive structural and molecular right ventricular remodelling in severe pulmonary hypertension: a genetically prone model for right heart failure. Cardiovasc Res 2019; 115: 788–799. doi: 10.1093/cvr/cvy258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JF, Clark VR, Banerjee S, et al. Transcriptomic analysis of right ventricular remodeling in two rat models of pulmonary hypertension: identification and validation of epithelial-to-mesenchymal transition in human right ventricular failure. Circ Heart Fail 2021; 14: e007058. doi: 10.1161/CIRCHEARTFAILURE.120.007058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prisco SZ, Hartweck LM, Rose L, et al. Inflammatory glycoprotein 130 signaling links changes in microtubules and junctophilin-2 to altered mitochondrial metabolism and right ventricular contractility. Circ Heart Fail 2022; 15: e008574. doi: 10.1161/CIRCHEARTFAILURE.122.009570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Qazazi R, Lima PDA, Prisco SZ, et al. Macrophage-NLRP3 activation promotes right ventricle failure in pulmonary arterial hypertension. Am J Respir Crit Care Med 2022; 206: 608–624. doi: 10.1164/rccm.202110-2274OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vang A, da Silva Gonçalves Bos D, Fernandez-Nicolas A, et al. α7 Nicotinic acetylcholine receptor mediates right ventricular fibrosis and diastolic dysfunction in pulmonary hypertension. JCI Insight 2021; 6: e142945. doi: 10.1172/jci.insight.142945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian L, Wu D, Dasgupta A, et al. Epigenetic metabolic reprogramming of right ventricular fibroblasts in pulmonary arterial hypertension: a pyruvate dehydrogenase kinase-dependent shift in mitochondrial metabolism promotes right ventricular fibrosis. Circ Res 2020; 126: 1723–1745. doi: 10.1161/CIRCRESAHA.120.316443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omura J, Habbout K, Shimauchi T, et al. Identification of long noncoding RNA H19 as a new biomarker and therapeutic target in right ventricular failure in pulmonary arterial hypertension. Circulation 2020; 142: 1464–1484. doi: 10.1161/CIRCULATIONAHA.120.047626 [DOI] [PubMed] [Google Scholar]
- 22.Nayakanti SR, Friedrich A, Sarode P, et al. Targeting Wnt-β-catenin-FOSL signaling ameliorates right ventricular remodeling. Circ Res 2023; 132: 1468–1485. doi: 10.1161/CIRCRESAHA.122.321725 [DOI] [PubMed] [Google Scholar]
- 23.Boehm M, Tian X, Mao Y, et al. Delineating the molecular and histological events that govern right ventricular recovery using a novel mouse model of pulmonary artery de-banding. Cardiovasc Res 2020; 116: 1700–1709. doi: 10.1093/cvr/cvz310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prins KW, Archer SL, Pritzker M, et al. Interleukin-6 is independently associated with right ventricular function in pulmonary arterial hypertension. J Heart Lung Transplant 2017; 37: 376–384. doi: 10.1016/j.healun.2017.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boucherat O, Yokokawa T, Krishna V, et al. Identification of LTBP-2 as a plasma biomarker for right ventricular dysfunction in human pulmonary arterial hypertension. Nat Cardiovasc Res 2022; 1: 748–760. doi: 10.1038/s44161-022-00113-w [DOI] [PubMed] [Google Scholar]
- 26.Khassafi F, Chelladurai P, Valasarajan C, et al. Transcriptional profiling unveils molecular subgroups of adaptive and maladaptive right ventricular remodeling in pulmonary hypertension. Nat Cardiovasc Res 2023; 2: 917–936. doi: 10.1038/s44161-023-00338-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cubero Salazar IM, Lancaster AC, Jani VP, et al. Poor cardiac output reserve in pulmonary arterial hypertension is associated with right ventricular stiffness and impaired interventricular dependence. Eur Respir J 2024; 64: 2400420. doi: 10.1183/13993003.00420-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jankowich M, Abbasi SA, Vang A, et al. Right ventricular fibrosis is related to pulmonary artery stiffness in pulmonary hypertension: a cardiac magnetic resonance imaging study. Am J Respir Crit Care Med 2019; 200: 776–779. doi: 10.1164/rccm.201903-0580LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brittain EL, Talati M, Fessel JP, et al. Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hypertension. Circulation 2016; 133: 1936–1944. doi: 10.1161/CIRCULATIONAHA.115.019351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agrawal V, Hemnes AR, Shelburne NJ, et al. l-Carnitine therapy improves right heart dysfunction through Cpt1-dependent fatty acid oxidation. Pulm Circ 2022; 12: e12107. doi: 10.1002/pul2.12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kazmirczak F, Hartweck LM, Vogel NT, et al. Intermittent fasting activates AMP-kinase to restructure right ventricular lipid metabolism and microtubules. JACC Basic Transl Sci 2023; 8: 239–254. doi: 10.1016/j.jacbts.2022.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prisco SZ, Eklund M, Raveendran R, et al. With no lysine kinase 1 promotes metabolic derangements and RV dysfunction in pulmonary arterial hypertension. JACC Basic Transl Sci 2021; 6: 834–850. doi: 10.1016/j.jacbts.2021.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimauchi T, Boucherat O, Yokokawa T, et al. PARP1-PKM2 axis mediates right ventricular failure associated with pulmonary arterial hypertension. JACC Basic Transl Sci 2022; 7: 384–403. doi: 10.1016/j.jacbts.2022.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hester J, Ventetuolo C, Lahm T. Sex, gender, and sex hormones in pulmonary hypertension and right ventricular failure. Compr Physiol 2019; 10: 125–170. doi: 10.1002/cphy.c190011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tello K, Richter MJ, Yogeswaran A, et al. Sex differences in right ventricular-pulmonary arterial coupling in pulmonary arterial hypertension. Am J Respir Crit Care Med 2020; 202: 1042–1046. doi: 10.1164/rccm.202003-0807LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Wezenbeek J, Groeneveldt JA, Llucià-Valldeperas A, et al. Interplay of sex hormones and long-term right ventricular adaptation in a Dutch PAH-cohort. J Heart Lung Transplant 2022; 41: 445–457. doi: 10.1016/j.healun.2021.11.004 [DOI] [PubMed] [Google Scholar]
- 37.Frump AL, Albrecht ME, Yakubov B, et al. 17β-Estradiol and estrogen receptor α protect right ventricular function in pulmonary hypertension via BMPR2 and apelin. J Clin Invest 2021; 131: e129433. doi: 10.1172/JCI129433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng TC, Philip JL, Tabima DM, et al. Estrogen receptor-α prevents right ventricular diastolic dysfunction and fibrosis in female rats. Am J Physiol Heart Circ Physiol 2020; 319: H1459–H1473. doi: 10.1152/ajpheart.00247.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wessels JN, Mouratoglou SA, van Wezenbeek J, et al. Right atrial function is associated with right ventricular diastolic stiffness: RA-RV interaction in pulmonary arterial hypertension. Eur Respir J 2022; 59: 2101454. doi: 10.1183/13993003.01454-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcus JT, Westerhof BE, Groeneveldt JA, et al. Vena cava backflow and right ventricular stiffness in pulmonary arterial hypertension. Eur Respir J 2019; 54: 1900625. doi: 10.1183/13993003.00625-2019 [DOI] [PubMed] [Google Scholar]
- 41.Wessels JN, van Wezenbeek J, de Rover J, et al. Right atrial adaptation to precapillary pulmonary hypertension: pressure-volume, cardiomyocyte, and histological analysis. J Am Coll Cardiol 2023; 82: 704–717. doi: 10.1016/j.jacc.2023.05.063 [DOI] [PubMed] [Google Scholar]
- 42.Jiang H, Salmon RM, Upton PD, et al. The prodomain-bound form of bone morphogenetic protein 10 is biologically active on endothelial cells. J Biol Chem 2016; 291: 2954–2966. doi: 10.1074/jbc.M115.683292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goetze JP, Bruneau BG, Ramos HR, et al. Cardiac natriuretic peptides. Nat Rev Cardiol 2020; 17: 698–717. doi: 10.1038/s41569-020-0381-0 [DOI] [PubMed] [Google Scholar]
- 44.Llucià-Valldeperas A, de Man FS, Bogaard HJ. Adaptation and maladaptation of the right ventricle in pulmonary vascular diseases. Clin Chest Med 2021; 42: 179–194. doi: 10.1016/j.ccm.2020.11.010 [DOI] [PubMed] [Google Scholar]
- 45.Keranov S, Dörr O, Jafari L, et al. CILP1 as a biomarker for right ventricular maladaptation in pulmonary hypertension. Eur Respir J 2021; 57: 1901192. doi: 10.1183/13993003.01192-2019 [DOI] [PubMed] [Google Scholar]
- 46.Zelt JGE, Cadete V, Deng Y, et al. Right ventricular maladaptation to pressure overload in Fischer rats is associated with profound deficiency in adenylate kinase 1 and impaired ventricular energetics. Hypertension 2022; 79: 2774–2786. doi: 10.1161/HYPERTENSIONAHA.122.19300 [DOI] [PubMed] [Google Scholar]
- 47.Humbert M, McLaughlin V, Gibbs JSR, et al. Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med 2021; 384: 1204–1215. doi: 10.1056/NEJMoa2024277 [DOI] [PubMed] [Google Scholar]
- 48.Souza R, Badesch DB, Ghofrani HA, et al. Effects of sotatercept on haemodynamics and right heart function: analysis of the STELLAR trial. Eur Respir J 2023; 62: 2301107. doi: 10.1183/13993003.01107-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waxman AB, Systrom DM, Manimaran S, et al. SPECTRA phase 2b study: impact of sotatercept on exercise tolerance and right ventricular function in pulmonary arterial hypertension. Circ Heart Fail 2024; 17: e011227. doi: 10.1161/CIRCHEARTFAILURE.123.011227 [DOI] [PubMed] [Google Scholar]
- 50.Russo I, Cavalera M, Huang S, et al. Protective effects of activated myofibroblasts in the pressure-overloaded myocardium are mediated through Smad-dependent activation of a matrix-preserving program. Circ Res 2019; 124: 1214–1227. doi: 10.1161/CIRCRESAHA.118.314438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talati MH, Brittain EL, Fessel JP, et al. Mechanisms of lipid accumulation in the bone morphogenic protein receptor 2 mutant right ventricle. Am J Respir Crit Care Med 2016; 194: 719–728. doi: 10.1164/rccm.201507-1444OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoeper MM, Badesch DB, Ghofrani HA, et al. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med 2023; 388: 1478–1490. doi: 10.1056/NEJMoa2213558 [DOI] [PubMed] [Google Scholar]
- 53.Guihaire J, Haddad F, Noly PE, et al. Right ventricular reserve in a piglet model of chronic pulmonary hypertension. Eur Respir J 2015; 45: 709–717. doi: 10.1183/09031936.00081314 [DOI] [PubMed] [Google Scholar]
- 54.Ukita R, Tumen A, Stokes JW, et al. Progression toward decompensated right ventricular failure in the ovine pulmonary hypertension model. ASAIO J 2022; 68: e29–e33. doi: 10.1097/MAT.0000000000001417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agger P, Hyldebrandt JA, Hansen ESS, et al. Magnetic resonance hyperpolarization imaging detects early myocardial dysfunction in a porcine model of right ventricular heart failure. Eur Heart J Cardiovasc Imaging 2020; 21: 93–101. doi: 10.1093/ehjci/jez074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown RD, Hunter KS, Li M, et al. Functional and molecular determinants of right ventricular response to severe pulmonary hypertension in a large animal model. Am J Physiol Heart Circ Physiol 2023; 324: H804–H820. doi: 10.1152/ajpheart.00614.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mendelson JB, Sternbach JD, Doyle MJ, et al. Multi-omic and multispecies analysis of right ventricular dysfunction. J Heart Lung Transplant 2024; 43: 303–313. doi: 10.1016/j.healun.2023.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tello K, Dalmer A, Vanderpool R, et al. Cardiac magnetic resonance imaging-based right ventricular strain analysis for assessment of coupling and diastolic function in pulmonary hypertension. JACC Cardiovasc Imaging 2019; 12: 2155–2164. doi: 10.1016/j.jcmg.2018.12.032 [DOI] [PubMed] [Google Scholar]
- 59.Yoshida K, van Wezenbeek J, Wessels JN, et al. Tricuspid regurgitation in pulmonary arterial hypertension: a right ventricular volumetric and functional analysis. Eur Respir J 2024; 63: 2301696. doi: 10.1183/13993003.01696-2023 [DOI] [PubMed] [Google Scholar]
- 60.Yoshida K, Axelsen JB, Saku K, et al. How to incorporate tricuspid regurgitation in right ventricular-pulmonary arterial coupling. J Appl Physiol 2023; 135: 53–59. doi: 10.1152/japplphysiol.00081.2023 [DOI] [PubMed] [Google Scholar]
- 61.Tamborini G, Muratori M, Brusoni D, et al. Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. Eur J Echocardiogr 2009; 10: 630–634. doi: 10.1093/ejechocard/jep015 [DOI] [PubMed] [Google Scholar]
- 62.Maus TM. TAPSE: a red herring after cardiac surgery. J Cardiothorac Vasc Anesth 2018; 32: 779–781. doi: 10.1053/j.jvca.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 63.Naeije R, Tello K, D'Alto M. Tricuspid regurgitation: right ventricular volume versus pressure load. Curr Heart Failure Repo 2023; 20: 208–217. doi: 10.1007/s11897-023-00599-w [DOI] [PubMed] [Google Scholar]
- 64.Beaubien-Souligny W, Rola P, Haycock K, et al. Quantifying systemic congestion with point-of-care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J 2020; 12: 16. doi: 10.1186/s13089-020-00163-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rako ZA, Yogeswaran A, Yildiz S, et al. Liver stiffness is associated with right heart dysfunction, cardiohepatic syndrome, and prognosis in pulmonary hypertension. J Heart Lung Transplant 2024; 43: 1105–1115. doi: 10.1016/j.healun.2024.02.013 [DOI] [PubMed] [Google Scholar]
- 66.Tello K, Dalmer A, Axmann J, et al. Reserve of right ventricular-arterial coupling in the setting of chronic overload. Circ Heart Fail 2019; 12: 005512. doi: 10.1161/CIRCHEARTFAILURE.118.005512 [DOI] [PubMed] [Google Scholar]
- 67.Tello K, Kremer N, Richter MJ, et al. Inhaled iloprost improves right ventricular load-independent contractility in pulmonary hypertension. Am J Respir Crit Care Med 2022; 206: 111–114. doi: 10.1164/rccm.202201-0095LE [DOI] [PubMed] [Google Scholar]
- 68.Badagliacca R, Reali M, Poscia R, et al. Right intraventricular dyssynchrony in idiopathic, heritable, and anorexigen-induced pulmonary arterial hypertension: clinical impact and reversibility. JACC Cardiovasc Imaging 2015; 8: 642–652. doi: 10.1016/j.jcmg.2015.02.009 [DOI] [PubMed] [Google Scholar]
- 69.Marcus JT, Gan CT, Zwanenburg JJ, et al. Interventricular mechanical asynchrony in pulmonary arterial hypertension: left-to-right delay in peak shortening is related to right ventricular overload and left ventricular underfilling. J Am Coll Cardiol 2008; 51: 750–757. doi: 10.1016/j.jacc.2007.10.041 [DOI] [PubMed] [Google Scholar]
- 70.Mukherjee M, Chung S-E, Ton VK, et al. Unique abnormalities in right ventricular longitudinal strain in systemic sclerosis patients. Circ Cardiovasc Imaging 2016; 9: e003792. doi: 10.1161/CIRCIMAGING.115.003792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mukherjee M, Mercurio V, Hsu S, et al. Assessment of right ventricular reserve utilizing exercise provocation in systemic sclerosis. Int J Cardiovasc Imaging 2021; 37: 2137–2147. doi: 10.1007/s10554-021-02237-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Addetia K, Miyoshi T, Amuthan V, et al. Normal values of three-dimensional right ventricular size and function measurements: results of the World Alliance Societies of Echocardiography study. J Am Soc Echocardiogr 2023; 36: 858–866. doi: 10.1016/j.echo.2023.04.011 [DOI] [PubMed] [Google Scholar]
- 73.Carvalho Singulane C, Singh A, Miyoshi T, et al. Sex-, age-, and race-related normal values of right ventricular diastolic function parameters: data from the World Alliance Societies of Echocardiography study. J Am Soc Echocardiogr 2022; 35: 426–434. doi: 10.1016/j.echo.2021.10.006 [DOI] [PubMed] [Google Scholar]
- 74.Soulat-Dufour L, Addetia K, Miyoshi T, et al. Normal values of right atrial size and function according to age, sex, and ethnicity: results of the World Alliance Societies of Echocardiography study. J Am Soc Echocardiogr 2021; 34: 286–300. doi: 10.1016/j.echo.2020.11.004 [DOI] [PubMed] [Google Scholar]
- 75.Kawel-Boehm N, Hetzel SJ, Ambale-Venkatesh B, et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J Cardiovasc Magn Reson 2020; 22: 87. 10.1186/s12968-020-00683-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewis RA, Johns CS, Cogliano M, et al. Identification of cardiac magnetic resonance imaging thresholds for risk stratification in pulmonary arterial hypertension. Am J Respir Crit Care Med 2020; 201: 458–468. doi: 10.1164/rccm.201909-1771OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 78.Celant LR, Wessels JN, Kianzad A, et al. Restoration of right ventricular function in the treatment of pulmonary arterial hypertension. Heart 2023; 109: 1844–1850. doi: 10.1136/heartjnl-2023-322742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Celestin BE, Bagherzadeh SP, Ichimura K, et al. Identifying consistent echocardiographic thresholds for risk stratification in pulmonary arterial hypertension. Pulm Circ 2024; 14: e12361. doi: 10.1002/pul2.12361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tello K, Wan J, Dalmer A, et al. Validation of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular-arterial coupling in severe pulmonary hypertension. Circ Cardiovasc Imaging 2019; 12: e009047. doi: 10.1161/CIRCIMAGING.119.009047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haddad F, Contrepois K, Amsallem M, et al. The right heart network and risk stratification in pulmonary arterial hypertension. Chest 2022; 161: 1347–1359. doi: 10.1016/j.chest.2021.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kanwar MK, Gomberg-Maitland M, Hoeper M, et al. Risk stratification in pulmonary arterial hypertension using Bayesian analysis. Eur Respir J 2020; 56: 2000008. doi: 10.1183/13993003.00008-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghio S, Mercurio V, Fortuni F, et al. A comprehensive echocardiographic method for risk stratification in pulmonary arterial hypertension. Eur Respir J 2020; 56: 2000513. doi: 10.1183/13993003.00513-2020 [DOI] [PubMed] [Google Scholar]
- 84.Fine NM, Chen L, Bastiansen PM, et al. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovasc Imaging 2013; 6: 711–721. doi: 10.1161/CIRCIMAGING.113.000640 [DOI] [PubMed] [Google Scholar]
- 85.El-Kersh K, Zhao C, Elliott G, et al. Derivation of a risk score (REVEAL-ECHO) based on echocardiographic parameters of patients with pulmonary arterial hypertension. Chest 2023; 163: 1232–1244. doi: 10.1016/j.chest.2022.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vonk Noordegraaf A, Channick R, Cottreel E, et al. The REPAIR study: effects of macitentan on RV structure and function in pulmonary arterial hypertension. JACC Cardiovasc Imaging 2022; 15: 240–253. doi: 10.1016/j.jcmg.2021.07.027 [DOI] [PubMed] [Google Scholar]
- 87.Alabed S, Garg P, Alandejani F, et al. Establishing minimally important differences for cardiac MRI end-points in pulmonary arterial hypertension. Eur Respir J 2023; 62: 2202225. doi: 10.1183/13993003.02225-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Badagliacca R, Poscia R, Pezzuto B, et al. Prognostic relevance of right heart reverse remodeling in idiopathic pulmonary arterial hypertension. J Heart Lung Transplant 2017; 37: 195–205. Doi: 10.1016/j.healun.2017.09.026 [DOI] [PubMed] [Google Scholar]
- 89.Badagliacca R, Raina A, Ghio S, et al. Influence of various therapeutic strategies on right ventricular morphology, function and hemodynamics in pulmonary arterial hypertension. J Heart Lung Transplant 2018; 37: 365–375. doi: 10.1016/j.healun.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 90.Badagliacca R, Papa S, Manzi G, et al. Usefulness of adding echocardiography of the right heart to risk-assessment scores in prostanoid-treated pulmonary arterial hypertension. JACC Cardiovasc Imaging 2020; 13: 2054–2056. doi: 10.1016/j.jcmg.2020.04.005 [DOI] [PubMed] [Google Scholar]
- 91.van Wolferen SA, van de Veerdonk MC, Mauritz GJ, et al. Clinically significant change in stroke volume in pulmonary hypertension. Chest 2011; 139: 1003–1009. doi: 10.1378/chest.10-1066 [DOI] [PubMed] [Google Scholar]
- 92.Weatherald J, Boucly A, Launay D, et al. Haemodynamics and serial risk assessment in systemic sclerosis associated pulmonary arterial hypertension. Eur Respir J 2018; 52: 1800678. doi: 10.1183/13993003.00678-2018 [DOI] [PubMed] [Google Scholar]
- 93.Swift AJ, Capener D, Johns C, et al. Magnetic resonance imaging in the prognostic evaluation of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2017; 196: 228–239. doi: 10.1164/rccm.201611-2365OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van de Veerdonk MC, Marcus JT, Westerhof N, et al. Signs of right ventricular deterioration in clinically stable patients with pulmonary arterial hypertension. Chest 2015; 147: 1063–1071. doi: 10.1378/chest.14-0701 [DOI] [PubMed] [Google Scholar]
- 95.Amsallem M, Sweatt AJ, Aymami MC, et al. Right heart end-systolic remodeling index strongly predicts outcomes in pulmonary arterial hypertension: comparison with validated models. Circ Cardiovasc Imaging 2017; 10: e005771. doi: 10.1161/CIRCIMAGING.116.005771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ryo K, Goda A, Onishi T, et al. Characterization of right ventricular remodeling in pulmonary hypertension associated with patient outcomes by 3-dimensional wall motion tracking echocardiography. Circ Cardiovasc Imaging 2015; 8: e003176. doi: 10.1161/CIRCIMAGING.114.003176 [DOI] [PubMed] [Google Scholar]
- 97.Alenezi F, Mandawat A, Il'Giovine ZJ, et al. Clinical utility and prognostic value of right atrial function in pulmonary hypertension. Circ Cardiovasc Imaging 2018; 11: e006984. doi: 10.1161/CIRCIMAGING.117.006984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700889. doi: 10.1183/13993003.00889-2017 [DOI] [PubMed] [Google Scholar]
- 99.D'Alto M, Badagliacca R, Argiento P, et al. Risk reduction and right heart reverse remodeling by upfront triple combination therapy in pulmonary arterial hypertension. Chest 2020; 157: 376–383. doi: 10.1016/j.chest.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 100.Swift AJ, Lu H, Uthoff J, et al. A machine learning cardiac magnetic resonance approach to extract disease features and automate pulmonary arterial hypertension diagnosis. Eur Heart J Cardiovasc Imaging 2021; 22: 236–245. doi: 10.1093/ehjci/jeaa001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abdi AH, Luong C, Tsang T, et al. Automatic quality assessment of echocardiograms using convolutional neural networks: feasibility on the apical four-chamber view. IEEE Trans Med Imaging 2017; 36: 1221–1230. doi: 10.1109/TMI.2017.2690836 [DOI] [PubMed] [Google Scholar]
- 102.Tokodi M, Staub L, Budai A, et al. Partitioning the right ventricle into 15 segments and decomposing its motion using 3D echocardiography-based models: the updated ReVISION method. Front Cardiovasc Med 2021; 8: 622118. doi: 10.3389/fcvm.2021.622118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hsia BC, Lai A, Singh S, et al. Validation of American Society of Echocardiography guideline – recommended parameters of right ventricular dysfunction using artificial intelligence compared with cardiac magnetic resonance imaging. J Am Soc Echocardiogr 2023; 36: 967–977. doi: 10.1016/j.echo.2023.05.015 [DOI] [PubMed] [Google Scholar]
- 104.Kawut SM, Lima JA, Barr RG, et al. Sex and race differences in right ventricular structure and function: the Multi-ethnic Study of Atherosclerosis – Right Ventricle Study. Circulation 2011; 123: 2542–2551. doi: 10.1161/CIRCULATIONAHA.110.985515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duke JW, Lewandowski AJ, Abman SH, et al. Physiological aspects of cardiopulmonary dysanapsis on exercise in adults born preterm. J Physiol 2022; 600: 463–482. doi: 10.1113/JP281848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mohamed A, Lamata P, Williamson W, et al. Multimodality imaging demonstrates reduced right-ventricular function independent of pulmonary physiology in moderately preterm-born adults. JACC Cardiovasc Imaging 2020; 13: 2046–2048. doi: 10.1016/j.jcmg.2020.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wauters A, Vicenzi M, De Becker B, et al. At high cardiac output, diesel exhaust exposure increases pulmonary vascular resistance and decreases distensibility of pulmonary resistive vessels. Am J Physiol Heart Circ Physiol 2015; 309: H2137–H2144. doi: 10.1152/ajpheart.00149.2015 [DOI] [PubMed] [Google Scholar]
- 108.Lewandowski AJ, Lamata P, Francis JM, et al. Breast milk consumption in preterm neonates and cardiac shape in adulthood. Pediatrics 2016; 138: e20160050. doi: 10.1542/peds.2016-0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mulchrone A, Bellofiore A, Douwes JM, et al. Impaired right ventricular-vascular coupling in young adults born preterm. Am J Respir Crit Care Med 2020; 201: 615–618. doi: 10.1164/rccm.201904-0767LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leary PJ, Kaufman JD, Barr RG, et al. Traffic-related air pollution and the right ventricle. The Multi-ethnic Study of Atherosclerosis. Am J Respir Crit Care Med 2014; 189: 1093–1100. doi: 10.1164/rccm.201312-2298OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Budts W, Roos-Hesselink J, Rädle-Hurst T, et al. Treatment of heart failure in adult congenital heart disease: a position paper of the Working Group of Grown-Up Congenital Heart Disease and the Heart Failure Association of the European Society of Cardiology. Eur Heart J 2016; 37: 1419–1427. doi: 10.1093/eurheartj/ehv741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jone PN, Ivy DD, Hauck A, et al. Pulmonary hypertension in congenital heart disease: a scientific statement from the American Heart Association. Circ Heart Fail 2023; 16: e00080. doi: 10.1161/HHF.0000000000000080 [DOI] [PubMed] [Google Scholar]
- 113.van Dissel AC, Opotowsky AR, Burchill LJ, et al. End-stage heart failure in congenitally corrected transposition of the great arteries: a multicentre study. Eur Heart J 2023; 44: 3278–3291. doi: 10.1093/eurheartj/ehad511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wei X, Wu B, Zhao J, et al. Myocardial hypertrophic preconditioning attenuates cardiomyocyte hypertrophy and slows progression to heart failure through upregulation of S100A8/A9. Circulation 2015; 131: 1506–1517. doi: 10.1161/CIRCULATIONAHA.114.013789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen X, Quan R, Qian Y, et al. 10-year survival of pulmonary arterial hypertension associated with connective tissue disease: insights from a multicentre PAH registry. Rheumatology 2023; 62: 3555–3564. doi: 10.1093/rheumatology/kead103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tedford RJ, Mudd JO, Girgis RE, et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail 2013; 6: 953–963. doi: 10.1161/CIRCHEARTFAILURE.112.000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hsu S, Kokkonen-Simon KM, Kirk JA, et al. Right ventricular myofilament functional differences in humans with systemic sclerosis-associated versus idiopathic pulmonary arterial hypertension. Circulation 2018; 137: 2360–2370. doi: 10.1161/CIRCULATIONAHA.117.033147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hsu S, Houston BA, Tampakakis E, et al. Right ventricular functional reserve in pulmonary arterial hypertension. Circulation 2016; 133: 2413–2422. doi: 10.1161/CIRCULATIONAHA.116.022082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Braams NJ, van Leeuwen JW, Vonk Noordegraaf A, et al. Right ventricular adaptation to pressure-overload: differences between chronic thromboembolic pulmonary hypertension and idiopathic pulmonary arterial hypertension. J Heart Lung Transplant 2021; 40: 458–466. doi: 10.1016/j.healun.2021.02.018 [DOI] [PubMed] [Google Scholar]
- 120.Ruigrok D, Meijboom LJ, Westerhof BE, et al. Right ventricular load and function in chronic thromboembolic pulmonary hypertension: differences between proximal and distal chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2019; 199: 1163–1166. doi: 10.1164/rccm.201808-1562LE [DOI] [PubMed] [Google Scholar]
- 121.Fukumitsu M, Westerhof BE, Ruigrok D, et al. Early return of reflected waves increases right ventricular wall stress in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol 2020; 319: H1438–H1450. doi: 10.1152/ajpheart.00442.2020 [DOI] [PubMed] [Google Scholar]
- 122.Fukumitsu M, Groeneveldt JA, Braams NJ, et al. When right ventricular pressure meets volume: the impact of arrival time of reflected waves on right ventricle load in pulmonary arterial hypertension. J Physiol 2022; 600: 2327–2344. doi: 10.1113/JP282422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Braams NJ, Kianzad A, van Wezenbeek J, et al. Long-term effects of pulmonary endarterectomy on right ventricular stiffness and fibrosis in chronic thromboembolic pulmonary hypertension. Circ Heart Fail 2023; 16: e010336. doi: 10.1161/CIRCHEARTFAILURE.122.010336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Howden EJ, Ruiz-Carmona S, Claeys M, et al. Oxygen pathway limitations in patients with chronic thromboembolic pulmonary hypertension. Circulation 2021; 143: 2061–2073. doi: 10.1161/CIRCULATIONAHA.120.052899 [DOI] [PubMed] [Google Scholar]
- 125.Claessen G, La Gerche A, Dymarkowski S, et al. Pulmonary vascular and right ventricular reserve in patients with normalized resting hemodynamics after pulmonary endarterectomy. J Am Heart Assoc 2015; 4: e001602. doi: 10.1161/JAHA.114.001602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guazzi M, Naeije R. Pulmonary hypertension in heart failure: pathophysiology, pathobiology, and emerging clinical perspectives. J Am Coll Cardiol 2017; 69: 1718–1734. doi: 10.1016/j.jacc.2017.01.051 [DOI] [PubMed] [Google Scholar]
- 127.Di Salvo TG, Mathier M, Semigran MJ, et al. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol 1995; 25: 1143–1153. doi: 10.1016/0735-1097(94)00511-N [DOI] [PubMed] [Google Scholar]
- 128.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 2001; 37: 183–188. doi: 10.1016/S0735-1097(00)01102-5 [DOI] [PubMed] [Google Scholar]
- 129.Guazzi M, Bandera F, Pelissero G, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol 2013; 305: H1373–H1381. doi: 10.1152/ajpheart.00157.2013 [DOI] [PubMed] [Google Scholar]
- 130.Bosch L, Lam CSP, Gong L, et al. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail 2017; 19: 1664–1671. doi: 10.1002/ejhf.873 [DOI] [PubMed] [Google Scholar]
- 131.Padang R, Chandrashekar N, Indrabhinduwat M, et al. Aetiology and outcomes of severe right ventricular dysfunction. Eur Heart J 2020; 41: 1273–1282. doi: 10.1093/eurheartj/ehaa037 [DOI] [PubMed] [Google Scholar]
- 132.Gerges M, Gerges C, Pistritto AM, et al. Pulmonary hypertension in heart failure. Epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med 2015; 192: 1234–1246. doi: 10.1164/rccm.201503-0529OC [DOI] [PubMed] [Google Scholar]
- 133.Naeije R, Badagliacca R. The overloaded right heart and ventricular interdependence. Cardiovasc Res 2017; 113: 1474–1485. doi: 10.1093/cvr/cvx160 [DOI] [PubMed] [Google Scholar]
- 134.van Wezenbeek J, Kianzad A, van de Bovenkamp A, et al. Right ventricular and right atrial function are less compromised in pulmonary hypertension secondary to heart failure with preserved ejection fraction: a comparison with pulmonary arterial hypertension with similar pressure overload. Circ Heart Fail 2022; 15: e008726. doi: 10.1161/CIRCHEARTFAILURE.121.008726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pagnamenta A, Dewachter C, McEntee K, et al. Early right ventriculo-arterial uncoupling in borderline pulmonary hypertension on experimental heart failure. J Appl Physiol 2010; 109: 1080–1085. doi: 10.1152/japplphysiol.00467.2010 [DOI] [PubMed] [Google Scholar]
- 136.Hubesch G, Dewachter C, Chomette L, et al. Early alteration of right ventricle-pulmonary artery coupling in experimental heart failure with preserved ejection fraction. J Am Heart Assoc 2024; 13: e032201. doi: 10.1161/JAHA.123.032201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Aslam MI, Hahn VS, Jani V, et al. Reduced right ventricular sarcomere contractility in heart failure with preserved ejection fraction and severe obesity. Circulation 2021; 143: 965–967. doi: 10.1161/CIRCULATIONAHA.120.052414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Borbély A, van der Velden J, Papp Z, et al. Cardiomyocyte stiffness in diastolic heart failure. Circulation 2005; 111: 774–781. doi: 10.1161/01.CIR.0000155257.33485.6D [DOI] [PubMed] [Google Scholar]
- 139.Guazzi M, Naeije R. Right heart phenotype in heart failure with preserved ejection fraction. Circ Heart Fail 2021; 14: e007840. doi: 10.1161/CIRCHEARTFAILURE.120.007840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Paulus WJ, Zile MR. From systemic inflammation to myocardial fibrosis: the heart failure with preserved ejection fraction paradigm revisited. Circ Res 2021; 128: 1451–1467. doi: 10.1161/CIRCRESAHA.121.318159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nijst P, Martens P, Dupont M, et al. Intrarenal flow alterations during transition from euvolemia to intravascular volume expansion in heart failure patients. JACC Heart Fail 2017; 5: 672–681. doi: 10.1016/j.jchf.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 142.Scott JV, Moutchia J, McClelland RL, et al. Novel liver injury phenotypes and outcomes in clinical trial participants with pulmonary hypertension. Am J Respir Crit Care Med 2024; 210: 1045–1056. doi: 10.1164/rccm.202311-2196OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kremer N, Roller FC, Kremer S, et al. Native hepatic T1-time as a non-invasive predictor of diastolic dysfunction and a monitoring tool for disease progression and treatment response in patients with pulmonary hypertension. Int J Cardiol 2024; 409: 132189. doi: 10.1016/j.ijcard.2024.132189 [DOI] [PubMed] [Google Scholar]
- 144.Omote K, Sorimachi H, Obokata M, et al. Pulmonary vascular disease in pulmonary hypertension due to left heart disease: pathophysiologic implications. Eur Heart J 2022; 43: 3417–3431. doi: 10.1093/eurheartj/ehac184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.D'Alto M, Liccardo B, Di Maio M, et al. Lung ultrasound, echocardiography, and fluid challenge for the differential diagnosis of pulmonary hypertension. J Am Soc Echocardiogr 2023; 36: 1181–1189. doi: 10.1016/j.echo.2023.07.010 [DOI] [PubMed] [Google Scholar]
- 146.Singh I, Rahaghi FN, Naeije R, et al. Right ventricular-arterial uncoupling during exercise in heart failure with preserved ejection fraction: role of pulmonary vascular dysfunction. Chest 2019; 156: 933–943. doi: 10.1016/j.chest.2019.04.109 [DOI] [PubMed] [Google Scholar]
- 147.Singh I, Oliveira RKF, Heerdt PM, et al. Sex-related differences in dynamic right ventricular-pulmonary vascular coupling in heart failure with preserved ejection fraction. Chest 2021; 159: 2402–2416. doi: 10.1016/j.chest.2020.12.028 [DOI] [PubMed] [Google Scholar]
- 148.Gargani L, Pugliese NR, De Biase N, et al. Exercise stress echocardiography of the right ventricle and pulmonary circulation. J Am Coll Cardiol 2023; 82: 1973–1985. doi: 10.1016/j.jacc.2023.09.807 [DOI] [PubMed] [Google Scholar]
- 149.Dankmeijer JHF, Ibrahim M, Reid DD, et al. Chronic cor pulmonale. Report of an expert committee. WHO Technical Report Series No. 213. Circulation 1963; 27: 94–615. [Google Scholar]
- 150.Fishman AP. State of the art: chronic cor pulmonale. Am Rev Respir Dis 1976; 114: 775–794. doi: 10.1164/arrd.1976.114.4.775 [DOI] [PubMed] [Google Scholar]
- 151.Hoeper MM, Barberà JA, Channick RN, et al. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol 2009; 54: S85–S96. doi: 10.1016/j.jacc.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 152.Yogeswaran A, Kuhnert S, Gall H, et al. Relevance of cor pulmonale in COPD with and without pulmonary hypertension: a retrospective cohort study. Front Cardiovasc Med 2022; 9: 826369. doi: 10.3389/fcvm.2022.826369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Matthay RA, Berger HJ, Davies RA, et al. Right and left ventricular exercise performance in chronic obstructive pulmonary disease: radionuclide assessment. Ann Intern Med 1980; 93: 234–239. doi: 10.7326/0003-4819-93-2-234 [DOI] [PubMed] [Google Scholar]
- 154.MacNee W, Wathen CG, Flenley DC, et al. The effects of controlled oxygen therapy on ventricular function in patients with stable and decompensated cor pulmonale. Am Rev Respir Dis 1988; 137: 1289–1295. doi: 10.1164/ajrccm/137.6.1289 [DOI] [PubMed] [Google Scholar]
- 155.Hilde JM, Skjørten I, Grøtta OJ, et al. Right ventricular dysfunction and remodeling in chronic obstructive pulmonary disease without pulmonary hypertension. J Am Coll Cardiol 2013; 62: 1103–1111. doi: 10.1016/j.jacc.2013.04.091 [DOI] [PubMed] [Google Scholar]
- 156.D'Andrea A, Stanziola A, Di Palma E, et al. Right ventricular structure and function in idiopathic pulmonary fibrosis with or without pulmonary hypertension. Echocardiography 2016; 33: 57–65. doi: 10.1111/echo.12992 [DOI] [PubMed] [Google Scholar]
- 157.Lamia B, Muir JF, Molano LC, et al. Altered synchrony of right ventricular contraction in borderline pulmonary hypertension. Int J Cardiovasc Imaging 2017; 33: 1331–1339. doi: 10.1007/s10554-017-1110-6 [DOI] [PubMed] [Google Scholar]
- 158.Kawut SM, Poor HD, Parikh MA, et al. Cor pulmonale parvus in chronic obstructive pulmonary disease and emphysema: the MESA COPD study. J Am Coll Cardiol 2014; 64: 2000–2009. doi: 10.1016/j.jacc.2014.07.991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Reichek N. Cor pulmonale parvus: patting the elephant. J Am Coll Cardiol 2014; 64: 2010–2012. doi: 10.1016/j.jacc.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 160.France AJ, Prescott RJ, Biernacki W, et al. Does right ventricular function predict survival in patients with chronic obstructive lung disease? Thorax 1988; 43: 621–626. doi: 10.1136/thx.43.8.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tello K, Ghofrani HA, Heinze C, et al. A simple echocardiographic estimate of right ventricular-arterial coupling to assess severity and outcome in pulmonary hypertension on chronic lung disease. Eur Respir J 2019; 54: 1802435. doi: 10.1183/13993003.02435-2018 [DOI] [PubMed] [Google Scholar]
- 162.Prins KW, Rose L, Archer SL, et al. Disproportionate right ventricular dysfunction and poor survival in group 3 pulmonary hypertension. Am J Respir Crit Care Med 2018; 197: 1496–1499. doi: 10.1164/rccm.201712-2405LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Han Y, Forfia P, Vaidya A, et al. Ranolazine improves right ventricular function in patients with precapillary pulmonary hypertension: results from a double-blind, randomized, placebo-controlled trial. J Card Fail 2021; 27: 253–257. doi: 10.1016/j.cardfail.2020.10.006 [DOI] [PubMed] [Google Scholar]
- 164.Han QJ, Forfia P, Vaidya A, et al. Effects of ranolazine on right ventricular function, fluid dynamics, and metabolism in patients with precapillary pulmonary hypertension: insights from a longitudinal, randomized, double-blinded, placebo controlled, multicenter study. Front Cardiovasc Med 2023; 10: 1118796. doi: 10.3389/fcvm.2023.1118796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brittain EL, Niswender K, Agrawal V, et al. Mechanistic phase II clinical trial of metformin in pulmonary arterial hypertension. J Am Heart Assoc 2020; 9: e018349. doi: 10.1161/JAHA.120.018349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lurz P, Orban M, Besler C, et al. Clinical characteristics, diagnosis, and risk stratification of pulmonary hypertension in severe tricuspid regurgitation and implications for transcatheter tricuspid valve repair. Eur Heart J 2020; 41: 2785–2795. doi: 10.1093/eurheartj/ehaa138 [DOI] [PubMed] [Google Scholar]
- 167.Kassis N, Layoun H, Goyal A, et al. Mechanistic insights into tricuspid regurgitation secondary to pulmonary arterial hypertension. Am J Cardiol 2022; 175: 97–105. doi: 10.1016/j.amjcard.2022.04.010 [DOI] [PubMed] [Google Scholar]
- 168.Kresoja KP, Rommel KP, Lücke C, et al. Right ventricular contraction patterns in patients undergoing transcatheter tricuspid valve repair for severe tricuspid regurgitation. JACC Cardiovasc Interv 2021; 14: 1551–1561. doi: 10.1016/j.jcin.2021.05.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01321-2024.Shareable (328.1KB, pdf)