Abstract
Chronic thromboembolic pulmonary hypertension is a complication of pulmonary embolism and a treatable cause of pulmonary hypertension. The pathology is a unique combination of mechanical obstruction due to failure of clot resolution, and a variable degree of microvascular disease, that both contribute to pulmonary vascular resistance. Accordingly, multiple treatments have been developed to target the disease components. However, accurate diagnosis is often delayed. Evaluation includes high-quality imaging modalities, necessary for disease confirmation and for appropriate treatment planning. All patients with chronic thromboembolic pulmonary disease, and especially those with pulmonary hypertension, should be referred to expert centres for multidisciplinary team decision on treatment. The first decision remains assessment of operability, and the best improvement in symptoms and survival is achieved by the mechanical therapies, pulmonary endarterectomy and balloon pulmonary angioplasty. With the advances in multimodal therapies, excellent outcomes can be achieved with 3-year survival of >90%.
Shareable abstract
Treatment of chronic thromboembolic pulmonary hypertension requires a multidisciplinary team approach with tailored treatment across three therapeutic modalities: pulmonary endarterectomy, balloon pulmonary angioplasty and PH targeted medical therapy https://bit.ly/4cH37WC
Introduction
Chronic thromboembolic pulmonary disease (CTEPD) is the umbrella term describing a treatable form of chronic pulmonary vascular obstruction. CTEPD includes chronic thromboembolic pulmonary hypertension (CTEPH) and CTEPD without pulmonary hypertension (PH) [1]. CTEPH is the main diagnosis within PH classification group 4 [2]. Its incidence, which had been estimated at 5–6 cases per million inhabitants per year, is reaching 13 cases per million inhabitants per year when a systematic pulmonary embolism follow-up is organised [3]. The haemodynamic thresholds defining PH in CTEPH are adopted from the revised and now accepted thresholds based on definition of normal versus abnormal pulmonary haemodynamic parameters [4, 5]. Without effective treatment, patients with CTEPH can progress to right heart failure and premature death. CTEPD without PH may not necessarily progress to CTEPH [6]. However, select cases may achieve symptomatic benefit with treatments developed for CTEPH including pulmonary endarterectomy (PEA) and balloon pulmonary angioplasty (BPA) [7, 8].
Since the last World Symposium on Pulmonary Hypertension (WSPH) in 2018, there have been ongoing advances across broad topics pertaining to CTEPD. This article highlights these advances, points out remaining gaps and emphasises key reminders paramount in the optimal diagnosis and management of patients with CTEPH.
Components of disease
The development of CTEPH involves several components and factors that contribute to its manifestation and progression. Anatomically, two different vascular lesions participate in the increase in pulmonary vascular resistance (PVR) in CTEPH patients: an obstruction of pulmonary arteries by unresolved organised fibrothrombotic clots and a secondary microvasculopathy observed in both obstructed and nonobstructed lung areas [9]. While after acute pulmonary embolism (PE) most thrombi resolve with treatment, in some cases, clots fail to dissolve completely. In CTEPH, incomplete thrombus resolution triggers an abnormal healing response in the pulmonary arteries. This response involves the formation of scar-like tissue and vascular remodelling, which narrows and stiffens the arteries, further obstructing pulmonary blood flow, resulting in a persistent obstruction of pulmonary arteries. Different mechanisms are potentially responsible for incomplete clot resolution: abnormalities in fibrinogen structure and function, inflammatory thrombosis and inhibition of intrathrombus angiogenesis [10]; a component of in situ thrombosis is potentially also contributing to CTEPH pathogenesis [11, 12]. These fibrothrombotic lesions are visualised as slits/tails, webs, stenoses or pouching on vascular imaging and are the target of mechanical treatment with PEA or BPA.
The secondary microvasculopathy, resembling changes seen in pulmonary arterial hypertension (PAH), predominates in nonobstructed lung regions and results from a redistribution of flow and increased pressure and shear stress [13]. A microvasculopathy is also observed in completely or partially obstructed lung areas and is attributed to the development and hypertrophy of systemic bronchial arteries anastomosing with pulmonary arterioles and venules [14]. The presence of microvasculopathy is suspected when mechanical obstruction does not correlate with the haemodynamic severity [15]. The microvasculopathy may explain clinical deterioration of some patients in the absence of PE recurrence, and the persistence of PH after PEA. Medical therapy with PH drugs in CTEPH aims to target the microvasculopathy.
Risk factors for CTEPH include 1) thrombotic risk factors with a predominance of antiphospholipid syndrome and increased factor VIII, as opposed to acute PE; 2) medical conditions such as splenectomy, chronic inflammatory disorders, ventriculoatrial shunts, infections and cancer; and 3) non-O blood groups [16]. When acute PE is diagnosed and thrombus load is considerable, it has been hypothesised that systemic thrombolysis could prevent the development of CTEPH. However, it did not in intermediate high-risk PE patients treated in the Pulmonary Embolism Thrombolysis (PEITHO) trial [17]. Consequently, we have currently no preventive strategy available for CTEPH. Earlier recognition and diagnosis of CTEPH at milder stages may be feasible with a systematic strategy and follow-up after acute PE [3]. In the region with proximity to the centre utilising such a screening strategy after PE, the diagnostic rate of CTEPH and referral for PEA were both notably higher [3].
Concerning the haemodynamic alterations in CTEPH, it has been shown that patients may have left ventricular (LV) dysfunction and somewhat increased LV filling pressures contributing to PH in older age [18]. With regard to CTEPH molecular and cellular mechanisms, additional evidence has been reported on 1) the inflammatory thrombosis hypothesis with involvement of neutrophil extracellular traps, elevated plasma concentration of von Willebrand factor, NF-κB signalling and upregulation of inflammatory signalling by macrophages and T-cells identified by single-cell RNA sequencing [12, 19–22]; 2) the angiogenesis-driven clot resolution hypothesis; 3) the genetic hypothesis [23]; and 4) a potential role of air pollution [24]. Concerning risk factors, a CTEPH prevalence of 3.8% has been observed in patients with antiphospholipid syndrome (APS) [25]. Primary APS, prior PE, recurrent venous thromboembolism (VTE), thrombocytopenia and specific antiphospholipid antibodies predict CTEPH in APS. Active assessment for CTEPH in APS patients may be considered.
Further research is ongoing to identify biomarkers of fibrotic thrombus transformation that could help to identify patients with CTEPH earlier. Integration of clinical and -omic measurements is needed to identify clinically valuable diagnostic signatures and pathways for potential future therapeutic interventions. It is still unclear whether the systemic collateral circulation, supposedly developed to preserve pulmonary parenchyma and airways from ischaemia/infarction, is a friend or foe due to competing flow in pulmonary arteries. It is not known whether bronchial collateral coiling before or after PEA is beneficial or harmful, and in which circumstances, apart from haemoptysis, it should be considered. Advanced imaging modalities may allow separate analyses of pulmonary and systemic collateral circulations, and changes if any after treatments. While systemic thrombolysis does not reduce CTEPH incidence after acute submassive PE [17], it is not known whether mechanical intervention by aspiration or by combined local thrombolysis and ultrasonic clot disruption will do. Catheter-based therapy studies need long-term evaluation for CTEPH incidence and careful adjudication of acute PE versus acute clot on top of existing CTEPH at time of initial intervention.
Diagnostic directions and considerations
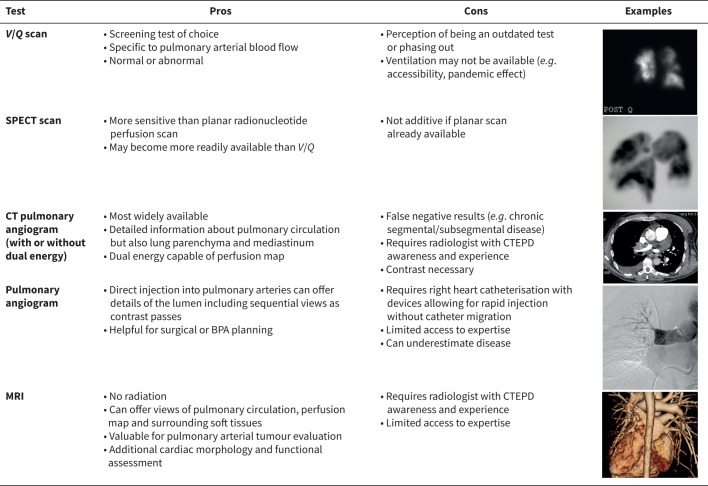
Despite improvements in imaging techniques (figure 1), a ventilation/perfusion (V/Q) lung scan should be performed as the first examination when suspecting CTEPD [26, 27]. In some regions, and also due to post-pandemic practice, the ventilation portion may not be available. In such a scenario, the perfusion scan alone would still be of value when evaluating patients with PH. In the 6th WSPH, the V/Q scan was confirmed as a first step in the diagnosis of CTEPH, and since then, this role has been incorporated into the ERS statement [1]. The guidelines for the diagnosis and management of acute PE support V/Q lung scan as the first-line test for diagnosing CTEPH following PE in the presence of dyspnoea or functional limitation and high probability of PH by echocardiography [28]. However, additional diagnostic imaging is required, as unmatched perfusion defects may also be seen in other pulmonary vascular disease such as acute PE, pulmonary veno-occlusive disease, pulmonary artery tumours, pulmonary vasculitis, peripheral pulmonary artery stenosis and pulmonary vein stenosis [29, 30]. Single photon emission computed tomography (SPECT) V/Q has been shown to be superior to planar V/Q scan in the detection of both acute PE and CTEPD, because of its ability to generate three-dimensional imaging data (resolving the problem of overlapping structures).
FIGURE 1.
Chronic thromboembolic pulmonary disease (CTEPD) imaging modalities. V/Q: ventilation/perfusion; SPECT: single photon emission computed tomography; CT: computed tomography; MRI: magnetic resonance imaging; BPA: balloon pulmonary angioplasty.
Although computed tomography pulmonary angiography (CTPA) may not be the initial test for the evaluation of CTEPH, it is important in the next step of the diagnostic algorithm and for treatment decision-making. In everyday clinical practice, CTPA is easily available. Mural thrombus, complete arterial occlusions, intravascular webs or bands, and artery narrowing or retraction are all signs of CTEPD and can be visualised accurately on CTPA [31]. In addition to CTEPD, obstructive defects involving the proximal pulmonary arteries can be seen with pulmonary artery sarcoma, and less commonly other tumours, pulmonary vasculitis, congenital pulmonary artery stenoses and hydatidosis [5]. Very peripheral disease is difficult to recognise on CTPA due to the small calibre of the vessels, but visualisation is very important for optimal treatment planning, and perfusion defects can be detected even in the absence of a visible arterial abnormality [32]. In this respect, advanced computed tomography (CT) technologies, such as dual-energy CT (DECT), can provide excellent morphological images of the pulmonary arteries with perfusion images of the lungs based on iodine maps which have been considered surrogate markers of lung perfusion [33]. When an analysis of iodine maps is combined with standard CT images of pulmonary arterial morphology, DECT has a sensitivity and specificity of 100% for a diagnosis of CTEPH [33]. Despite what guidelines recommend and the improvement in technology, the infrequent use of V/Q lung scanning in daily practice and the lack of expertise required to evaluate CTPA are still responsible for the underdiagnosis of CTEPH. Indeed, it is still frequent that patients initially referred as idiopathic PAH may have their diagnosis changed to CTEPH after obtaining a perfusion scan or re-evaluation of a CTPA by an expert radiologist.
Although there is increasing interest in noninvasive haemodynamic evaluation, given the complexity of pathophysiological changes occurring in CTEPH, right heart catheterisation (RHC) and catheter-based pulmonary angiography are still mandatory to confirm the diagnosis. Indeed, despite advanced imaging, the degree of pre-capillary microvasculopathy can only be estimated by considering the discrepancy between obstructive disease seen on imaging and haemodynamic severity. In this regard, a complete haemodynamic evaluation by RHC including cardiac output is recommended, because the calculated PVR is important to assess the prognosis and the risks associated with PEA [34]. Measurement of pulmonary arterial wedge pressure (PAWP) is necessary to exclude post-capillary PH resulting from comorbidities. Intravascular obstructions might confound the correct estimation of PAWP in some patients, and if unreliable, left ventricular end-diastolic pressure should be obtained by left ventricular catheterisation. High-quality pulmonary angiography, with or without digital subtraction, is still considered valuable especially for planning BPA [27]. In terms of angiographic technique, multiple selective injections are not required for the diagnosis of CTEPH, whereas they are essential for BPA planning and during treatment sessions. Biplane acquisition provides optimal anatomical detail; the lateral projection providing more detailed definition of lobar and segmental anatomy compared to the anteroposterior view alone.
CTPA is still the initial imaging test of choice for acute PE, but not for CTEPH. Whenever CTPA is performed in the suspicion of acute PE, signs such as increased calibre of the pulmonary artery with intravascular bands or webs, the presence of mosaic oligemia, hypertrophy of the bronchial arteries and right ventricle are suggestive of a pre-existing CTEPH [35, 36]. For the first time, guidelines for the diagnosis and management of acute PE have provided awareness of imaging characteristics of chronic thromboembolism, and these have supported the awareness of the diagnosis of CTEPH at the time of “acute” PE [28]. When obtaining CTPA for the workup of CTEPH, proper protocol and acquisition are critical to ensure visualisation of the more subtle findings in CTEPH compared with PE.
Cone beam and area detector CT allow for more accurate visualisation of subsegmental vasculature and have been shown to be more useful for procedural guidance for BPA rather than for diagnosis of CTEPH. However, the benefits of the technology require validation in prospective trials before recommendation for routine clinical use [1]. Three-dimensional contrast-enhanced lung perfusion magnetic resonance imaging (MRI) is a more recent and advanced imaging technique that can be deployed to search for perfusion defects in the pulmonary vascular bed. MRI was compared with SPECT lung scintigraphy in a series of 74 patients with PH (30 of the patients had CTEPH and 10 had CTEPD without PH). MRI showed a trend to increased sensitivity when compared to SPECT perfusion scintigraphy in the detection of CTEPH (97% SPECT, 100% MRI) [37]. Despite being a reliable and safe diagnostic tool (no radiation exposure in comparison with CTPA), its use is still restricted to specialised centres.
Multidisciplinary team definition and role
The concept of importance of a “CTEPH team” for determining operability was first stated at the 5th WSPH and was incorporated into the CTEPH treatment algorithm. We stated the team should consist of an experienced surgeon and CTEPH physicians. This predated the acceptance of BPA as an established treatment modality [26]. Although there is no scientific evidence for the multidisciplinary team (MDT) definition, the MDT concept is used successfully in all experienced CTEPH centres internationally and is arguably more important today compared with 2013, as the treatment options and combinations for CTEPH patients have increased. This was expanded in the 6th WSPH to include the need for a multidisciplinary assessment in the treatment algorithm, with a larger “expert CTEPH team” including an experienced surgeon, PH specialist, BPA interventionalist and CTEPH-trained radiologist to reflect the additional treatment modalities [38]. The critical importance of the contribution of an expert radiologist is perhaps under-recognised with respect both to the CTEPH diagnosis and determining treatment options, and CTEPH is sometimes misdiagnosed on CTPA [39]. MDT decision-making is still mainly based on relatively subjective clinical experience.
The European Respiratory Society (ERS) statement made clear that some patients with CTEPH benefit from multimodality treatment, but there were few published data, no direct randomised controlled trial (RCT) comparisons and no consensus on eligibility criteria, just the requirement for an expert centre and MDT approach [1]. The International Society for Heart and Lung Transplantation consensus statement on the management of CTEPH patients also suggested that patients with CTEPH require multidisciplinary care at expert CTEPH centres, but with no new evidence [40]. The latest European Society of Cardiology (ESC)/ERS guidelines specified that treatment assessment should be by an expert MDT (class IC), including experienced PEA surgeons, at a CTEPH centre to evaluate operability, and indicated that a multimodality approach should be considered for patients with persistent PH after PEA and for patients with inoperable CTEPH (class IIaC). These recommendations were broadly based on the 6th WSPH conclusions rather than on any new published evidence. The guidelines confirmed that the CTEPH MDT should include PEA surgeons, BPA interventionalists, PH specialists and thoracic radiologists, all trained in high-volume centres [5]. There has been little published on the specific role of the MDT. An article from Switzerland describing the introduction of a national MDT concept has shown feasibility, although the numbers were small and <100 patients were reported [41].
Therefore, further confirmation of the MDT concept was repeated in all major statements, but without significant interval advances or substantial new evidence since the 6th WSPH. Current best practice proposed that the CTEPH MDT include experienced PH radiologists, experienced PEA surgeons, CTEPH/PH physicians, BPA interventionalists, a coordinator, PEA/PH specialist nurses and MDT administration. The latter is required for minuting decision-making. Ideally, the team should have two clinicians from each interventional subspecialty to allow more than one opinion. In principle, the role of CTEPH MDT is 1) confirmation of CTEPH diagnosis; 2) determination of operability; 3) determination of BPA potential; 4) determination of appropriateness of medical therapy and/or multimodal approach; and 5) follow-up/review of previously treated patients, both to determine the need for additional therapy and as a feedback loop for the MDT.
However, there remain numerous gaps regarding CTEPH MDTs: 1) no evidence for a clinically useful objective radiological classification/description tool; 2) no evidence for an accurate pre-operative surgical or BPA risk-scoring system; 3) no trial evidence on objectivity of inter- and intra-MDT decision-making; and 4) no trial evidence for direct comparison between BPA and PEA to aid MDT decision-making. There is a need for more objective radiological reporting to aid MDT decision-making and to help compare treatment outcomes. Furthermore, there should be an emphasis placed on the important feedback loop to the surgeon or BPA team of limits and consequences of their treatments including with longer term follow-up [5].
The application of MDT in CTEPH cannot be overstated and is foundational for optimal management of each case (table 1). The following sections provide overviews of each CTEPH therapy in the current management and based on MDT determination.
TABLE 1.
Steps to recovery in chronic thromboembolic pulmonary hypertension: AMEND
| A | Anticoagulation |
| M | Multidisciplinary team review |
| E | Pulmonary Endarterectomy |
| N | Nonsurgical treatments: medical therapy, balloon pulmonary angioplasty |
| D | Don't forget follow-up assessment after any intervention |
Pulmonary endarterectomy
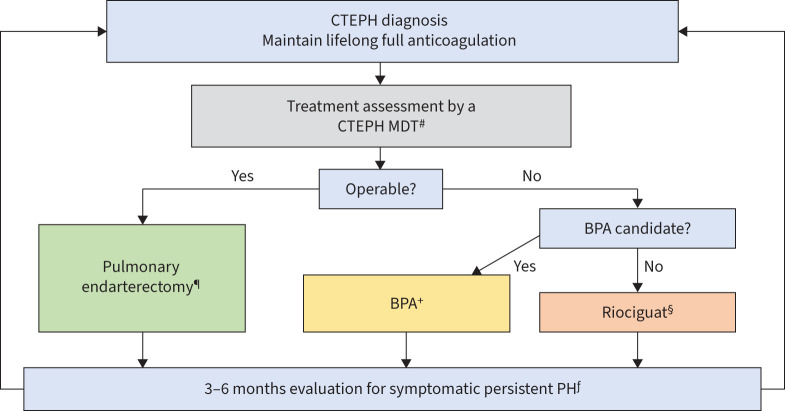
Surgical resection of occlusive chronic thromboembolic material, by means of PEA, also referred to as pulmonary thromboendarterectomy (PTE) is the guideline-recommended treatment of choice (class IB) and the first step when evaluating patients with CTEPH [5] (figure 2). The procedure can normalise pulmonary artery pressures in ∼70–75% of patients and carries an operative mortality of ∼2% at expert centres [42–44]. Currently, survival for PTE/PEA is >90% at 3 years, 87% at 5 years and 78% at 10 years; however, disease-specific survival is 91% at 5 years and 85% at 10 years [45, 46]. In comparison, survival was only 70% at 3 years in those CTEPH patients not operated and before availability of BPA and riociguat [47]. In the current era of multimodality treatment with BPA and medical therapy, 5-year survival in inoperable patients has improved significantly, and is approaching 90%, but it remains unchanged at ∼70% for patients who did not undergo either intervention (M. Delcroix, UZ Leuven, Leuven, Belgium; personal communication) [47]. Operability assessment and patient selection remain the Achilles’ heel in prediction of excellent long-term outcome. Certain patient characteristics can help with appropriate treatment strategy [48].
FIGURE 2.
Chronic thromboembolic pulmonary hypertension (CTEPH) treatment algorithm. MDT: multidisciplinary team; BPA: balloon pulmonary angioplasty; PH: pulmonary hypertension. #: CTEPH MDT requires pulmonary endartectomy surgeon, PH expert, BPA specialist and chest radiologist; ¶: treatment of choice for technically operable disease; +: riociguat therapy prior to BPA: mean pulmonary arterial pressure ≥40 mmHg or pulmonary vascular resistance >4 Wood Units; §: other PH medications approved in select regions; ƒ: structured follow-up; may include imaging and haemodynamic assessment.
Historically, high PVR was considered a potential contraindication to surgery [49]; however, even in patients with the most severe forms of disease, characterised by a pre-operative PVR of >1000 dyn·s·cm−5, the operative mortality has decreased to <5% [43, 46]. Furthermore, advanced age (>80 years), morbid obesity or repeat operations are not considered contraindications to surgery [50–55]. In addition, recent data show that although females have similar short- and long-term outcomes, there is slightly higher incidence of residual PH requiring medical therapy [56]. There is now clear evidence that in expert centres and with advancements in diagnosis, imaging, surgical techniques and instruments, distal endarterectomy in segmental and subsegmental vessels is feasible with comparable short- and long-term outcomes [45, 57]. As a result, a new intraoperative surgical classification is now widely accepted to better designate the location of thromboembolic disease and surgical resection [57, 58].
Advancements in extracorporeal membrane oxygenation (ECMO) technology, utilisation and management has had a significant impact on treatment of patients with CTEPH. This has also translated into improved outcomes, not only in high-risk surgical patients allowing better pre-operative optimisation and perioperative management, but also in rare circumstances when devastating post-operative complications such as severe reperfusion injury or airway haemorrhage are encountered [46, 59–64]. Pioneered at the University of California San Diego, minimally invasive PEA is a new technique where the procedure is performed through bilateral or occasionally unilateral mini-anterior thoracotomy incisions, and can be offered to patients to avoid sternotomy, or are otherwise considered at high risk of wound-healing complications [58, 65, 66].
What determines operability is multifactorial, complex and dependent on centre expertise [28, 67]. Patients can be technically operable, but refuse surgery or have prohibitive comorbidities. Some may have severe discordance between amount of disease and severity of PH, and yet others may have disease that is beyond the reach of expert surgeons. There is no evidence that operable patients benefit from delaying surgery for a trial of bridging therapy with either PH drugs or with BPA [47, 68]. There are now reports of combining PEA with BPA, both sequentially [69–71] or in a hybrid manner [72] where surgery is performed on the operable side and BPA on the inoperable side. However, caution is advised in interpreting these reports, as despite significant advancement in imaging, it is hard to predict the exact level of disease; and obstruction within pulmonary vasculature may be underestimated, thereby potentially denying the patient the opportunity for complete resection of disease.
What constitutes the definition of a CTEPH surgical expert centre at a regional or at a national level is debatable, challenging and may depend on paucity or surplus of other CTEPH centres. But there are certain important characteristics and goals which must be taken into consideration. This has become even more relevant with wide availability of medical therapy and easy access to percutaneous therapies such as BPA for all patients, including those who would clearly benefit from surgery. A three-step stratified definition of expert surgical centre is proposed which factors the following important goals: surgical mortality (<3%), surgical volume (>50 per year), ability to perform segmental endarterectomy [34, 45, 57] and availability of ECMO support if necessary. Furthermore, in this era of a multidisciplinary approach and multimodality treatment of CTEPH, an expert centre should be capable of evaluating and offering any/all established treatment modalities according to individual need.
Balloon pulmonary angioplasty
At the 6th WSPH, BPA was described as “an important component of the CTEPH treatment algorithm”, mainly based on single-centre trials from Japan [73–75], showing promising improvements of haemodynamics, symptoms, exercise capacity and right ventricular function. Shortly before the 6th WSPH, publications from European centres confirmed the lower rates of severe complications compared to the first series published in 2001 [76–82]; however, the benefits were less pronounced than those achieved in Japanese centres. A steep learning curve with extensive training and case experience led to the recommendation of treatment in expert centres [83]. BPA should be considered for symptomatic patients ineligible for PEA due to distally localised pulmonary artery lesions or patients with persistent/recurrent PH after surgery. Therefore, the MDT is deemed obligatory for treatment decision-making. Target lesions for BPA are visualised by pulmonary angiography [84]; the intervention may be augmented using intravascular imaging and pressure gradient analysis [85]. The 6th WSPH summarised the most common complications of BPA and pointed out that vascular injury (mostly caused by wire perforation) may be far more common than the capillary leak syndrome known from PEA surgery [84, 86, 87]. Mid-term results were promising, but the according evidence was scarce, and all trials derived from Japanese centres [88, 89].
Over the past 5 years, BPA has become a guideline-recommended treatment (class IB) for inoperable CTEPH patients or patients with residual PH after PEA [5]. Two randomised controlled trials have been published, comparing the effectiveness of the interventional treatment with drug therapy using riociguat [90, 91]. Furthermore, two multicentre registries [89, 92] have confirmed the findings of several single centre reports [69, 71, 93, 94]. In conclusion, BPA leads to significant improvements of pulmonary haemodynamics and physical capacity with 3-year survival rates of 92–95% [10, 90, 91, 95]. Importantly, it has been shown that the number of successfully treated lesions predicts treatment response, and especially the number of successfully treated occlusions (particularly chronic total occlusions) have the largest impact on relative change in mean pulmonary artery pressure (mPAP) [93]. The rate of restenosis was very low with 0.6% of the treated lesions in a median follow-up of 1.9 years [96]. By far the most common peri-interventional complication with a rate of ∼10% (of the sessions) is lung injury, usually caused by wire perforation [69, 95, 97], with more severe lung injury occurring in cases with mPAP ≥40 mmHg [98]. Accordingly, extra caution has been advised for BPA intervention, including balloon sizing for patients with mPAP ≥40 mmHg [95]. In a systematic review of BPA publications, the rate of lung injury complication was down to 7.7% in the more recent period spanning from 2018 to 2022 [99]. The improved complication rate is probably associated with the learning curve previously reported from the major French series [69]. Importantly, the occurrence of this complication does not affect the outcome of BPA in expert centres [97]. As a promising management strategy, the instillation of gel foam has been described besides proximal vessel occlusion (using a guiding or balloon catheter) [100]. Another important finding was that medical pre-treatment reduced the rate of severe complications of BPA in patients with a PVR >4 Wood Units (WU) in a randomised controlled trial [90]. Furthermore, the combination of riociguat and BPA leads to long-term survival rates of 92.9% after 3 years and 90% after 5 years in inoperable CTEPH patients with significant improvement in quality of life [94, 101].
Not all patients with technically operable disease are amenable to PEA. Treating surgically accessible lesions by BPA leads to significantly less improvement of pulmonary haemodynamics and physical capacity compared to interventional treatment of technically inoperable CTEPH. However, the cumulative survival rates at 1, 5 and 10 years after BPA were not significantly different (92.5%, 86.1%, 84.3%, respectively, versus 96.5%, 92.9%, 90.1%) [102, 103]. As a certain strategy for patients with high-risk for PEA, pre-operative BPA has been used to decrease right ventricular afterload and thus to reduce the perioperative risk: in nine selected CTEPH patients with mixed (proximal and distal) anatomical lesions and baseline PVR >10 WU, all three therapeutic modalities were combined in a sequential multimodal treatment concept [70]. The intraoperative use of BPA in regions that could not be surgically cleared has already been described, but remains a single-centre experience [72]. However, BPA for residual/recurrent PH after PEA appears promising with growing evidence [104–106]. Lastly, a first consensus paper driven by European and Japanese centres summarises a standardised approach to BPA including diagnostic workup, treatment decision-making, procedural techniques and treatment goals [95].
In the 2022 ESC/ERS guidelines [5], BPA was recommended in patients who are technically inoperable or have residual PH after PEA. However, BPA has a weaker recommendation (class IIbC) in patients who are technically operable, but have a high proportion of distal disease and an unfavourable risk/benefit ratio for PEA. In this regard, an ongoing prospective randomised trial (GO-CTEPH; clinicaltrials.gov identifier NCT05110066) compares surgery and intervention in patients eligible for both PEA and BPA [107]. Although current guidelines recommend medical therapy prior to intervention, they do not address whether medical therapy should be continued after achieving normal haemodynamics with BPA. Another ongoing prospective randomised trial (THERAPY-HYBRID-BPA; clinicaltrials.gov identifier NCT04600492) may contribute to developing future recommendations for the long-term treatment strategy after BPA [108]. Although BPA has been expanding globally over the past 5 years, no specific interventional equipment has yet been developed for worldwide use. This is one reason why the interventional technique and its appropriate training has not yet been fully standardised. BPA programmes in most centres developed according to initial reports of refined BPA in 2012, and therefore, long-term survival >10 years after BPA and its determinants have not yet been elucidated. To entirely standardise BPA-specific treatment goals, it is necessary to determine to what extent improvements in haemodynamic parameters after BPA, such as mPAP and PVR values, are sufficient to improve long-term survival in CTEPH patients.
The experience in performing BPA has grown worldwide with numerous single-centre reports, several national registries and RCTs comparing riociguat with BPA. Due to the global expansion of BPA programmes, differences in technical performance, treatment goals and nomenclature of complications need to be addressed. Hopefully, the international BPA registry will shed some light and help guide more uniformity (clinicaltrials.gov identifier NCT03245268) [109].
Medical therapy
In the management of CTEPH, lifelong therapeutic anticoagulation is recommended to prevent recurrent VTE. The established standard of care for anticoagulation in CTEPH involves the utilisation of vitamin K antagonists (VKAs), which have garnered widespread acceptance for this purpose. VKAs are particularly recommended for anticoagulation in CTEPH patients who also present with antiphospholipid syndrome, a condition affecting ∼10% of individuals with CTEPH [5]. In recent years, there has been a rising trend in the utilisation of non-vitamin K oral anticoagulants (NOACs) in the management of CTEPH. Both retrospective and prospective studies have consistently demonstrated comparable major bleeding rates between VKAs and NOACs in CTEPH patients [110–112]. However, specific investigations have reported higher recurrence rates of VTE with NOACs in comparison to VKAs [111, 113]. In particular, a study conducted by Bunclark et al. [113] identified a higher recurrence of VTE in patients treated with NOACs following PEA. A study conducted by Jeong et al. [114] revealed a significant correlation between the use of NOACs and the presence of acute or subacute thrombi at the time of surgery. Furthermore, the prospective EXPERT study reported a higher incidence of embolic or thrombotic events in the NOAC group compared to the VKA group [111]. Importantly, RCTs such as the KABUKI trial and registry data have not demonstrated any significant impact on haemodynamics or survival in patients with CTEPH receiving anticoagulant treatment with VKAs or NOACs [110, 112, 113, 115].
Treating hypoxaemia is suggested for patients with CTEPH to mitigate the consequences of reduced oxygen delivery to organs and potentially prevent worsening of PH by inducing pulmonary vasoconstriction [116, 117]. Additionally, hypoxaemia negatively affects the exercise capacity of patients with PH [116]. A RCT has demonstrated that administering oxygen to patients who experience exercise-induced oxygen desaturation (defined as a decrease of ≥4% in peripheral oxygen saturation to ≤92% during a 6-min walk test) can lead to improvements in exercise capacity and quality of life [118]. An emerging perspective suggests that de-escalation of oxygen therapy is feasible in some patients following de-obstructive treatment [119].
There is a rationale for utilising drugs approved for PAH to address microvasculopathy in inoperable CTEPH patients or in operable patients with residual PH after PEA. For the 6th WSPH, three noteworthy RCTs were available. The BENEFiT study, which evaluated bosentan, a dual endothelin receptor antagonist, reported improvements in PVR, although no significant enhancement in the 6-min walk distance (6MWD) was observed [120]. The CHEST-1 study, evaluating riociguat, an oral soluble guanylate cyclase stimulator, demonstrated improvements in PVR, 6MWD and right ventricular function [121]. Both RCTs included inoperable CTEPH patients or operated patients with residual PH after PEA. The MERIT-1 study investigated macitentan, an endothelin receptor antagonist, in inoperable CTEPH patients and showed improvements in PVR, 6MWD and N-terminal pro-brain natriuretic peptide [122]. Notably, this study provided the initial evidence for combination drug therapy in CTEPH among patients with New York Heart Association functional class III–IV. Subsequently, the long-term benefits of riociguat were confirmed by the CHEST-2 study, and based on these findings, riociguat received approval for the treatment of inoperable CTEPH patients or operable patients with residual PH after PEA [123].
Since the 6th WSPH, two notable RCTs and several studies have provided evidence of the efficacy and safety of PH medical therapy in CTEPH. Post hoc analyses demonstrated the sustained long-term benefits of riociguat on right ventricle function [124, 125]. The CTREPH study, utilising subcutaneous continuous infusion of treprostinil, a stable prostacyclin analogue, showcased efficacy in reducing PVR and improving 6MWD in inoperable CTEPH patients or operated patients with residual PH after PEA [126]. Notably, some patients in this study received background therapy. Furthermore, the JapicCTI-163279 study assessed selexipag, an orally selective prostacyclin receptor agonist, revealing improvements in PVR, though no significant enhancement in 6MWD was observed. This study allowed the utilisation of combination medical therapy [127]. Still, several studies in CTEPH either terminated before completion or failed to demonstrate efficacy. The AMBER-1 study, exploring ambrisentan, an endothelin receptor antagonist in inoperable CTEPH patients, was prematurely halted due to insufficient enrolment [128]. A small randomised controlled trial testing sildenafil did not show improvement in 6MWD [129]. Two studies were interrupted due to futility: the SELECT study with selexipag, terminated before completion due to a lack of efficacy in treating inoperable CTEPH or residual PH after PEA (clinicaltrials.gov identifier NCT03689244); and the MACiTEPH study (clinicaltrials.gov identifier NCT04271475) evaluating macitentan in patients with CTEPH faced a similar outcome, closing early due to futility [130, 131].
There is evidence supporting the use of PH medical therapy before BPA in inoperable CTEPH patients with high PVR to mitigate procedural complications [90, 91]. The utilisation of PH medical therapy before PEA in operable CTEPH cases with high PVR is more controversial, and the task force remains divided on its role prior to PEA. Although there has been an interval safety signal using combination of PH targeted therapies in CTEPH from available RCTs, more robust evidence is needed in CTEPH. The ongoing IMPACT-CTEPH study (clinicaltrials.gov identifier NCT04780932) is positioned to provide valuable information to address this gap in the context of inoperable CTEPH before BPA [132]. There is a lack of data guiding withdrawal of medical therapies after successful BPA and/or PEA. A structured long-term follow-up is required to facilitate such an investigation.
Multimodal therapy
Given the availability of three effective treatments targeting different lesions at different levels of the pulmonary vasculature, the current approach to treatment has evolved to incorporate a multimodal management strategy including the sequential combination of at least two of the three treatment modalities. The importance of a multimodal, individualised approach in CTEPH management at expert centres integrating surgical, interventional, imaging and medical PH expertise was first stated at the 6th WSPH [38]. Then, the ERS statement made clear that some patients may have mixed anatomical lesions (i.e. organised fibrotic clots, proximally at lobar to segmental level, or distally at subsegmental level and microvasculopathy) and may therefore benefit from multimodal therapy, but without consensus on eligibility criteria [1]. The latest ESC/ERS guidelines advocated for a comprehensive review of all CTEPH patients by a multidisciplinary CTEPH team capable of multimodal management, to formulate an effective therapeutic strategy including a combined approach with PEA, BPA and medical treatment to target the different vascular components of the disease [5].
PEA is the recommended first-line treatment for operable patients [5]. However, despite advancements in the surgical technique, PEA remains a challenging surgery, especially for patients with high pre-operative PVR (table 2), as this has been associated with an increase in early post-operative mortality. However, current evidence does not support the use of pharmacological treatment prior to PEA to improve post-operative outcomes [47, 68]. In contrast, the beneficial effect of a combined approach with BPA (prior to or at the same time as surgery) and PEA has been shown in selected patients with high-risk haemodynamics with unilateral operable and contralateral inoperable disease [70, 72, 133].
TABLE 2.
Approach to chronic thromboembolic pulmonary hypertension with severe pulmonary haemodynamics
| Pros | Cons | |
|---|---|---|
| PH medical therapy prior to PEA | • Improving haemodynamics, perceived lowering the risk of PEA • Uncontrolled, anecdotal data • Potential strategy to stabilise PH waiting for access |
• No controlled data • Delays in treatment • Unknown effects of medications on operable disease |
| Combined PEA and BPA (hybrid approach) | • Feasibility published • Access to disease out of reach of PEA at time of surgery |
• Restricted to expert centres • Risk of BPA complication higher than average |
| BPA prior to planned PEA | • Anecdotal data • May allow improvements in haemodynamics prior to PEA |
• Restricted to expert centres • Risk of BPA complication higher than average • PEA may be more challenging in area of prior BPA |
| PEA followed by BPA | • Clearance of reachable disease bilaterally • What remains is inoperable |
• Restricted to expert centres • Higher risk of PEA • Delay with BPA due to surgical recovery • BPA may be challenging due to surgical changes |
| PH medical therapy prior to planned BPA | • Supported by RCTs • Improved haemodynamics before BPA • Lower BPA complications |
• Access/reimbursement of riociguat • Mono- versus combination therapies unclear, but being investigated • Risk of patients continuing on medical therapy unnecessarily post-BPA |
PH: pulmonary hypertension; PEA: pulmonary endartectomy; BPA: balloon pulmonary angioplasty; RCT: randomised controlled trial.
In contrast, BPA, most often in combination with pharmacological therapy, has become an established treatment for selected patients with residual PH post-PEA. Residual PH after PEA, defined at that time as mPAP ≥25 mmHg, has been reported in 17–51% of patients [47, 134]. Single-centre series with small numbers of patients have demonstrated improvements in functional class and haemodynamics at follow-up in patients with residual PH post-PEA who were treated by BPA [105, 106, 135–137]. However, haemodynamic improvement was less pronounced than that in primary BPA. In addition, some reports described very hard occlusions after PEA, which were challenging to dilate [106], potentially resulting in BPA-related complications such as haemoptysis [135]. In a single-institution series, clinically relevant residual PH adversely affecting long-term survival after PEA was noted when mPAP was ≥38 mmHg and PVR was ≥5 WU 3–6 months after surgery. In the same study, the authors also showed that a mPAP ≥30 mmHg correlated with the initiation of medical treatment post-PEA and proposed this threshold value to define clinically significant PH post-PEA for which BPA may be considered [134].
Until the RACE RCT, there was no convincing evidence that medical therapy introduced prior to BPA intervention can improve outcome (haemodynamics, exercise capacity and safety during BPA procedures) in inoperable CTEPH [90]. The MR BPA and RACE studies showed that both BPA and riociguat lowered PVR, with mPAP decreasing more with BPA, and cardiac output increasing more with riociguat in patients with inoperable CTEPH [90, 91]. The RACE ancillary study also demonstrated that riociguat and BPA, used sequentially, had complementary effects and that pre-treatment with riociguat for 6 months prior to BPA decreased the incidence of serious adverse events related to BPA by optimising pre-BPA pulmonary haemodynamics [90]. As the rates of interventional complications can be reduced by pharmacological pre-treatment, the latest ESC/ERS guidelines specified that in patients who are candidates for BPA and have a PVR of >4 WU, medical therapy should be considered prior to the intervention, although the quality of evidence is low [5]. Long-term follow-up is recommended by the latest ESC/ERS guidelines. Regular follow-up, including high-quality imaging techniques and RHC 3–6 months after intervention (PEA or BPA), is required for consideration of a multimodal treatment [5]. These significant advancements in multimodal therapeutic management have resulted in a 3-year survival rate >90% for both operated and nonoperated patients with CTEPH (M. Delcroix, UZ Leuven, Leuven, Belgium; personal communication) [47, 69, 94, 138].
Data regarding the therapeutic target after PEA/BPA or medical therapy are scarce. The goals of CTEPH treatment are alleviation of symptoms, improvement in exercise capacity and quality of life and normalisation or near normalisation of pulmonary haemodynamics at rest [5]. Because of the prognostic threshold of a mPAP of 30 mmHg [134, 139], the minimum haemodynamic goal of BPA should aim for final mPAP <30 mmHg. However, this has not been validated and experts may differ on their goal criteria. It is currently unclear if an initial dual oral combination therapy is more effective than a monotherapy, a concept previously established in PAH. This question is being explored in an ongoing study (IMPACT-CTEPH) [132].
Best practice and expert definitions
The management of CTEPH has become more demanding, but more satisfying, with the advances in multimodal therapies. Once treated primarily with one modality with even the prospect of lung transplantation [26, 140], the field has evolved to obviate the need for lung transplantation. However, successful CTEPH management demands standards which have been reinforced since the last WSPH.
Table 3 summarises best practice recommendations from the expert panel. As emphasised repeatedly, MDT is critical and consists of all four subspecialties attending to each case. The inherent steep learning curve for both PEA and BPA requires proper training and ongoing volume/experience to deliver the best results. A PEA centre should achieve in-hospital mortality rates <5%, with expert centres achieving rates <3% with the capability of treating segmental and subsegmental disease. A BPA centre should achieve procedure-related mortality <3%, with expert BPA centres now reporting mortality rates <1%. For both interventions, higher volume may correlate with improved outcomes. ECMO support should be available for high-risk patients and if needed for major complications. Lastly, a comprehensive CTEPH centre has combined PEA, BPA and PH expertise available to apply any and all modes of CTEPH therapy best suited to the individual case. For any of these centres, a structured follow-up should become standard of care and an important aspect of disease management.
TABLE 3.
Chronic thromboembolic pulmonary hypertension (CTEPH) multidisciplinary team (MDT) and centre expertise
| Requirements | |
|---|---|
| MDT | PEA surgeon + BPA specialist + PH expert + pulmonary vascular radiologist |
| PEA centre | ≥20 surgeries per year with post-operative mortality rate <5%, ECMO support |
| Expert PEA centre | 50 surgeries per year with mortality <3%, capable of treating segmental/subsegmental disease, ECMO support |
| BPA centre | ≥50 procedures per year with procedure related mortality <3% |
| Expert BPA centre | >100 procedures per year with mortality <1%, ECMO support |
| Comprehensive CTEPH centre | Combined PEA + BPA + PH + ECMO expertise available with treatments based on centre MDT |
PEA: pulmonary endartectomy; BPA: balloon pulmonary angioplasty; PH: pulmonary hypertension; ECMO: extracorporeal membrane oxygenation.
In addition, CTEPH centres ought to work on quality measures. To help achieve some of these goals, CTEPH centres are encouraged to network with expert centres for training opportunities and obtaining second opinion for challenging cases [26, 67]. Interested programmes in countries without an established reference centre should collaborate to conduct a national registry of CTEPH cases. This has the potential benefit of pooling both resources and expertise to optimise approach and management of these patients. Such a disease registry, or other collaborative efforts, may shed light on diagnostic and treatment gaps in need of attention. Some of the most informative work has come from such national and international collaborative efforts [49, 141–143].
Shareable PDF
Acknowledgements
The healthcare institutions of M. Delcroix, X. Jaïs, M. Jevnikar, D.P. Jenkins and M. Palazzini are members of the European Reference Network for Rare Lung Diseases (ERN-LUNG).
Footnotes
Conflict of interest: N.H. Kim reports consultancy fees from Bayer, Janssen, Merck, United Therapeutics and Pulnovo, payment or honoraria for lectures, presentations, manuscript writing or educational events from Bayer, Janssen and Merck, participation on a data safety monitoring board or advisory board with Bayer, Janssen, Merck and United Therapeutics, and leadership roles with International CTEPH Association and CTEPH.com. A.M. D'Armini reports payment or honoraria for lectures, presentations, manuscript writing or educational events from AOP Health and MSD, and participation on a data safety monitoring board or advisory board with AOP Health. M. Delcroix reports consultancy fees from Actelion/Janssen/J&J, Acceleron/MSD, Gossamer and Ferrer, and payment or honoraria for lectures, presentations, manuscript writing or educational events from Actelion/Janssen/J&J, Acceleron/MSD and Ferrer. X. Jaïs reports grants from Acceleron, Janssen, MSD and Bayer HealthCare, and consultancy fees and payment or honoraria for lectures, presentations, manuscript writing or educational events from MSD. M. Jevnikar reports payment or honoraria for lectures, presentations, manuscript writing or educational events and support for attending meetings from Janssen and MSD. M.M. Madani reports royalties or licences from Wexler Surgical, consultancy fees from Bayer/MSD and Actelion/Janssen/J&J, and participation on a data safety monitoring board or advisory board with International CTEPH Association. H. Matsubara reports grants from Nippon Shinyaku, payment or honoraria for lectures, presentations, manuscript writing or educational events from Bayer, Janssen, Kaneka Medix, Mochida, MSD, Nippon Shinyaku, Nipro and AOP Orphan, payment for expert testimony from MSD, participation on a data safety monitoring board or advisory board with Bayer, Janssen, Mochida and MSD, and a leadership role with International CTEPH Association. M. Palazzini reports consultancy fees, payment or honoraria for lectures, presentations, manuscript writing or educational events, and support for attending meetings from Janssen. C.B. Wiedenroth reports consultancy fees from J&J, OrphaCare and MSD, payment or honoraria for lectures, presentations, manuscript writing or educational events from J&J, AOP-Health, Bayer, Inari, MSD and Pfizer, and a leadership role with International CTEPH Association. G. Simonneau has no potential conflicts of interest to disclose. D.P. Jenkins reports consultancy fees and payment or honoraria for lectures, presentations, manuscript writing or educational events from Janssen, and and a leadership role with International CTEPH Association.
This article has an editorial commentary: https://doi.org/10.1183/13993003.01222-2024
Support statement: X. Jaïs and M. Jevnikar are supported by state funding managed by the National Research Agency according to the Investments for the Future programme integrated into France 2030, under the reference ANR-18-RHUS-0006 (DESTINATION 2024). C.B. Wiedenroth is supported by Central Project 01 of the DFG-funded CRC1213.
References
- 1.Delcroix M, Torbicki A, Gopalan D, et al. . ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J 2021; 57: 2002828. doi: 10.1183/13993003.02828-2020 [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Montani D, Celermajer DS, et al. . Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durrington C, Hurdman JA, Elliott CA, et al. . Systematic pulmonary embolism follow-up increases diagnostic rates of chronic thromboembolic pulmonary hypertension and identifies less severe disease: results from the ASPIRE Registry. Eur Respir J 2024; 63: 2300846. doi: 10.1183/13993003.00846-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovacs G, Berghold A, Scheidl S, et al. . Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J 2009; 34: 888–894. doi: 10.1183/09031936.00145608 [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, Kovacs G, Hoeper M, et al. . 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023; 61: 2200879. doi: 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 6.Reddy AS, Swietlik EM, Robertson L, et al. . Natural history of chronic thromboembolic pulmonary disease with no or mild pulmonary hypertension. J Heart Lung Transplant 2023; 42: 1275–1285. doi: 10.1016/j.healun.2023.04.016 [DOI] [PubMed] [Google Scholar]
- 7.Taboada D, Pepke-Zaba J, Jenkins DP, et al. . Outcome of pulmonary endarterectomy in symptomatic chronic thromboembolic disease. Eur Respir J 2014; 44: 1635–1645. doi: 10.1183/09031936.00050114 [DOI] [PubMed] [Google Scholar]
- 8.Wiedenroth CB, Olsson KM, Guth S, et al. . Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic disease. Pulm Circ 2018; 8: 2045893217753122. doi: 10.1177/2045893217753122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simonneau G, Dorfmüller P, Guignabert C, et al. . Chronic thromboembolic pulmonary hypertension: the magic of pathophysiology. Ann Cardiothorac Surg 2022; 11: 106–119. doi: 10.21037/acs-2021-pte-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delcroix M, de Perrot M, Jaïs X, et al. . Chronic thromboembolic pulmonary hypertension: realising the potential of multimodal management. Lancet Respir Med 2023; 11: 836–850. doi: 10.1016/S2213-2600(23)00292-8 [DOI] [PubMed] [Google Scholar]
- 11.Egermayer P, Peacock A. Is pulmonary embolism a common cause of chronic pulmonary hypertension? Limitations of the embolic hypothesis. Eur Respir J 2000; 15: 440–448. doi: 10.1034/j.1399-3003.2000.15.03.x [DOI] [PubMed] [Google Scholar]
- 12.Manz XD, Szulcek R, Pan X, et al. . Epigenetic modification of the von Willebrand factor promoter drives platelet aggregation on the pulmonary endothelium in chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2022; 205: 806–818. doi: 10.1164/rccm.202109-2075OC [DOI] [PubMed] [Google Scholar]
- 13.Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest 1993; 103: 685–692. doi: 10.1378/chest.103.3.685 [DOI] [PubMed] [Google Scholar]
- 14.Dorfmüller P, Günther S, Ghigna M-R, et al. . Microvascular disease in chronic thromboembolic pulmonary hypertension: a role for pulmonary veins and systemic vasculature. Eur Respir J 2014; 44: 1275–1288. doi: 10.1183/09031936.00169113 [DOI] [PubMed] [Google Scholar]
- 15.Azarian R, Wartski M, Collignon MA, et al. . Lung perfusion scans and hemodynamics in acute and chronic pulmonary embolism. J Nucl Med 1997; 38: 980–983. [PubMed] [Google Scholar]
- 16.Delcroix M, Kerr K, Fedullo P. Chronic thromboembolic pulmonary hypertension. epidemiology and risk factors. Ann Am Thorac Soc 2016; 13: Suppl. 3, S201–S206. doi: 10.1513/AnnalsATS.201509-621AS [DOI] [PubMed] [Google Scholar]
- 17.Konstantinides SV, Vicaut E, Danays T, et al. . Impact of thrombolytic therapy on the long-term outcome of intermediate-risk pulmonary embolism. J Am Coll Cardiol 2017; 69: 1536–1544. doi: 10.1016/j.jacc.2016.12.039 [DOI] [PubMed] [Google Scholar]
- 18.Gerges C, Pistritto A-M, Gerges M, et al. . Left Ventricular filling pressure in chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2023; 81: 653–664. doi: 10.1016/j.jacc.2022.11.049 [DOI] [PubMed] [Google Scholar]
- 19.Sharma S, Hofbauer TM, Ondracek AS, et al. . Neutrophil extracellular traps promote fibrous vascular occlusions in chronic thrombosis. Blood 2021; 137: 1104–1116. doi: 10.1182/blood.2020005861 [DOI] [PubMed] [Google Scholar]
- 20.Manz XD, Bogaard HJ, Aman J. Regulation of VWF (von Willebrand factor) in inflammatory thrombosis. Arterioscler Thromb Vasc Biol 2022; 42: 1307–1320. doi: 10.1161/ATVBAHA.122.318179 [DOI] [PubMed] [Google Scholar]
- 21.Viswanathan G, Kirshner HF, Nazo N, et al. . Single-cell analysis reveals distinct immune and smooth muscle cell populations that contribute to chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2023; 207: 1358–1375. doi: 10.1164/rccm.202203-0441OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao R, Dong X, Gong J, et al. . Examining the development of chronic thromboembolic pulmonary hypertension at the single-cell level. Hypertension 2022; 79: 562–574. doi: 10.1161/HYPERTENSIONAHA.121.18105 [DOI] [PubMed] [Google Scholar]
- 23.Liley J, Newnham M, Bleda M, et al. . Shared and distinct genomics of chronic thromboembolic pulmonary hypertension and pulmonary embolism. Am J Respir Crit Care Med 2024; 209: 1477–1485. doi: 10.1164/rccm.202307-1236OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swinnen K, Bijnens E, Casas L, et al. . Residential air pollution increases the risk for persistent pulmonary hypertension after pulmonary endarterectomy. Eur Respir J 2021; 57; 2002680. doi: 10.1183/13993003.02680-2020 [DOI] [PubMed] [Google Scholar]
- 25.Rosen K, Raanani E, Kogan A, et al. . Chronic thromboembolic pulmonary hypertension in patients with antiphospholipid syndrome: risk factors and management. J Heart Lung Transplant 2022; 41: 208–216. doi: 10.1016/j.healun.2021.10.016 [DOI] [PubMed] [Google Scholar]
- 26.Kim NH, Delcroix M, Jenkins DP, et al. . Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013; 62: Suppl. 25, D92–D99. doi: 10.1016/j.jacc.2013.10.024 [DOI] [PubMed] [Google Scholar]
- 27.Gopalan D, Delcroix M, Held M. Diagnosis of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160108. doi: 10.1183/16000617.0108-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konstantinides SV, Meyer G, Becattini C, et al. . 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020; 41: 543–603. doi: 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 29.Narechania S, Renapurkar R, Heresi GA. Mimickers of chronic thromboembolic pulmonary hypertension on imaging tests: a review. Pulm Circ 2020; 10: 2045894019882620. doi: 10.1177/2045894019882620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura Y, Tamura Y, Shigeta A, et al. . Adult-onset idiopathic peripheral pulmonary artery stenosis. Eur Respir J 2023; 62: 2300763. doi: 10.1183/13993003.00763-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remy-Jardin M, Ryerson CJ, Schiebler ML, et al. . Imaging of pulmonary hypertension in adults: a position paper from the Fleischner Society. Radiology 2021; 298: 531–549. doi: 10.1148/radiol.2020203108 [DOI] [PubMed] [Google Scholar]
- 32.Le Faivre J, Duhamel A, Khung S, et al. . Impact of CT perfusion imaging on the assessment of peripheral chronic pulmonary thromboembolism: clinical experience in 62 patients. Eur Radiol 2016; 26: 4011–4020. doi: 10.1007/s00330-016-4262-1 [DOI] [PubMed] [Google Scholar]
- 33.Masy M, Giordano J, Petyt G, et al. . Dual-energy CT (DECT) lung perfusion in pulmonary hypertension: concordance rate with V/Q scintigraphy in diagnosing chronic thromboembolic pulmonary hypertension (CTEPH). Eur Radiol 2018; 28: 5100–5110. doi: 10.1007/s00330-018-5467-2 [DOI] [PubMed] [Google Scholar]
- 34.Jenkins D, Madani M, Fadel E, et al. . Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160111. doi: 10.1183/16000617.0111-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barco S, Mavromanoli AC, Kreitner K-F, et al. . Preexisting chronic thromboembolic pulmonary hypertension in acute pulmonary embolism. Chest 2023; 163: 923–932. doi: 10.1016/j.chest.2022.11.045 [DOI] [PubMed] [Google Scholar]
- 36.Ende-Verhaar YM, Meijboom LJ, Kroft LJM, et al. . Usefulness of standard computed tomography pulmonary angiography performed for acute pulmonary embolism for identification of chronic thromboembolic pulmonary hypertension: results of the InShape III study. J Heart Lung Transplant 2019; 38: 731–738. doi: 10.1016/j.healun.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 37.Johns CS, Swift AJ, Rajaram S, et al. . Lung perfusion: MRI vs. SPECT for screening in suspected chronic thromboembolic pulmonary hypertension. J Magn Reson Imaging 2017; 46: 1693–1697. doi: 10.1002/jmri.25714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim NH, Delcroix M, Jais X, et al. . Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1801915. doi: 10.1183/13993003.01915-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hahn L, Papamatheakis D, Fernandes T,et al. . Multidisciplinary approach to chronic thromboembolic pulmonary hypertension: role of radiologists. Radiographics 2023; 43: e220078. doi: 10.1148/rg.220078 [DOI] [PubMed] [Google Scholar]
- 40.de Perrot M, Gopalan D, Jenkins D, et al. . Evaluation and management of patients with chronic thromboembolic pulmonary hypertension – consensus statement from the ISHLT. J Heart Lung Transplant 2021; 40: 1301–1326. doi: 10.1016/j.healun.2021.07.020 [DOI] [PubMed] [Google Scholar]
- 41.Opitz I, Lador F, Aubert JD, et al. . Introduction of a national multidisciplinary CTEPH board to improve operability assessment. Eur Respir J 2020; 56: Suppl. 64, 1541. doi: 10.1183/13993003.congress-2020.1541 [DOI] [Google Scholar]
- 42.Hsieh WC, Jansa P, Huang WC, et al. . Residual pulmonary hypertension after pulmonary endarterectomy: a meta-analysis. J Thorac Cardiovasc Surg 2018; 156: 1275–1287. doi: 10.1016/j.jtcvs.2018.04.110 [DOI] [PubMed] [Google Scholar]
- 43.Madani MM, Auger WR, Pretorius V, et al. . Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg 2012; 94: 97–103. doi: 10.1016/j.athoracsur.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 44.Jenkins DP. Pulmonary thromboendarterectomy in chronic pulmonary disease – the Royal Papworth Hospital experience. Ann Cardiothorac Surg 2022; 11: 175–176. doi: 10.21037/acs-2021-pte-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Perrot M, Donahoe L, McRae K, et al. . Outcome after pulmonary endarterectomy for segmental chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg 2022; 164: 696–707. doi: 10.1016/j.jtcvs.2021.10.078 [DOI] [PubMed] [Google Scholar]
- 46.de Perrot M, McRae K, Donahoe L, et al. . Pulmonary endarterectomy in severe chronic thromboembolic pulmonary hypertension: the Toronto experience. Ann Cardiothorac Surg 2022; 11: 133–142. doi: 10.21037/acs-2021-pte-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delcroix M, Lang I, Pepke-Zaba J, et al. . Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from a prospective international registry. Circulation 2016; 133: 859–871. doi: 10.1161/CIRCULATIONAHA.115.016522 [DOI] [PubMed] [Google Scholar]
- 48.Yang J, Madani MM, Mahmud E, et al. . Evaluation and management of chronic thromboembolic pulmonary hypertension. Chest 2023; 164: 490–502. doi: 10.1016/j.chest.2023.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pepke-Zaba J, Delcroix M, Lang I, et al. . Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124: 1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008 [DOI] [PubMed] [Google Scholar]
- 50.Newnham M, Hernández-Sánchez J, Dunning J, et al. . Age should not be a barrier for pulmonary endarterectomy in carefully selected patients. Eur Respir J 2017; 50: 1701804. doi: 10.1183/13993003.01804-2017 [DOI] [PubMed] [Google Scholar]
- 51.Grazioli V, Ghio S, Pin M, et al. . Pulmonary endarterectomy in the octogenarian population: safety and outcomes. J Cardiovasc Med 2021; 22: 567–571. doi: 10.2459/JCM.0000000000001138 [DOI] [PubMed] [Google Scholar]
- 52.Wiedenroth CB, Bandorski D, Ariobi K, et al. . Does age matter? Pulmonary endarterectomy in the elderly patient with CTEPH. Thorac Cardiovasc Surg 2022; 70: 663–670. doi: 10.1055/s-0041-1740559 [DOI] [PubMed] [Google Scholar]
- 53.Fernandes TM, Auger WR, Fedullo PF, et al. . Baseline body mass index does not significantly affect outcomes after pulmonary thromboendarterectomy. Ann Thorac Surg 2014; 98: 1776–1781. doi: 10.1016/j.athoracsur.2014.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ali JM, Dunning J, Ng C, et al. . The outcome of reoperative pulmonary endarterectomy surgery. Interact Cardiovasc Thorac Surg 2018; 26: 932–937. doi: 10.1093/icvts/ivx424 [DOI] [PubMed] [Google Scholar]
- 55.Astashchanka A, Kerr KM, Yang JZ, et al. . Repeat pulmonary thromboendarterectomy outcomes: a 15-year single-center retrospective review. J Thorac Cardiovasc Surg 2023; 166: 1512–1519. doi: 10.1016/j.jtcvs.2023.02.028 [DOI] [PubMed] [Google Scholar]
- 56.Chan JCY, Man HSJ, Asghar UM, et al. . Impact of sex on outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2023; 42: 1578–1586. doi: 10.1016/j.healun.2023.06.005 [DOI] [PubMed] [Google Scholar]
- 57.Fernandes TM, Kim NH, Kerr KM, et al. . Distal vessel pulmonary thromboendarterectomy: results from a single institution. J Heart Lung Transplant 2023; 42: 1112–1119. doi: 10.1016/j.healun.2023.02.1500 [DOI] [PubMed] [Google Scholar]
- 58.Madani MM. Pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension: state-of-the-art 2020. Pulm Circ 2021; 11: 20458940211007372. doi: 10.1177/20458940211007372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guth S, Wiedenroth CB, Wollenschläger M, et al. . Short-term venoarterial extracorporeal membrane oxygenation for massive endobronchial hemorrhage after pulmonary endarterectomy. J Thorac Cardiovasc Surg 2018; 155: 643–649. doi: 10.1016/j.jtcvs.2017.09.045 [DOI] [PubMed] [Google Scholar]
- 60.Chia AXF, Valchanov K, Ng C, et al. . Perioperative extracorporeal membrane oxygenation support for pulmonary endarterectomy: a 17-year experience from the UK national cohort. J Heart Lung Transplant 2024; 43: 241–250. doi: 10.1016/j.healun.2023.09.008 [DOI] [PubMed] [Google Scholar]
- 61.Ishisaka Y, Watanabe A, Takagi H, et al. . Use of perioperative extracorporeal membranous oxygenation in pulmonary endarterectomy cases: a systematic review and meta-analysis. J Intensive Care Med 2023; 38: 785–796. doi: 10.1177/08850666231178262 [DOI] [PubMed] [Google Scholar]
- 62.Abdelnour-Berchtold E, Donahoe L, McRae K, et al. . Central venoarterial extracorporeal membrane oxygenation as a bridge to recovery after pulmonary endarterectomy in patients with decompensated right heart failure. J Heart Lung Transplant 2022; 41: 773–779. doi: 10.1016/j.healun.2022.02.022 [DOI] [PubMed] [Google Scholar]
- 63.Kabadi AA, Fernandes TM, Papamatheakis DG, et al. . Airway hemorrhage complicating pulmonary thromboendarterectomy: risk factors and outcomes. Ann Thorac Surg 2023; 116: 121–128. doi: 10.1016/j.athoracsur.2022.11.003 [DOI] [PubMed] [Google Scholar]
- 64.Kelava M, Koprivanac M, Smedira N, et al. . Extracorporeal membrane oxygenation in pulmonary endarterectomy patients. J Cardiothorac Vasc Anesth 2019; 33: 60–69. doi: 10.1053/j.jvca.2018.06.025 [DOI] [PubMed] [Google Scholar]
- 65.Madani MM, Higgins JR. Minimally invasive pulmonary thromboendarterectomy: a novel technique. ISMICS Annual Scientific Meeting: Innovations, Technologies, and Techniques in Cardiothoracic and Cardiovascular/Vascular Surgery. 2019. https://meetings.ismics.org/abstracts/2019/C8.cgi.
- 66.Vekstein AM, Armstrong J, Haney J. Early experience with minimally invasive pulmonary thromboendarterectomy for high-risk patients. J Heart Lung Transplant 2023; 42: S19–S20. doi: 10.1016/j.healun.2023.02.036 [DOI] [Google Scholar]
- 67.Jenkins DP, Biederman A, D'Armini AM, et al. . Operability assessment in CTEPH: lessons from the CHEST-1 study. J Thorac Cardiovasc Surg 2016; 152: 669–674. doi: 10.1016/j.jtcvs.2016.02.062 [DOI] [PubMed] [Google Scholar]
- 68.Jensen KW, Kerr KM, Fedullo PF, et al. . Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation 2009; 120: 1248–1254. doi: 10.1161/CIRCULATIONAHA.109.865881 [DOI] [PubMed] [Google Scholar]
- 69.Brenot P, Jaïs X, Taniguchi Y, et al. . French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1802095. doi: 10.1183/13993003.02095-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jevnikar M, Solinas S, Brenot P, et al. . Sequential multimodal therapy in chronic thromboembolic pulmonary hypertension with mixed anatomical lesions: a proof of concept. Eur Respir J 2023; 62: 2300517. doi: 10.1183/13993003.00517-2023 [DOI] [PubMed] [Google Scholar]
- 71.Poch DS, Mahmud E, Patel M, et al. . Patient selection for balloon pulmonary angioplasty: six-year results from a high volume PTE surgical center. Pulm Circ 2022; 12: e12148. doi: 10.1002/pul2.12148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wiedenroth CB, Liebetrau C, Breithecker A, et al. . Combined pulmonary endarterectomy and balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2016; 35: 591–596. doi: 10.1016/j.healun.2015.10.030 [DOI] [PubMed] [Google Scholar]
- 73.Mizoguchi H, Ogawa A, Munemasa M, et al. . Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 748–755. doi: 10.1161/CIRCINTERVENTIONS.112.971077 [DOI] [PubMed] [Google Scholar]
- 74.Kataoka M, Inami T, Hayashida K, et al. . Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 756–762. doi: 10.1161/CIRCINTERVENTIONS.112.971390 [DOI] [PubMed] [Google Scholar]
- 75.Sugimura K, Fukumoto Y, Satoh K, et al. . Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J 2012; 76: 485–488. doi: 10.1253/circj.CJ-11-1217 [DOI] [PubMed] [Google Scholar]
- 76.Ogo T. Balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension. Curr Opin Pulm Med 2015; 21: 425–431. doi: 10.1097/MCP.0000000000000188 [DOI] [PubMed] [Google Scholar]
- 77.Fukui S, Ogo T, Morita Y, et al. . Right ventricular reverse remodelling after balloon pulmonary angioplasty. Eur Respir J 2014; 43: 1394–1402. doi: 10.1183/09031936.00012914 [DOI] [PubMed] [Google Scholar]
- 78.Fukui S, Ogo T, Goto Y, et al. . Exercise intolerance and ventilatory inefficiency improve early after balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Int J Cardiol 2015; 180: 66–68. doi: 10.1016/j.ijcard.2014.11.187 [DOI] [PubMed] [Google Scholar]
- 79.Feinstein JA, Goldhaber SZ, Lock JE, et al. . Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001; 103: 10–13. doi: 10.1161/01.CIR.103.1.10 [DOI] [PubMed] [Google Scholar]
- 80.Andreassen AK, Ragnarsson A, Gude E, et al. . Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart 2013; 99: 1415–1420. doi: 10.1136/heartjnl-2012-303549 [DOI] [PubMed] [Google Scholar]
- 81.Bouvaist H, Thony F, Jondot M, et al. . Balloon pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2014; 23: 393–395. doi: 10.1183/09059180.00000514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roik M, Wretowski D, Rowiński O, et al. . Refined balloon pulmonary angioplasty in inoperable chronic thromboembolic pulmonary hypertension – a multi-modality approach to the treated lesion. Int J Cardiol 2014; 177: e139–e141. doi: 10.1016/j.ijcard.2014.09.051 [DOI] [PubMed] [Google Scholar]
- 83.Ogawa A, Matsubara H. Balloon pulmonary angioplasty: a treatment option for inoperable patients with chronic thromboembolic pulmonary hypertension. Front Cardiovasc Med 2015; 2: 4. doi: 10.3389/fcvm.2015.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kawakami T, Ogawa A, Miyaji K, et al. . Novel angiographic classification of each vascular lesion in chronic thromboembolic pulmonary hypertension based on selective angiogram and results of balloon pulmonary angioplasty. Circ Cardiovasc Interv 2016; 9: e003318. doi: 10.1161/CIRCINTERVENTIONS.115.003318 [DOI] [PubMed] [Google Scholar]
- 85.Roik M, Wretowski D, Łabyk A, et al. . Refined balloon pulmonary angioplasty driven by combined assessment of intra-arterial anatomy and physiology – multimodal approach to treated lesions in patients with non-operable distal chronic thromboembolic pulmonary hypertension – technique, safety and efficacy of 50 consecutive angioplasties. Int J Cardiol 2016; 15: 228–235. doi: 10.1016/j.ijcard.2015.10.116 [DOI] [PubMed] [Google Scholar]
- 86.Kerr KM, Auger WR, Marsh JJ, et al. . The use of cylexin (CY-1503) in prevention of reperfusion lung injury in patients undergoing pulmonary thromboendarterectomy. Am J Respir Crit Care Med 2000; 162: 14–20. doi: 10.1164/ajrccm.162.1.9712142 [DOI] [PubMed] [Google Scholar]
- 87.Lang I, Meyer BC, Ogo T, et al. . Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160119. doi: 10.1183/16000617.0119-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Inami T, Kataoka M, Yanagisawa R, et al. . Long-term outcomes after percutaneous transluminal pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Circulation 2016; 134: 2030–2032. doi: 10.1161/CIRCULATIONAHA.116.024201 [DOI] [PubMed] [Google Scholar]
- 89.Ogawa A, Satoh T, Fukuda T, et al. . Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: results of a multicenter registry. Circ Cardiovasc Qual Outcomes 2017; 10: e004029. doi: 10.1161/CIRCOUTCOMES.117.004029 [DOI] [PubMed] [Google Scholar]
- 90.Jaïs X, Brenot P, Bouvaist H, et al. . Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACE): a multicentre, phase 3, open-label, randomised controlled trial and ancillary follow-up study. Lancet Respir Med 2022; 10: 961–971. doi: 10.1016/S2213-2600(22)00214-4 [DOI] [PubMed] [Google Scholar]
- 91.Kawakami T, Matsubara H, Shinke T, et al. . Balloon pulmonary angioplasty versus riociguat in inoperable chronic thromboembolic pulmonary hypertension (MR BPA): an open-label, randomised controlled trial. Lancet Respir Med 2022; 10: 949–960. doi: 10.1016/S2213-2600(22)00171-0 [DOI] [PubMed] [Google Scholar]
- 92.Darocha S, Roik M, Kopeć G, et al. . Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension: a multicentre registry. Eurointervention 2022; 17: 1104–1111. doi: 10.4244/EIJ-D-21-00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gerges C, Friewald R, Gerges M, et al. . Efficacy and safety of percutaneous pulmonary artery subtotal occlusion and chronic total occlusion intervention in chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2021; 14: e010243. doi: 10.1161/CIRCINTERVENTIONS.120.010243 [DOI] [PubMed] [Google Scholar]
- 94.Wiedenroth CB, Rolf A, Steinhaus K, et al. . Riociguat and balloon pulmonary angioplasty improve prognosis in patients with inoperable chronic thromboembolic pulmonary Hypertension. J Heart Lung Transplant 2023; 42: 134–139. doi: 10.1016/j.healun.2022.08.011 [DOI] [PubMed] [Google Scholar]
- 95.Lang IM, Andreassen AK, Andersen A, et al. . Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: a clinical consensus statement of the ESC working group on pulmonary circulation and right ventricular function. Eur Heart J 2023; 44: 2659–2671. doi: 10.1093/eurheartj/ehad413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tabuchi I, Ogawa A, Shigetoshi M, et al. . Low incidence of restenosis after successful balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Cardiovasc Interv Ther 2023; 38: 231–240. doi: 10.1007/s12928-022-00866-y [DOI] [PubMed] [Google Scholar]
- 97.Wiedenroth CB, Deissner H, Adameit MSD, et al. . Complications of balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension: impact on the outcome. J Heart Lung Transplant 2022; 41: 1086–1094. doi: 10.1016/j.healun.2022.05.002 [DOI] [PubMed] [Google Scholar]
- 98.Ejiri K, Ogawa A, Fujii S, et al. . Vascular injury is a major cause of lung injury after balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2018; 11: e005884. doi: 10.1161/CIRCINTERVENTIONS.117.005884 [DOI] [PubMed] [Google Scholar]
- 99.Jain N, Sheikh MA, Bajaj D, et al. . Periprocedural complications with balloon pulmonary angioplasty: analysis of global studies. JACC Cardiovasc Interv 2023; 16: 976–983. doi: 10.1016/j.jcin.2023.01.361 [DOI] [PubMed] [Google Scholar]
- 100.Ejiri K, Ogawa A, Shimokawahara H, et al. . Treatment of vascular injury during balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. JACC Asia 2022; 2: 831–842. doi: 10.1016/j.jacasi.2022.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wiedenroth CB, Steinhaus K, Rolf A, et al. . Patient -reported long-term outcome of balloon pulmonary angioplasty for inoperable CTEPH. Thorac Cardiovasc Surg 2023; in press [ 10.1055/s-0043-1772770]. doi: [DOI] [PubMed] [Google Scholar]
- 102.Nishihara T, Shimokawahara H, Ogawa A, et al. . Comparison of the safety and efficacy of balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension patients with surgically accessible and inaccessible lesions. J Heart Lung Transplant 2023; 42: 786–794. doi: 10.1016/j.healun.2023.01.003 [DOI] [PubMed] [Google Scholar]
- 103.Darocha S, Araszkiewicz A, Kurzyna M, et al. . Balloon pulmonary angioplasty in technically operable and technically inoperable chronic thromboembolic pulmonary hypertension. J Clin Med 2021; 10: 1038. doi: 10.3390/jcm10051038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shimura N, Kataoka M, Inami T, et al. . Additional percutaneous transluminal pulmonary angioplasty for residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol 2015; 183: 138–142. doi: 10.1016/j.ijcard.2015.01.034 [DOI] [PubMed] [Google Scholar]
- 105.Yanaka K, Nakayama K, Shinke T, et al. . Sequential hybrid therapy with pulmonary endarterectomy and additional balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. J Am Heart Assoc 2018; 7: e008838. doi: 10.1161/JAHA.118.008838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Araszkiewicz A, Darocha S, Pietrasik A, et al. . Balloon pulmonary angioplasty for the treatment of residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol 2019; 278: 232–237. doi: 10.1016/j.ijcard.2018.10.066 [DOI] [PubMed] [Google Scholar]
- 107.Balloon Pulmonary anGiOplasty Versus Pulmonary Endarterectomy in Patients With Chronic ThromboEmbolic Pulmonary Hypertension: a Non-inferiority Randomized Trial (GO-CTEPH). https://clinicaltrials.gov/study/NCT05110066. Date last updated: 29 April 2024.
- 108.The Effect of Riociguat for Peak Cardiac Index on Cardiopulmonary Exercise Test in CTEPH Patients After Normalization of Pulmonary Artery Pressure by Combination Treatment of Riociguat and Balloon Pulmonary Angioplasty (THERAPY-HYBRID-BPA Trial). https://clinicaltrials.gov/study/NCT04600492. Date last updated: 13 October 2020.
- 109.International BPA Registry. https://clinicaltrials.gov/study/NCT03245268. Date last updated: 10 June 2022.
- 110.Barati S, Amini H, Ahmadi ZH, et al. . Evaluating the efficacy and safety of rivaroxaban as a warfarin alternative in chronic thromboembolic pulmonary hypertension patients undergoing pulmonary endarterectomy: a randomized clinical trial. Rev Port Cardiol 2023; 42: 139–144. doi: 10.1016/j.repc.2021.09.023 [DOI] [PubMed] [Google Scholar]
- 111.Humbert M, Simonneau G, Pittrow D, et al. . Oral anticoagulants (NOAC and VKA) in chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2022; 41: 716–721. doi: 10.1016/j.healun.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 112.Hosokawa K, Watanabe H, Taniguchi Y, et al. . A multicenter, single-blind, randomized, warfarin-controlled trial of edoxaban in patients with chronic thromboembolic pulmonary hypertension: KABUKI trial. Circulation 2024; 149: 406–409. doi: 10.1161/CIRCULATIONAHA.123.067528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bunclark K, Newnham M, Chiu Y-D, et al. . A multicenter study of anticoagulation in operable chronic thromboembolic pulmonary hypertension. J Thromb Haemost 2020; 18: 114–122. doi: 10.1111/jth.14649 [DOI] [PubMed] [Google Scholar]
- 114.Jeong I, Alotaibi M, Fernandes TM, et al. . Direct oral anticoagulants in patients with chronic thromboembolic pulmonary hypertension and the presence of recent thrombus during pulmonary endarterectomy. Pulm Circ 2022; 12: e12110. doi: 10.1002/pul2.12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hosokawa K, Abe K, Funakoshi K, et al. . Long-term outcome of chronic thromboembolic pulmonary hypertension using direct oral anticoagulants and warfarin: a Japanese prospective cohort study. J Thromb Haemost 2023; 21: 2151–2162. doi: 10.1016/j.jtha.2023.03.036 [DOI] [PubMed] [Google Scholar]
- 116.Sun XG, Hansen JE, Oudiz RJ, et al. . Exercise pathophysiology in patients with primary pulmonary hypertension. Circulation 2001; 104: 429–435. doi: 10.1161/hc2901.093198 [DOI] [PubMed] [Google Scholar]
- 117.Marshall C, Marshall B. Site and sensitivity for stimulation of hypoxic pulmonary vasoconstriction. J Appl Physiol Environ Exerc Physiol 1983; 55: 711–716. doi: 10.1152/jappl.1983.55.3.711 [DOI] [PubMed] [Google Scholar]
- 118.Ulrich S, Saxer S, Hasler ED, et al. . Effect of domiciliary oxygen therapy on exercise capacity and quality of life in patients with pulmonary arterial or chronic thromboembolic pulmonary hypertension: a randomised, placebo-controlled trial. Eur Respir J 2019; 54: 1900276. doi: 10.1183/13993003.002762019 [DOI] [PubMed] [Google Scholar]
- 119.Kimura M, Kohno T, Shinya Y, et al. . De-escalation of oxygen therapy and medication in patients with chronic thromboembolic pulmonary hypertension after balloon pulmonary angioplasty. Can J Cardiol 2023; 39: 637–645. doi: 10.1016/j.cjca.2023.01.014 [DOI] [PubMed] [Google Scholar]
- 120.Jaïs X, D'Armini AM, Jansa P, et al. . Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol 2008; 52: 2127–2134. doi: 10.1016/j.jacc.2008.08.059 [DOI] [PubMed] [Google Scholar]
- 121.Ghofrani H-A, D'Armini AM, Grimminger F, et al. . Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319–329. doi: 10.1056/NEJMoa1209657 [DOI] [PubMed] [Google Scholar]
- 122.Ghofrani H-A, Simonneau G, D'Armini AM, et al. . Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med 2017; 5: 785–794. doi: 10.1016/S2213-2600(17)30305-3 [DOI] [PubMed] [Google Scholar]
- 123.Simonneau G, D'Armini AM, Ghofrani H-A, et al. . Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST-2 open-label, randomised, long-term extension trial. Lancet Respir Med 2016; 4: 372–380. doi: 10.1016/S2213-2600(16)30022-4 [DOI] [PubMed] [Google Scholar]
- 124.Marra AM, Halank M, Benjamin N, et al. . Right ventricular size and function under riociguat in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (the RIVER study). Respir Res 2018; 19: 258. doi: 10.1186/s12931-018-0957-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Benza RL, Ghofrani HA, Grünig E, et al. . Effect of riociguat on right ventricular function in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2021; 40: 1172–1180. doi: 10.1016/j.healun.2021.06.020 [DOI] [PubMed] [Google Scholar]
- 126.Sadushi-Kolici R, Jansa P, Kopec G, et al. . Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTREPH): a double-blind, phase 3, randomised controlled trial. Lancet Respir Med 2019; 7: 239–248. doi: 10.1016/S2213-2600(18)30367-9 [DOI] [PubMed] [Google Scholar]
- 127.Ogo T, Shimokawahara H, Kinoshita H, et al. . Selexipag for the treatment of chronic thromboembolic pulmonary hypertension. Eur Respir J 2022; 60: 2101694. doi: 10.1183/13993003.01694-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Escribano-Subias P, Bendjenana H, Curtis PS, et al. . Ambrisentan for treatment of inoperable chronic thromboembolic pulmonary hypertension (CTEPH). Pulm Circ 2019; 9: 2045894019846433. doi: 10.1177/2045894019846433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suntharalingam J, Treacy CM, Doughty NJ, et al. . Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest 2008; 134: 229–236. doi: 10.1378/chest.07-2681 [DOI] [PubMed] [Google Scholar]
- 130.A Study to Find Out if Selexipag is Effective and Safe in Patients With Chronic Thromboembolic Pulmonary Hypertension When the Disease is Inoperable or Persistent/Recurrent After Surgery and/or Interventional Treatment (SELECT). https://clinicaltrials.gov/study/NCT03689244. Date last updated: 21 June 2021.
- 131.A Prospective, Randomized, Double-blind, Multicenter, Placebo-controlled, Parallel Group, Adaptive Phase 3 Study With Open-label Extension to Evaluate Efficacy and Safety of Macitentan 75 mg in Inoperable or Persistent/Recurrent Chronic Thromboembolic Pulmonary Hypertension (MACiTEPH). https://clinicaltrials.gov/study/NCT04271475. Date last updated: 2 February 2024.
- 132.Initial Dual Oral coMbination Therapy Versus Standard-of-care Initial Oral Monotherapy Prior to Balloon Pulmonary Angioplasty in Patients With Inoperable Chronic Thromboembolic Pulmonary hypertension (IMPACT-CTEPH). https://clinicaltrials.gov/study/NCT04780932. Date last updated: 20 June 2024.
- 133.Shimahara Y, Suzuki S, Fujiyoshi T, et al. . Balloon pulmonary angioplasty followed by pulmonary endarterectomy: combination treatment for high-surgical-risk patients with chronic thromboembolic pulmonary hypertension. Interdiscip Cardiovasc Thorac Surg 2023; 36: ivad031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cannon JE, Su L, Kiely DG, et al. . Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the UK national cohort. Circulation 2016; 133: 1761–1771. doi: 10.1161/CIRCULATIONAHA.115.019470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Andersen A, Hansen JV, Dragsbaek SJ, et al. . Balloon pulmonary angioplasty for patients with chronic thromboembolic pulmonary hypertension previously operated by pulmonary endarterectomy. Pulm Circ 2022; 12: e12115. doi: 10.1002/pul2.12115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nishiyama M, Inoue Y, Sasaki H, et al. . Long-term outcomes of combined pulmonary endarterectomy and additional balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Gen Thorac Cardiovasc Surg 2023; 71: 291–298. doi: 10.1007/s11748-022-01872-w [DOI] [PubMed] [Google Scholar]
- 137.Kirby LC, Rodgers MS, Amaral-Almeida L, et al. . Balloon pulmonary angioplasty outcomes in pateints previously treated by pulmonary endarterectomy surgery are inferior to those of inoperable patients. Pulm Circ 2023; 13: e12265. doi: 10.1002/pul2.12265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Taniguchi Y, Jaïs X, Jevnikar M, et al. . Predictors of survival in patients with not-operated chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2019; 38: 833–842. doi: 10.1016/j.healun.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 139.Riedel M, Stanek V, Widimsky J, et al. . Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 1982; 81: 151–158. doi: 10.1378/chest.81.2.151 [DOI] [PubMed] [Google Scholar]
- 140.Hoeper MM, Barberà JA, Channick RN, et al. . Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol 2009; 54: S85–S96. doi: 10.1016/j.jacc.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 141.Mayer E, Jenkins D, Lindner J, et al. . Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011; 141: 702–710. doi: 10.1016/j.jtcvs.2010.11.024 [DOI] [PubMed] [Google Scholar]
- 142.Kerr KM, Elliott CG, Chin K, et al. . Results from the United States chronic thromboembolic pulmonary hypertension registry: enrollment characteristics and 1-year follow-up. Chest 2021; 160: 1822–1831. doi: 10.1016/j.chest.2021.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guth S, D'Armini AM, Delcroix M, et al. . Current strategies for managing chronic thromboembolic pulmonary hypertension: results of the worldwide prospective CTEPH registry. ERJ Open Res 2021; 7: 00850-2020. doi: 10.1183/23120541.00850-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01294-2024.Shareable (380.1KB, pdf)