Abstract
Pulmonary arterial hypertension leads to significant impairment in haemodynamics, right heart function, exercise capacity, quality of life and survival. Current therapies have mechanisms of action involving signalling via one of four pathways: endothelin-1, nitric oxide, prostacyclin and bone morphogenetic protein/activin signalling. Efficacy has generally been greater with therapeutic combinations and with parenteral therapy compared with monotherapy or nonparenteral therapies, and maximal medical therapy is now four-drug therapy. Lung transplantation remains an option for selected patients with an inadequate response to therapies.
Shareable abstract
In this document from the 7th World Symposium on Pulmonary Hypertension, an updated treatment algorithm is outlined. Medications targeting four pathways are now available, and initial combination therapy with early and frequent reassessment is recommended. https://bit.ly/46ctth4
Introduction
Medications approved to treat pulmonary arterial hypertension (PAH) lead to improvement in functional class, exercise capacity, haemodynamics, right heart function and brain natriuretic peptide (BNP)/N-terminal pro-BNP (NT-proBNP) levels, among others [1–3]. These benefits have been seen in clinical trials across a wide range of PAH severities and across multiple PAH subgroups.
Compared with monotherapy, larger improvements are seen with combination therapy and thus most patients with PAH are candidates for oral or oral plus parenteral combination therapy from the time of diagnosis, along with early reassessment and escalation in therapy when indicated (figure 1).
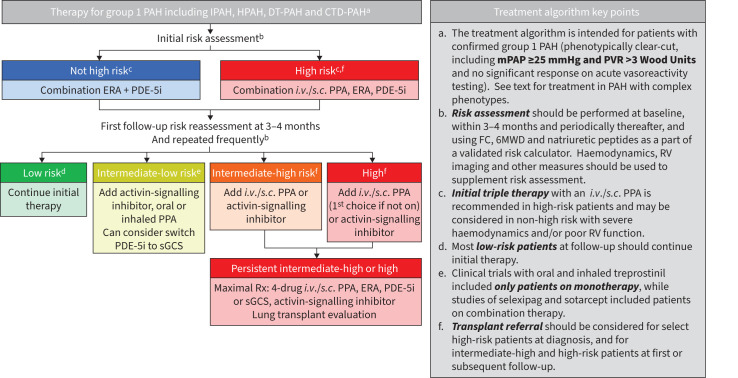
FIGURE 1.
Treatment algorithm. PAH: pulmonary arterial hypertension; IPAH: idiopathic PAH; HPAH: hereditary PAH; DT: drug and toxin; CTD: connective tissue disease; ERA: endothelin-1 receptor antagonist; PDE-5i: phosphodiesterase-5 inhibitor; i.v.: intravenous; s.c.: subcutaneous; PPA: prostacyclin pathway agent; sGCS: soluble guanylyl cyclase stimulator; Rx: prescription; mPAP: mean pulmonary artery pressure; PVR: pulmonary vascular resistance; FC: functional class; 6MWD: 6-min walk distance; RV: right ventricle.
Trials utilising a time-to-clinical-worsening composite as a primary or secondary end-point have demonstrated that PAH therapies also improve longer-term outcomes. Results have been driven most strongly by reductions in PAH clinical worsening and in PAH-related hospitalisations, but in meta-analyses a modest reduction in all-cause mortality has also been reported [4]. Indeed, survival from the time of diagnosis appears to be improving in observational studies as well, although the median survival time remains <10 years [5, 6]. There remains a need for improved utilisation of existing therapies, particularly parenteral prostacyclin pathway agents (PPAs), as well as the targeting of novel therapeutic pathways.
The addition of sotatercept, an activin signalling inhibitor, to the treatment armamentarium has brought significant optimism, as it is the first treatment to act on a completely novel pathway in nearly two decades [7, 8], and four-pathway drug combination therapy is now an option (figure 2). Longer-term studies are still underway and will be important in providing a better understanding of the overall benefits and safety profile of this medication.
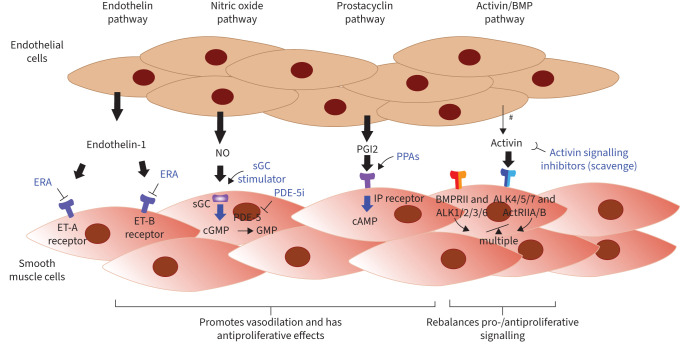
FIGURE 2.
Pulmonary arterial hypertension (PAH) therapies work through four major pathways: endothelin-1 receptor antagonists (ERAs) block the endothelin (ET)-1 receptor. Phosphodiesterase-5 inhibitors (PDE-5i) and soluble guanylyl cyclase (sGC) stimulators increase signalling in the nitric oxide (NO) and cyclic GMP (cGMP) pathway, resulting in increased cGMP levels, and prostacyclin (PGI2) and other prostacyclin pathway agents (PPAs) bind the prostacyclin receptor (IP receptor), promoting the production of cAMP, leading to vasodilation and inhibiting vascular cell growth. Sotatercept, a novel biologic agent targeting the transforming growth factor-β superfamily, acts as a ligand trap for activins and related growth factors. This helps rebalance growth-promoting and growth-inhibiting signalling pathways, with multiple downstream effects. Signalling is shown as proceeding from endothelial cell to smooth muscle cell for simplicity, but is bidirectional. BMPR: bone morphogenetic protein receptor; ALK: anaplastic lymphocyte kinase; ActR: activin receptor. #: in addition, signalling mediators also originate from multiple other cell types, particularly for activin.
In this expert consensus document from the World Symposium on Pulmonary Hypertension (WSPH), we aim to:
1) outline overall treatment recommendations for PAH (figure 1);
2) provide recommendations for specific PAH subgroups;
3) review clinical trials completed since the last WSPH in 2018 (table 1 [7–17]);
4) review medication profiles (table 2), and discuss transitions from one medication to another;
5) discuss knowledge gaps and proposals for future research.
TABLE 1.
Key clinical trials with approved pulmonary arterial hypertension (PAH) medications since the 6th World Symposium on Pulmonary Hypertension in 2018
| Aetiology Subjects n |
Groups | Weeks n | Primary end-point |
Primary end-point results (95% CI) |
Positive primary end-point | Other end-points met | |
|---|---|---|---|---|---|---|---|
| Initial combination therapy (all or majority) | |||||||
| TRITON [9] | PAH 247 |
Macitentan, tadalafil, selexipag versus macitentan, tadalafil, placebo | 26 | PVR | GMR: 0.96 (0.86–1.07) | No | NA |
| A DUE [10] | PAH 187 |
Macitentan + tadalafil FDC versus macitentan or tadalafil# | 16 | PVR | GMR: macitentan + tadalafil versus macitentan: 0.71 (0.61–0.82) GMR: macitenan + tadalafil versus tadalafil: 0.72 (0.64–0.8) |
Yes | NT-proBNP |
| Sequential combination therapy | |||||||
| FREEDOM-EV [11] | PAH 690 |
Oral treprostinil versus placebo | NA | TTCW | HR: 0.74 (0.56–0.97) | Yes | FC, Borg dyspnoea score, NT-proBNP |
| Novel therapies | |||||||
| PULSAR [7, 12] | PAH 106 |
Sotatercept 0.3 mg, 0.7 mg, placebo | 24 | PVR | LSM: 0.3 mg −145.8 (−241.0–−50.6), 0.7 mg −239.5 (−329.3–−149.7) | Yes | 6MWD, NT-proBNP |
| STELLAR [8] | PAH 323 |
Sotatercept versus placebo | 24 | Walk | HLE: 40.8 (27.5–54.1) m | Yes | NT-proBNP, FC, TTCW, others |
| Specific subgroups | |||||||
| PORTICO [13] | PoPH 85 |
Macitentan versus placebo | 12 | PVR | GMR: 0.65 (0.0.59–0.72) | Yes | mPAP, cardiac index |
| MAESTRO [14] | Eisenmenger syndrome 226 |
Macitentan versus placebo | 16 | Walk | −4.7 (−22.8–13.5) m | No | NA |
| New end-points | |||||||
| REPLACE [15] | PAH 226 |
Open-label riociguat versus continued PDE-5i | 24 | Clinical improvement | OR: 2.78 (1.53–5.06) | Yes | Clinical worsening events |
| TRACE [16] | PAH 108 |
Selexipag versus placebo | 24 | Actigraphy | 3 primary end-points, no difference (time, steps) | No | NA |
| AFFILIATE [17] | PAH 385 |
Sildenafil 5, 20, 80 mg three times daily | NA | Mortality 5 mg versus 80 mg (noninferiority) | Mortality 80 mg versus 5 mg: HR 0.51 (99.7% CI 0.22–1.21) | Yes¶ | 80 mg superior to 5 mg for TTCW, 6MWD |
PVR: pulmonary vascular resistance; GMR: geometric mean ratio; NA: not applicable; FDC: fixed-dose combination (of macitentan and tadalafil); NT-proBNP: N-terminal pro-brain natriuretic peptide; TTCW: time to clinical worsening; HR: hazard ratio; FC: functional class; LSM: least-squares mean; 6MWD: 6-min walk distance, HLE: Hodges–Lehmann estimate; PoPH: portopulmonary hypertension; mPAP: mean pulmonary artery pressure; PDE-5i: phosphodiesterase-5 inhibitor. #: in A DUE, the macitentan + tadalafil comparison groups were 1) former treatment-naïve or endothelin-1 receptor antagonist (ERA) patients, for the comparison versus macitentan and 2) former treatment-naïve or PDE-5i patients, for the comparison versus tadalafil. ¶: the AFFILIATE study met its primary end-point of noninferiority between sildenafil 80 mg three times daily and 5 mg three times daily on mortality, based on prespecified noninferiority thresholds. However, the study was halted early because of the numeric imbalance in mortality (26.4%, 19.5% and 14.8% in the 5 mg, 20 mg and 80 mg groups, respectively).
TABLE 2.
Medications for pulmonary arterial hypertension
| Medications | Common adverse reactions | Other important information | |
|---|---|---|---|
| Oral medications | |||
| PDE-5i [18–21] | Sildenafil, tadalafil | Headache Flushing Dyspepsia Epistaxis |
Rare loss of vision or hearing Avoid with nitrates, riociguat |
| Guanylyl cyclase stimulators [22] | Riociguat | Headache Dyspepsia Dizziness Hypotension |
Avoid in pregnancy#, avoid with nitrates, PDE-5i Monitor for hypotension; may require dose adjustment based on systemic SBP |
| Endothelin-1 receptor antagonists [21, 23–27] | Ambrisentan, bosentan, macitentan | Peripheral oedema Nasal congestion Anaemia¶ |
Avoid in pregnancy#, monitor haemoglobin (all), liver function (monthly for bosentan, as clinically indicated for others) |
| Prostacyclin receptor agonists [28] | Selexipag | Prostanoid-type AEs+ | Data on selexipag in pregnancy are not available |
| Prostanoids, p.o. [11, 29–32] | Treprostinil, beraprost | Prostanoid-type AEs+ | |
| Inhaled medications | |||
| Prostanoids, inhaled [33, 34] | Iloprost, treprostinil | Cough Prostanoid-type AEs+ |
|
| Parenteral medications | |||
| Prostanoids, parenteral [35, 36] | Epoprostenol (i.v.), treprostinil (i.v., s.c.) | Prostanoid-type AEs+ | Sudden discontinuation of parenteral prostanoids can be life-threatening |
| Activin-signalling inhibitor [7, 8] | Sotatercept (s.c.) | Headache Diarrhoea Nosebleed Bleeding events Telangiectasia |
Avoid in pregnancy#; potential risk of reduced future fertility based on animal studies; monitor for thrombocytopenia and increased haemoglobin for first five doses and periodically |
PDE-5i: phosphodiesterase-5 inhibitor; SBP: systolic blood pressure; AE: adverse events; i.v.: intravenous; s.c.: subcutaneous. #: highly reliable contraception and monthly pregnancy testing required for all individuals of childbearing potential due to risk of teratogenicity; ¶: while adverse reactions to endothelin-1 receptor antagonists tend to be class effects, there is some variability and switching within the same class can be considered; +: prostanoid-type AEs include flushing, headache, jaw pain, nausea/vomiting, diarrhoea.
Treatment goals and risk stratification
A wide range of prognostic measures have been identified in PAH, with functional class, 6-min walk distance (6MWD), NT-proBNP, right ventricular imaging and haemodynamics among the strongest. Patients achieving a lower-risk status based on these measures have better outcomes than patients who fail to do so [37], and PAH treatment guidelines have traditionally utilised these severity measures as a means for choosing PAH therapies, with a goal of achieving a low-risk status.
While this approach has made sense based on both the order in which PAH therapies were discovered as well as the inclusion criteria and trial design for most early studies where only patients of certain functional classes were included, achieving a low-risk status in the short-term has not been found to be a strong surrogate for longer-term clinical worsening outcomes [38].
It is therefore likely that alternative strategies will be needed in the future, optimally determined empirically and through randomised studies to identify optimal treatment strategies.
What future treatment algorithms may look like remains to be seen: will early or upfront initiation of three or even four medications be shown to be beneficial, leading to a strategy similar to guideline directed medical therapy in heart failure [39]? Or will a targeted stepwise approach be recommended, perhaps with alternative goals such as normalisation or near-normalisation of mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR)?
At the present time, we lack evidence to support either of these approaches. Indeed, initial triple oral therapy was found not to be superior in the TRITON study, which evaluated combination therapy with tadalafil, macitentan, and selexipag versus tadalafil, macitentan and placebo [9]. Continued use of a predominantly risk-based treatment approach is therefore recommended, but with the anticipation that this will likely change as additional studies are completed.
Details on risk stratification can be found in the WSPH task force article on PAH risk stratification and treatment goals [40]. In general, recommendations are for initial treatment based on stratification as high versus not high risk, while beginning at first follow-up a “four-tier” approach is recommended, stratifying patients into low risk, intermediate-low, intermediate-high and high risk based on risk calculators. The most commonly utilised include those developed from the Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL registry), including REVEAL 2.0 and REVEAL lite 2.0 [41], and the European Society of Cardiology (ESC)/European Respiratory Society (ERS) calculator, studied with slight variations across multiple cohorts [42–46]. Noninvasive prognostic measures included in all the commonly used risk calculators include functional class, 6MWD and BNP or NT-proBNP levels. Patients with good results on all three measures have excellent 1-year outcomes and these results can be utilised as a core component in decisions regarding PAH medical therapies. However, optimal risk reassessment for making treatment decisions requires other measures including serial cardiac imaging and haemodynamics, particularly when considering major treatment changes. These measures are important in order to more fully characterise overall disease status and because factors including age, comorbidities, musculoskeletal disease and other factors beyond PAH severity can also affect the noninvasive results.
Supportive measures
Supportive measures recommended for PAH patients are summarised in table 3 [47–53]. Although no major new supportive care recommendations are made when compared to the most recent WSPH and ESC/ERS PAH guidelines [47, 48], attention to these measures remains important. In particular, female patients of childbearing potential should be offered highly effective birth control, and recommendations against pregnancy for most patients are maintained, due to unacceptably high maternal risk in PAH; see also pregnancy recommendations in the WSPH article on PAH management in special conditions [54]. As general healthcare measures, all PAH patients should receive vaccinations, with particular attention to respiratory pathogens including influenza, Streptococcus pneumoniae and severe acute respiratory syndrome coronavirus 2 [47], and vaccination against respiratory syncytial virus should be recommended in patients aged >60 years and can be considered in PAH in general [49]. Data from randomised controlled clinical trials (RCTs) on anticoagulation in PAH are lacking and available data derived from observational studies and registries have demonstrated conflicting results regarding efficacy in idiopathic (IPAH), hereditary (HPAH) or drug- or toxin-induced (DT)-PAH [55–58]. Therefore, anticoagulation is not generally recommended but may be considered on an individual basis [47]. In contrast, registry data have more consistently suggested an absence of benefit and probable harm with anticoagulation in CTD-PAH, and its routine use is not recommended [47]. Finally, pre-transplant counselling on issues such as weight optimisation, avoidance of tobacco and illicit drugs, and attention to treatment of comorbidities is recommended, in order to ensure potential future barriers to transplant are addressed [59].
TABLE 3.
| Supervised exercise training |
| Psychological support |
| Immunisation against SARS-CoV-2, influenza, Streptococcus pneumoniae and consider vaccination against RSV |
| Diuretic treatment in patients with fluid retention |
| Continuous LTOT when arterial blood oxygen pressure is consistently <8 kPa (60 mmHg) |
| Correction of iron status in patients with iron-deficiency anaemia |
| Advise against pregnancy |
| Clear contraceptive advice |
| Pre-transplant counselling |
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; RSV: respiratory syncytial virus; LTOT: long-term oxygen therapy.
Vasoreactivity and calcium channel blockers
It is recommended that patients with IPAH, HPAH or DT-PAH undergo acute vasoreactivity testing during the index right heart catheterisation in order to identify patients who should be treated with calcium channel blockers (CCBs). Acute vasoreactivity is defined by a decrease of the mPAP by ≥10 mmHg to reach an absolute mPAP <40 mmHg, without a decrease in cardiac output [47]. A recent multicentre study evaluating acute vasoresponders has found that patients meeting these haemodynamic criteria plus having a large improvement in pulmonary arterial compliance were more likely to have a long-term response, but we do not recommend a change in the acute vasoresponder criteria at this time. Significant heterogeneity in long-term response was also found in this study, emphasising the need for early comprehensive risk-reassessment at 3–4 months [60]. CCBs should be initiated at a low doses followed by uptitration based on overall response including haemodynamic measures; to achieve an optimal response, high doses may be required. Treatment goals include a low-risk profile on noninvasive testing and near normalisation in haemodynamics. Former CCB responders should be managed as per the standard (nonvasodilator) PAH algorithm; whether to discontinue CCBs is not well studied.
Patients with PAH and mPAP 21–24 mmHg and/or PVR <3 Wood Units
The 6th WSPH recommended lowering the mPAP threshold for the diagnosis of PH from ≥25 mmHg to >20 mmHg [61]. Subsequently, the ESC/ERS guidelines recommended lowering the threshold for abnormal from >3 Wood Units (WU) to >2 WU [47]. Taken together, these modifications to the haemodynamic definition of pulmonary hypertension (PH) will change the expected incidence and prevalence of PH, although the magnitude of this change has not been well characterised to date [62]. Importantly, while the new definition of PH identifies a larger cohort of patients with pulmonary vascular disease, current therapies for PAH were studied in populations fulfilling the old definition. As such, there are no approved therapies for patients with mPAP between 21 and 24 mmHg. There are few current studies examining the impact of vasodilator therapy in patients with mildly elevated pressures (clinicaltrials.gov identifier NCT04797286); most of the completed studies included patients with scleroderma and exercise PH and thus lack generalisability to PH patients with mildly elevated pressures [63–65]. A consensus is therefore lacking on whether patients with mildly elevated pulmonary arterial pressures and PVR should be considered for initiation of PAH therapies. Clinical trial enrolment is recommended, if available, and all patients should be closely monitored for progression. If a decision to initiate therapy is made despite a lack of robust trial data, initial monotherapy is recommended over combination therapy.
Treatment algorithm: initial treatment, patient selection and choice of therapy
The majority of patients included in PAH clinical trials have had IPAH, HPAH, DT-PAH or connective tissue disease (CTD)-associated PAH, and therefore the treatment algorithm presented is focused on these patients. This algorithm is also intended for patients with phenotypically clear-cut PAH, similar to the majority of patients enrolled in PAH clinical trials, and for patients with a mPAP ≥25 mmHg and PVR >3 WU; see also the section on comorbidities and phenotypes herein and in the WSPH article on definition, classification and diagnosis of pulmonary hypertension [66].
Initial therapy following diagnosis should be based on an evaluation of risk status. For patients not classified as being at high risk at baseline, initial therapy with an endothelin-1 receptor antagonist (ERA) and phosphodiesterase-5 inhibitor (PDE-5i) combination is recommended [47]. Same-day initiation of both medications is well tolerated and is a recommended option, but sequential initiation over a short period of time (1–2 weeks) to assess tolerability is also acceptable. Use of tadalafil in combination with macitentan or ambrisentan is preferred, based on stronger evidence of efficacy for these combinations [9, 10, 21] versus combinations containing bosentan or sildenafil [67]. When using sildenafil, initiation with 20 mg orally three times daily is recommended, but uptitration to 80 mg can be considered if needed, though at a cost of a higher rate of adverse events [17, 18].
Patients with severe PAH and classified as high risk at the time of diagnosis should receive a parenteral PPA in combination with an ERA and a PDE-5i [47]. Although parenteral therapies (intravenous epoprostenol, subcutaneous or i.v. treprostinil) were initially evaluated as monotherapy [35, 36, 68], well-conducted observational studies have suggested significantly improved outcomes, including survival, for initial triple combination therapy that includes a parenteral PPA in high-risk patients [5, 69]. A discussion about lung transplant and potential referral should also be considered at diagnosis in high-risk patients, depending on patient, centre- and region-specific criteria; see also the WSPH article on transplantation and bridging and support technologies in pulmonary hypertension [70]. Patients classified as non-high risk, but having some high-risk features may also be considered for initial parenteral therapy, based on observational data showing excellent and possibly superior long-term outcomes in some subgroups of patients with this approach [5].
Initial triple oral therapy with selexipag, a PDE-5i and an ERA is not recommended based on the TRITON trial, which found no difference in PVR or other end-points at 26 weeks [9, 71]. Finally, while inhaled therapies (inhaled treprostinil, inhaled iloprost) and oral riociguat may be considered as a component of initial therapies, the overall evidence for this based on patient number, duration and types of studies is lower compared with initial ERA–PDE-5i combination therapy [21, 23].
Initial reassessment for potential therapy escalation: early is preferred
The optimal timing of the first follow-up reassessment and potential treatment escalation has not been fully established, but 3–4 months appears to be a reasonable compromise allowing most prognostic measures to have reached or neared a plateau [72]. There is some evidence that patients receiving add-on therapy earlier following their diagnosis may have a more robust response versus those much later in the disease process, although this is more speculative [73]. The 3–4-month time point for reassessment applies only to patients who are stable or improving during this time; those with clear-cut deterioration should be considered for treatment escalation promptly. For patients initially receiving monotherapy, the addition of a second agent at this time (or earlier) should be considered for most patients, including in particular patients who have not reached a low-risk status.
Low risk at follow-up: is additional therapy indicated?
Most patients currently classified as low risk still have significant symptoms and reduced exercise capacity. Risk of progression is also present; in a combined analysis of the AMBITION, GRIPHON and SERAPHIN trials, >20% of patients classified as low risk on multiparameter risk scores developed a clinical worsening event within 3 years of study entry [38]. These results suggest that simply achieving a low-risk status based on current risk calculators alone is insufficient to ensure optimal symptom control or an adequate long-term clinical response in all patients.
However, clinical trials have also found smaller short-term improvements in functional class and 6MWD in PAH patients with low-risk scores [74]. And while the relative risk reduction in clinical worsening in PAH RCTs appears similar in magnitude in lower-risk versus higher-risk patients, the absolute risk reduction in lower-risk patients is small [75]. Additional PAH therapy may also lead to additional side-effects and expense.
Individualised management may therefore be considered. Expanded goals of therapy have been proposed, including a PVR <5 WU [76], large declines in the PVR (e.g. >50–60%) [77–79], improvement in stroke volume index or other haemodynamic measures [80] and achieving a normal or near-normal right heart size and function on cardiac imaging [81]. However, longer-term studies are needed in order to better determine the extent to which these measures add value on top of an already low risk status.
Intermediate-low risk at follow-up: treatment escalation
Patients already on combination oral therapy who are at intermediate-low risk on a four-tiered risk scale should be considered for add-on or transitions in therapy, including add-on therapy with sotatercept, with an oral PPA (selexipag, oral treprostinil), with an inhaled PPA, or with transition from an oral PDE-5 inhibitor to riociguat. Table 4 summarises key trials performed with these medications among patients already on one or more therapies. Studies of both sotatercept and selexipag included large numbers of patients on combination background therapy, providing a higher level of evidence for their use in this setting, while studies of oral and inhaled treprostinil included only patients on monotherapy.
TABLE 4.
Key randomised trials of prostacyclin pathway agents, riociguat and sotatercept in studies of patients on background endothelin-1 antagonists and/or phosphodiesterase-5 inhibitors
| Study [reference] drug |
Subjects n | Blinded | Duration weeks | Background medical treatments % | Primary end-point |
Primary end-point results (95% CI) | Positive primary end-point | Other key positive end-points |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||||||
|
GRIPHON [28]
selexipag |
1156 | Yes | ∼71 | 20 | 47 | 33 | 0 | TTCW | HR 0.60 (0.46–0.78) | Yes | 6MWD, NT-proBNP |
|
STELLAR [8]
sotatercept |
323 | Yes | 24 | 0 | 4 | 35 | 61 | 6MWD | HLE 40.8 (27.5–54.1) m | Yes | 6MWD, PVR, NT-proBNP, FC, TTCW, others |
|
TRIUMPH [33]
inhaled treprostinil |
235 | Yes | 12 | 0 | 100 | 0 | 0 | 6MWD | HLE 20.0 (8.0–32.8) m | Yes | 6MWD, NT-proBNP, QoL |
| FREEDOM-EV [11] treprostinil p.o. | 690 | Yes | ∼52 | 0 | 100 | 0 | 0 | TTCW | HR 0.74 (0.56–0.97) | Yes | NT-proBNP, FC |
|
REPLACE [15]
riociguat |
226 | No | 24 | 0 | 29 | 71 | 0 | Clinical improvement | OR 2.78 (1.53–5.06) | Yes | 6MWD, FC |
TTCW: time to clinical worsening; HR: hazard ratio; 6MWD: 6-min walk distance; NT-proBNP: N-terminal pro-brain natriuretic peptide; HLE: Hodges–Lehmann estimate; PVR: pulmonary vascular resistance; FC: functional class; QoL: quality of life.
Differences in route of administration, types of background therapy, trial designs and safety profiles for these medications make comparisons difficult, with the impressive and more robust improvement with sotatercept countered by larger and longer clinical trials focused on the prevention of clinical worsening for the oral PPAs.
Specifically, in STELLAR (n=323), patients receiving sotatercept showed a placebo-corrected improvement in 6MWD of 41 m (p<0.001, primary end-point) at 24 weeks, along with improvement versus placebo in health-related quality of life, functional class, haemodynamics, echocardiography and NT-proBNP [82]. In addition, patients in the sotatercept arm had significantly fewer clinical worsening events over a median follow-up of 33 weeks (hazard ratio 0.16, 95% CI 0.08–0.35) [8]. Generally consistent results were also seen across multiple subgroups. All patients were on background PAH therapy including 4% on one, 35% on two and 61% on three approved background PAH therapies. Additionally, 40% were receiving a parenteral PPA.
By contrast, studies targeting the prostacyclin pathway with oral agents, GRIPHON (selexipag) and FREEDOM-EV (oral treprostinil) were larger, longer-term studies (median follow-up >1 year) with event-driven composite primary end-points. Both studies showed a significant reduction in worsening events, with a 40% (p<0.001) reduction in GRIPHON and 26% (p=0.03) reduction in FREEDOM-EV [11, 28]. Change in 6MWD was more modest, with an improvement of 12 m (p=0.003) in GRIPHON and 8 m (p=0.12) in FREEDOM-EV (both placebo-corrected). Patients in GRIPHON were treatment-naïve (20%), on oral monotherapy (47%) or on oral combination therapy (33%), while patients in FREEDOM-EV were receiving background oral monotherapy with an ERA, soluble guanylate cyclase stimulator or PDE-5i.
The safety and adverse event profiles are similarly difficult to compare due to a lack of head-to-head data, differences in study design and duration, differences in sample sizes and significant differences in types and frequency of adverse events. For the sotatercept trials, adverse events leading to sotatercept discontinuation were uncommon (2% in STELLAR), but a number of adverse events including serious adverse events have been associated with sotatercept use. Bleeding events occurred in 22% of the sotatercept arm and 13% of the control arm in STELLAR, usually as minor mucosal bleeding (nosebleeds, gingival bleeding), but serious events have also been reported across the sotatercept studies including gastrointestinal bleeding, central nervous system bleeding and haemoptysis. Other adverse events of potential importance included the development or worsening of telangiectasias, increases in haemoglobin and decreases in platelets. In contrast, the most common PPA-associated adverse events seen in these studies were those common to all prostacyclins and include headache, flushing, jaw pain, extremity pain, nausea and diarrhoea. These PPA associated adverse events tend to occur early on after medication initiation and may improve over time or with dose reduction.
Overall, these differences make it difficult to make any strong recommendations. In general, one might consider sotatercept in those with greater symptom burden or greater impairment in exercise capacity, given the improvement in these measures seen in STELLAR. In contrast, one might consider selexipag or oral treprostinil as a simpler approach initially in those at higher bleeding risk, such as with hereditary haemorrhagic telangiectasia, or when aiming for a reduction in long-term clinical worsening events and/or in those who are more comfortable with the more established long-term safety profile.
Inhaled PPA (inhaled treprostinil or iloprost) [33, 34, 83] are also options as add-on therapy for patients at intermediate-low risk. However, as a less convenient option due to route of delivery and the number of treatments required per day (four for treprostinil, between six and nine for iloprost), and as medications studied either as monotherapy (iloprost) or added-on to monotherapy (inhaled treprostinil), these medications are most often considered when issues with tolerability arise with other medications, or in patients felt to be at higher risk of adverse events with oral medications. Positives include a lower frequency of prostacyclin-type side effects versus other routes, and potentially fewer systemic effects in general [84].
Switching from a PDE-5i to riociguat may also be considered, based on a positive randomised but unblinded study evaluating the likelihood of clinical improvement in patients previously treated with a PDE-5i who were randomised to either switch to riociguat versus continuing a PDE-5i [15, 85]. Clinical improvement was defined as absence of clinical worsening and improvement in at least two of three of the following: 6MWD, World Health Organization functional class and NT-proBNP. Although the study was positive, the lack of blinding as well as the use of an unvalidated outcome measure lowers the strength of this recommendation.
Intermediate-high and high risk at follow-up: maximal therapy and transplant
Patients at intermediate-high risk at follow-up should receive a parenteral PPA or be considered for add-on sotatercept, while those at highest risk should receive a parenteral PPA, if not already receiving. Parenteral PPA have been studied as monotherapy [35, 36, 68], double combination [19] and triple combination therapy (observational), including in patients with functional class IV symptoms and other signs of severe disease [5, 79]. Maximal medical therapy is now four-drug therapy; this is based on the sotatercept RCTs where a majority of patients studied were receiving background triple therapy [7, 8].
Timing of referral, evaluation and listing for transplant will depend on many factors including potential barriers to transplant that may need to be addressed, local criteria for listing, local waitlist and prioritisation factors. Patients should be considered for referral for lung transplant evaluation with any of the following: progressive disease or hospitalisation for PAH despite therapy, need for parenteral PPA, known or suspected pulmonary veno-occlusive disease (PVOD)/pulmonary capillary haemangiomatosis (PCH) or the development of secondary liver or kidney dysfunction. Transplant evaluation is generally considered in high-risk patients at diagnosis, and is recommended in all follow-up patients with progression to or persistence of intermediate-high- or high-risk features [86]. Additional criteria which may also be considered for referral and listing are discussed in the WSPH article on transplantation and bridging and support technologies in PH [70].
Among those listed for transplant, further prioritisation (ranking on the priority list) can be difficult in PAH as traditional measures utilised for transplant prioritisation have not performed very well in PAH in this setting, and PAH patients have higher rates of waitlist mortality compared with patients with parenchymal lung disease [87, 88]. Alternative strategies, such as moving acutely decompensated PAH patients who are failing to improve with initial intensive care treatments to the top of the waitlist, may reduce mortality [89]. Appeal for a higher lung allocation score may also be an option in regions where this strategy is available [87].
Comorbidities and other patient populations at higher risk of adverse response to medications
Given the large variability in pathophysiology and phenotypes for patients with PH and comorbidities, a separate “comorbidities” arm has not been included in the PAH treatment algorithm. Instead, a very careful approach to these patients is recommended, as detailed later, in order to identify patients who have definite/phenotypically clear-cut PAH and may benefit from PAH therapies, including potentially upfront combination therapy as per the treatment algorithm, as well as others who are unlikely to benefit and may be harmed by PAH therapies.
PAH patients with comorbidities are increasingly seen in clinical practice and have been found in several studies to have a higher rate of adverse events with therapy [90] and possibly a less robust treatment response. Potential contributors include differences in PAH phenotype as well as the occurrence of occult group 2 PH, where patients meet typical haemodynamic criteria for pre-capillary PH at rest, but manifest group 2 haemodynamics with provocative manoeuvres such as exercise or fluid challenge, resulting in misclassification.
Clinical, haemodynamic and imaging findings may raise concern for occult group 2 PH. This includes catheterisation findings of a relatively higher pulmonary capillary wedge pressure (12–15 mmHg range) and relatively lower PVR (3–5 WU), echocardiographic findings of left atrial enlargement, grade 2 or higher diastolic dysfunction, a reduced left ventricular ejection fraction or left-sided valve dysfunction, and medical conditions associated with left heart disease, including hypertension, diabetes, obesity, sleep apnoea, atrial fibrillation and coronary artery disease [91].
While many patients with these features will have group 2 PH and should not receive PAH therapies at all, a subset of patients may have PAH, but with a high comorbidity burden. In this setting, initiating therapy as monotherapy may be preferred in order to monitor for tolerability and response to therapy. Although head-to-head data are lacking, the rate of adverse events and medication discontinuation rate may be higher for the ERAs versus the PDE-5i in this setting, so starting therapy with a PDE-5i may be preferred [92].
Another subset of patients present with more clear-cut group 1 PAH, despite having one or more comorbidities. This patient population is increasing in clinical trials, mirroring the prevalence of comorbidities in the general population. For example, in GRIPHON, 51% of all patients had at least one cardiovascular comorbidity, including hypertension (33%), obesity (27%), diabetes (11%), and coronary artery disease (10%), while in AMBITION, 40% had hypertension, 10% diabetes and 4% coronary artery disease [90]. Subgroup analyses can be difficult to interpret, but reductions in risk of clinical worsening in the “comorbidities” patients in these two studies were generally similar to the overall results [90, 93].
These findings demonstrate the potential benefits of PAH therapy in patients with comorbidities, including the use of combination therapy in some patients with comorbidities who meet the rigorous inclusion criteria typical of PAH clinical trials. However, caution is warranted, as treatment-associated adverse events were more common and improvement in symptoms and walk distance end-points were more modest [90, 93].
Another common setting where concern for a non-group 1 diagnosis may occur is with significant pre-capillary PH in combination with lung disease. PAH clinical trials have typically excluded those with moderate or severe lung disease, but not mild lung disease. Subgroup analyses focused on lung function and/or oxygen use from PAH clinical trials are lacking. Registry data suggests that patients with both a smoking history and a diffusing capacity of the lung for carbon monoxide <45% may have a less robust improvement in 6MWD and shorter average survival times after PAH therapy (and other unique phenotypic findings), but clinical trial outcomes focused on this subgroup are needed [94]. Criteria for approaching the diagnosis and treatment of group 3 PH as well as details on differentiating group 1 PAH versus group 3 PH are discussed separately in the WSPH article on PH associated with lung diseases [95].
When PAH therapies are started in PAH patients with comorbidities, close follow-up is needed due to higher adverse event rates. There is also the potential for an alternative and sometimes non-group 1 PH diagnosis to be revealed only following therapy initiation. In this setting, PAH therapies should generally be discontinued and treatment focused on the underlying condition.
In all the settings described, evaluation at an expert centre is recommended. While PAH therapies may be of benefit to a subset of patients with these more complicated phenotypes, harm or possible harm with PAH therapies has been seen across a wide range of pulmonary and cardiac conditions, including ERAs and riociguat in interstitial lung disease, ERAs in heart failure with preserved ejection fraction (HFPEF), PDE-5i in group 2 PH with a history of left-sided valve disease or HFPEF and PDE-5i in PH associated with sickle cell anaemia [92, 96–105]. Management recommendations are described in the WSPH article on PH associated with left heart disease [106].
PAH subgroups
CTD-PAH
Patients with CTD-PAH comprise the second-largest PAH subgroup included in clinical trials of PAH therapy, after IPAH, accounting for 30–40% of the study population [107, 108]. The majority of these patients have scleroderma-related PAH (SSc-PAH). Despite a lower disease prevalence in the general population than other CTDs such as rheumatoid arthritis or systemic lupus erythematosus, PAH is more commonly seen in SSc, with 8–12% of patients developing this disease [109, 110]. Outcomes for patients with CTD-PAH vary, with the worst outcomes noted for SSc-PAH compared to other CTDs [111]. In general, survival in SSc-PAH is worse than IPAH, but has improved over the past decade, with median survival approaching 8 years in SSc-PAH [112]. Similarly, relative improvements in other outcomes, such as time to clinical worsening and 6MWD, have been observed, although the magnitude tends to be smaller than that seen in IPAH populations [107]. Reasons for these differences may include limitations of the outcome measures for CTD-PAH patients where comorbidities contribute to time to clinical worsening and functional limitations and lower thresholds for clinically relevant changes as seen in the 6MWD [113]. Furthermore, pulmonary venular involvement leading to a PVOD phenotype may be under-recognised in SSc-PAH and is associated with poor response to vasodilators and poorer outcomes overall [114, 115]. Despite these potential modifiers of response to therapy, post hoc analysis of the AMBITION study demonstrates similarly robust improvements in time to clinical worsening and 6MWD with initial PDE-5i and ERA therapy in CTD-PAH patients as IPAH patients [116]. As such, previous consensus recommendations have supported application of the same general treatment algorithm for CTD-PAH as other forms of PAH.
One caveat to this treatment approach based on risk assessment is the limitation of risk assessment tools in CTD-PAH and specifically SSc-PAH populations, where model performance may not be as robust as in IPAH [117]. Given the differences in treatment response and outcomes in CTD-PAH compared to other forms of PAH, CTD-specific tools may be needed to better assess risk in this population, along with close attention to haemodynamics and right ventricular imaging.
In addition to differences in treatment response, CTD-PAH patients have a higher incidence of treatment-related adverse events compared to other PAH types, which may limit use of certain therapies in this population [107]. Caution should be exercised with initiation of medications that may increase bleeding risk, such as sotatercept, in CTD patients who are predisposed to gastrointestinal bleeding due to vascular anomalies. In addition, digital contractures and impaired manual dexterity may impact the ability of some CTD-PAH patients to manage parenteral therapies, further limiting treatment options.
Congenital heart disease associated PAH with unrepaired defects
Congenital heart disease (CHD)-associated PAH includes four subtypes: Eisenmenger syndrome, PAH associated with persistent systemic-to-pulmonary shunts, PAH with small/coincidental defects and PAH associated with repaired defects. Many clinical trials of PAH therapies included CHD patients with repaired defects as long as >1 year since the time of repair, and these patients are typically treated similarly to those with IPAH.
In contrast, patients in the first two groups, having significant and unrepaired congenital heart defects, have more complicated pathophysiology and have been excluded from most PAH clinical trials. A comprehensive evaluation including assessment for potential closure, medical therapeutic strategy, complication monitoring and psychological support is important, and should be performed by multidisciplinary team in an experienced centre. Post-capillary PH must also be excluded prior to considering PAH therapies. Supportive therapies include assessment for iron deficiency, avoidance of routine phlebotomy, use of antibiotic prophylaxis for the prevention of endocarditis (for cyanotic CHD or with prosthetic material), and the use of air-filters with i.v. lines [118].
Compared with other group 1 PAH subgroups, particularly IPAH and CTD-associated PAH, clinical trial data on PAH therapies in patients with unrepaired CHD-PAH are limited. In the BREATHE-5 trial, greater functional and haemodynamic improvement was seen with bosentan versus placebo [119], while in the MAESTRO trial, macitentan failed to improve 6MWD versus placebo, but led to lower NT-proBNP (overall study) and improved haemodynamics (substudy) [14]. BREATHE-5 included only PAH-treatment-naïve patients, while 27% of patients in MAESTRO were on a background PDE-5i. Improvement in exercise capacity, symptoms and survival in CHD-PAH has also been suggested with PAH therapies more generally, including double and triple combination therapy [120–127], but these data are largely observational. Larger and higher-quality studies in CHD-PAH are needed.
Initiation of PAH therapies in a sequential and symptom-oriented fashion is generally recommended (initial monotherapy, add-on therapy as needed) in both Eisenmenger syndrome and in PAH associated with persistent systemic-to-pulmonary shunts, especially in those patients with preserved exercise capacity. However, greater haemodynamic improvement has been reported with use of combination therapy, and in recent years the use of combination therapy has become more common [127, 128]. For patients with persistent high-risk clinical features, lung or heart–lung transplantation should be considered [129, 130].
For patients in whom closure is not initially considered feasible, a number of reports have suggested that closure might be considered after treatment with targeted drug therapy for PAH, if sufficient improvement is seen [131]. However, there is still no evidence for a long-term benefit from this approach, and caution is warranted. Decisions on shunt closure should not be made on haemodynamic parameters alone, and a multiparametric strategy should be followed. There is a need for future prospective studies, ideally with randomisation, to ascertain the benefit and safety of this approach; see also the WSPH article on paediatric PH [132].
Drug- and toxin-associated PAH
A number of medications are associated with the development of PAH, covered in greater detail in the WSPH article on the definition, classification and diagnosis of pulmonary hypertension [66]. Outcomes appear to be heterogeneous. Partial or full reversal of PAH has been reported after discontinuation of some agents, including dasatinib [133] and interferon [134], and thus for patients exposed to these medications and presenting with lower-risk features, discontinuation of the causative medication followed by close observation can be considered. If no improvement is seen over a period of 3–4 months, PAH therapy should be started. Some medications, particularly chemotherapy agents, have also been associated with PVOD/PCH, and this should be considered in the appropriate setting [135]. For stimulant-associated PAH, the most common form of drug/toxin-associated PAH in the current era, spontaneous remission is not typical, and patients with DT-PAH associated with stimulants also appear to have worse outcomes versus IPAH [136, 137]. Treatment recommendations include initiation of PAH therapy following the treatment algorithm (figure 1) and referral for treatment of substance use at the time of diagnosis.
HIV-PAH
Patients with PAH associated with HIV tend to be younger and are more likely to be male and to have more severe haemodynamic abnormalities versus patients with IPAH/HPAH [138, 139]. HIV itself is a risk factor for the development of PAH, although high rates of stimulant use and intravenous drug use, both risks for PAH, are also seen [86]. Treatment response to PAH therapies appears similar versus IPAH, based on open-label clinical studies [140] and observational data [138, 139, 141], and small numbers of HIV-PAH patients have been included in many of the large PAH RCTs [9, 21, 23, 24, 28]. Treatment with both highly active antiretroviral therapy (HAART) and PAH therapies is therefore recommended. This does require extra attention to potential medication interactions, particularly when protease inhibitors are being utilised as a component of HAART. Drug levels of both sildenafil and tadalafil, in particular, are increased significantly in this setting, and the manufacturers of both PDE-5i recommend against their use in PAH with any strong cytochrome P450 3A inhibitors. However, given the fatal nature of untreated/inadequately treated PAH, coadministration of protease inhibitors and reduced-dose PDE-5i has been described in the literature in a small number of case reports [142], and might be considered if no other options exist.
Portopulmonary hypertension
Portopulmonary hypertension (PoPH) is the occurrence of PAH in the setting of portal hypertension (hepatic venous pressure gradient >5 mmHg or indirect signs of portal hypertension). While most PoPH patients also have cirrhosis, PoPH can occur without cirrhosis and across all Child–Pugh classes [143]. The prevalence of PoPH in patients with cirrhosis or portal hypertension is not insignificant, accounting for ∼5–15% of all patients with PAH in the United States of America, France and Japan [144–147]. Conversely, the prevalence of PAH in patients with portal hypertension is reported to be between 2% and 6% [147–149].
The management of PoPH is unique. Prior to initiating treatment, it is important to distinguish true PoPH from other forms of PH that can occur in liver disease, including PH due to volume overload and/or high cardiac output states. Patients with PoPH will generally meet group 1 PAH haemodynamic criteria including a pulmonary artery wedge pressure ≤15 mmHg, but some degree of volume excess and high cardiac output may also be present and can complicate diagnosis. Optimising both volume state and treating the underlying liver disease are both important components of therapy.
Regarding PAH pharmacotherapy, the PORTICO study, utilising macitentan, is the only RCT with a placebo-controlled, double-blind design specifically targeting PoPH. This study compared PVR after 12 weeks of macitentan 10 mg versus placebo, finding a 35% reduction in PVR with macitentan [13]. Other smaller studies, mostly open-label case series, have shown improvement versus baseline for PoPH with other PAH therapies including greater improvement in haemodynamics with the use of two- and three-drug combination therapy [150–152]. Overall numbers of patients are low, particularly for Child–Pugh grade C disease. For those with more advanced liver disease, caution is recommended due to the higher potential for impaired drug metabolism.
A subset of patients with PoPH may be candidates for liver transplantation, and in observational studies, patients undergoing PAH medical therapy followed by liver transplantation have better survival rates versus PAH medical therapy alone [151, 153]. Haemodynamic targets prior to consideration of listing, as suggested by the International Liver Transplantation Society, include a mPAP <35 mmHg and a PVR <5 WU, or a mPAP 35–45 mmHg with a PVR <3 WU [152, 154]. These values are slightly more lenient than those utilised in earlier years. Pressures higher than this are generally considered an absolute contraindication to liver transplantation. Following liver transplant, PAH usually no longer progresses and some PAH patients have tolerated de-escalation or discontinuation of PAH therapies, but this is not universal [155].
Schistosomiasis
Schistosomiasis-associated PAH (Sch-PAH) is one of the most common causes of PAH worldwide [156, 157]. While Sch-PAH typically occurs in the setting of severe hepatosplenic schistosomiasis, the overall pathophysiology, prognosis and treatment response appear distinct from PoPH, albeit incompletely studied [156, 158]. Treatment with PAH therapies is associated with improvement in survival, with most reports describing treatment with PDE-5i or ERA monotherapy or combination therapy [159, 160]. Treatment of the underlying parasitic infection is also considered in all or some patients, although a haemodynamic benefit has not been established [159].
PVOD/PCH
PVOD/PCH represents a rare variant of PAH, distinguished by remodelling of the pulmonary venules and capillaries. Although PVOD/PCH and PAH share a similar clinical presentation with features of severe pre-capillary PH, it is important to differentiate these two conditions, as PVOD/PCH carries a worse prognosis and life-threatening pulmonary oedema may occur following the initiation of PAH therapy [47, 161–165]. To date, there is a lack of established evidence-based medical therapy for PVOD/PCH, making lung transplantation the preferred definitive treatment for eligible patients. It is strongly recommended that patients receive early referral to specialised centres upon diagnosis [72, 166].
The safety and efficacy of PAH-targeted drugs in the context of PVOD remain uncertain. Consequently, their use should be approached with great caution and limited to PH expert centres experienced in managing this complex condition.
A recent systematic review evaluating the safety and efficacy of PAH-targeted treatments in PVOD/PCH identified 14 single case reports, no RCTs and four case series involving between eight and 16 patients. These reports included patients treated with CCBs, ERAs, PDE-5i and low-dose parenteral epoprostenol, mostly as monotherapy [167]. While moderate clinical and haemodynamic improvement was reported in some individual cases, sustained long-term effects are generally lacking [166].
Limited-resource settings
Unfortunately, access to PH medications may be limited in some areas, whether due to a lack of resources in general or due to inequitable distribution of resources/healthcare coverage, as is even seen in some wealthier countries. Generally, symptomatic management with diuretics and general and supportive measures are universally available options. Unfortunately, the cost and national availability of PH medication makes use impossible for many, and monotherapy a default approach in others. Parenteral therapies are generally not available where resources are limited. On a positive note, the approval of generic medications for some types of PAH therapies is welcomed. Advocacy programmes by the broader PH community for access to cheaper and effective generics as well as education on approaches to PH where resources are limited are recommended. Atrial septostomy has shown significant benefit in PAH, most notably where access to medications is limited, but, unfortunately, familiarity with the procedure is uncommon globally [168].
Haemodynamic effects of PAH therapies
Medications other than sotatercept have both acute and chronic vasodilatory effects [169, 170]. Acutely, the decline in mPAP and PVR in response to PAH medications is often modest, presumably due to the extensive vascular remodelling that is often present in PAH. Greater haemodynamic improvement is observed long-term [171], and combination therapy leads to greater haemodynamic improvement versus monotherapy, particularly when given as upfront therapy. The largest overall declines in PVR have been reported for upfront triple combination therapy that includes a parenteral PPA [3].
Significant improvement in right atrial pressure (RAP), mPAP, PVR, cardiac index and mixed venous oxygen saturation (SvO2), among others, have been reported in clinical trials and observational studies [3, 172]. As nonselective vasodilators, PAH therapies also lead to modest declines in systemic vascular resistance [173]. Clinically significant decreases in systemic blood pressure are uncommon, but can be seen when PAH therapies are used in combination, or with use of riociguat, which appears to have more potent systemic effects [22].
In contrast, sotatercept also leads to long-term improvements in pulmonary haemodynamics, but it lacks acute vasodilatory effects in the pulmonary or systemic circulation, suggesting that long-term improvements in haemodynamics occur through other mechanisms. Significant improvements in RAP, mPAP, PVR and SvO2, but not cardiac index, have been seen in sotatercept clinical trials [7, 12].
The reasons for the lack of improvement in cardiac index are unclear, but sotatercept also leads to increases in haemoglobin and systemic vascular resistance, both of which would theoretically reduce the tendency for cardiac output to increase, and have been hypothesised to contribute to this finding. In addition, all patients in the sotatercept studies were on background PAH medications, and many had a cardiac index in the normal range at baseline. Conversely, even when looking at the subgroup of patients in the sotatercept studies with a low cardiac index, a statistically significant increase in cardiac index was not seen, although a small (nonsignificant) numeric increase was reported [82]. What this means from a clinical standpoint is not yet completely clear, as sotatercept leads to a robust reduction in PVR, improvements in right ventricular function on imaging, a lower NT-proBNP and clinically meaningful improvement in quality of life and exercise capacity.
Still, in patients presenting seriously ill and with a low cardiac index, the far greater experience with parenteral PPA in this setting suggests that these medications remain first-line. Rapid and pronounced improvement has been documented, including an early mortality benefit in the sickest patients [35].
Medication administration and adverse effects
More detailed information on the dosing and management of PAH therapies can be reviewed in prior WSPH publications and PH treatment guidelines [47, 48]. Medications and common adverse effects by pharmaceutical class are shown in table 2 [7, 8, 11, 18–27, 29–31, 33–36].
While most adverse events can be managed with supportive therapy and/or dose adjustment, when applicable, a few require special consideration. Volume overload is common in PAH as a part of the disease process, but can also develop in response to PAH therapies, particularly with the ERAs. Management involves frequent follow-up, adjusting diuretics, and occasionally discontinuation and transition to other medications. This effect tends to be magnified in patients with group 2 risk factors, and severe fluid retention with ERAs should prompt review of the overall PH diagnosis.
New or worsened hypoxia related to PAH therapy is not common based on clinical trial data, despite the potential for increased ventilation/perfusion mismatch with PAH medications seen in experimental studies [174].
In those developing more severe hypoxia, echocardiography with bubble study and high-resolution computed tomography should be considered, and if no other causes are identified, previously unrecognised PVOD/PCH, although rare, should be considered. Typical findings suggestive of PVOD/PCH include the development of new hypoxia in combination with increased septal lines and/or ground-glass opacities on imaging following the introduction of PAH therapies, as well as mediastinal adenopathy.
For sotatercept, the most recently approved PAH therapy, treatment is initiated at 0.3 mg·kg−1, and then increased to 0.7 mg·kg−1 for subsequent doses administered every 3 weeks. Downtitration to 0.3 mg·kg−1 can be considered when required for adverse events (particularly for elevated haemoglobin) or tolerability. Monitoring is recommended for increased haemoglobin, reduced platelets and the development of new telangiectasias, and an increased risk of bleeding events has been seen in trials. In addition, sotatercept carries a risk of fetal harm, and may have the potential to reduce future fertility and males and females, based on animal studies [175].
Parenteral PPAs and transitions between parenteral and nonparenteral PPAs
Parenteral PPAs are initiated at 1–2 ng·kg−1·min−1 for both epoprostenol and treprostinil, followed by uptitration over weeks to months, typically to an initial dose of 15–20 ng·kg−1·min−1 for epoprostenol [35, 173, 176, 177] and ≥40 ng·kg−1·min−1 for treprostinil [178, 179]. Additional uptitration may be considered if needed, but there is considerable variability across centres [178]. Higher targets are also advocated by some centres, mainly in Japan [180, 181]. Haemodynamic reassessment prior to additional dose escalation is recommended in order to avoid excessive side-effects as well as the potential for overdose and high cardiac output state [173, 182, 183].
Transitions between epoprostenol and treprostinil and/or changes in route of delivery between subcutaneous and intravenous administration may be required due to delivery-route complications (site pain, line infections) or other reasons. Transitions should be performed with close monitoring. There is no established dose conversion formula, but in general, higher doses of treprostinil relative to epoprostenol are utilised [184]. Acutely, modest under-dosing is preferred to over-dosing, such that many studies have utilised a relatively lower initial short-term transition target followed by a higher (outpatient) target based on tolerability and clinical response [185–191].
Transitions from parenteral PPAs to oral or inhaled PPA are also considered in some patients, often at patient request and usually in patients with a good response to current therapy (PVR <5 WU, normal cardiac index, low-risk findings). With the increasing armamentarium of PAH therapies, requests for this type of transition may increase in the coming years. However, while short-term case series describe mostly positive outcomes, these data are very limited with some patients worsening during or after the transition, and with a poor response to reinitiation of the parenteral PPA in some cases [192, 193].
For those choosing to proceed, downtitration of epoprostenol or treprostinil is typically completed over weeks to months. Many centres utilise catheterisation during downtitration while still on a very low dose of epoprostenol or treprostinil and/or at the time of discontinuation, and again 3–4 months following transition [193–195]. More rapid downtitration is also possible, particularly for transitions to oral treprostinil [196], but requires particularly careful management. Initiation of the oral or inhaled PPA often occurs during the parenteral therapy downtitration, most often with overlap of the two medications for a short time.
Shared decision-making
Before therapy is prescribed for a patient with PAH, a patient–clinician discussion should occur to promote shared decision-making. Topics discussed should include information on the underlying disease process(es), benefits of potential therapies, route of administration and administration burden, adverse effect profile, any medication interactions, comorbidities and patient preferences [197].
It is important during this process to ask the patient about their preferred level of participation in treatment decision-making, as some prefer a much more active role, while others prefer to leave decisions almost entirely to their physicians [198]. Decision-making is aided by an understanding of the patient's most important treatment goals, whether improvement in prognosis, improvement in symptoms, avoidance of burdensome therapies, other goals, or all of the above.
Knowledge gaps and future directions
In acute vasodilator responders with subsequent loss of response, it remains unknown whether CCBs should be continued, dosage reduced or discontinued.
Studies on treatment response for patients with mPAP 21–24 mmHg and PVR 2–3 WU and for those with exercise PH are needed, as there is a paucity of clinic trial information.
While combination therapy is more effective than monotherapy, additional studies on the timing and sequence of subsequent therapy escalation and optimal treatment goals are needed.
High-quality observational data may be of use to help to supplement clinical trial data, particularly for newer therapies and to assess safety; registries should strive to enhance phenotyping, to include patients across different medications, and particular attention should be paid to transitions between therapies including intravenous/subcutaneous PPA.
There remains concern that progressive subclinical deterioration in right ventricular function can occur despite favourable early clinical measures. Finding reliable markers of progression and response to escalation in therapies remains an important focus of research.
Interventions to increase the acceptance and timing of initiation of parenteral prostacyclins are needed.
With older median age at diagnosis and improved survival for patients with established PAH, study of the interaction between PAH therapies and new or previously diagnosed comorbid conditions is needed.
Some subgroups of PAH remain particularly under-studied. In particular, this includes PoPH, unrepaired CHD, schistosomiasis-associated PAH and PVOD/PCH.
Optimal treatment in resource-limited areas remains under-studied, and worldwide access to PAH therapies is a critical need.
Conclusions
Optimal treatment of PAH involves combination therapy for a majority of patients, including upfront combination therapy with an ERA and PDE-5i for most newly diagnosed patients. Exceptions include IPAH/HPAH/DT-PAH with a positive vasodilator response who should receive a CCB initially. Maximal medical therapy is now four-drug therapy. Reassessment and early escalation in therapy is recommended.
Shareable PDF
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.01222-2024
Conflict of interest: K.M. Chin reports grants and consultancy fees from Janssen, Merck, Gossamer Bio and United Therapeutics, support for attending meetings from Janssen, and is an associate editor for Circulation. S.P. Gaine reports grants from Janssen, Aerovent and Gossamer Bio, consultancy fees from Janssen, Merck, Gossamer Bio, United Therapeutics and Altavent, payment or honoraria for lectures, presentations, manuscript writing or educational events and support for attending meetings from Janssen, and participation on a data safety monitoring board or advisory board with Janssen. C. Gerges reports grants from OrphaCare, payment or honoraria for lectures, presentations, manuscript writing or educational events from AOPHealth, AstraZeneca, Janssen and Ferrer, and support for attending meetings from AOPHealth, AstraZeneca, Cordis, Janssen and MSD. Z-C. Jing has no potential conflicts of interest to disclose. S.C. Mathai reports consultancy fees from Janssen, United Therapeutics, Merck and Acceleron, participation on a data safety monitoring board or advisory board with Bayer, and a leadership role on the Patient Centered Outcomes Research Institute Rare Disease Advisory Panel. Y. Tamura reports grants from Nippon Shinyaku Co. Ltd and Mochida, consultancy fees from MSD, and payment or honoraria for lectures, presentations, manuscript writing or educational events from Nippon Shinyaku Co. Ltd and Janssen Pharmaceuticals. V.V. McLaughlin reports grants from Aerovate, Gossamer-Bio, Janssen, Keros, Merck and Sonovie, and consultancy fees from 35Pharma, Aerami, Aerovate, Caremark, L.L.C., Corvista, Gossamer Bio, Janssen, Keros, Merck, Riovant and United Therapeutics. O. Sitbon reports grants from Aerovate, AOP Orphan, Ferrer, Janssen and MSD, consultancy fees from Altavant/Enzyvant, AOP Orphan, Ferrer, Gossamer Bio, Janssen, Liquidia, MSD, Respira Therapeutics and Roivant Sciences, payment or honoraria for lectures, presentations, manuscript writing or educational events from Aerovate, AOP Orphan, Janssen, Ferrer and MSD, and participation on a data safety monitoring board or advisory board with Altavant/Enzyvant, Gossamer Bio, Janssen and Respira Therapeutics.
References
- 1.Gabler NB, French B, Strom BL, et al. . Validation of 6-minute walk distance as a surrogate end point in pulmonary arterial hypertension trials. Circulation 2012; 126: 349–356. doi: 10.1161/CIRCULATIONAHA.112.105890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savarese G, Paolillo S, Costanzo P, et al. . Do changes of 6-minute walk distance predict clinical events in patients with pulmonary arterial hypertension? A meta-analysis of 22 randomized trials. J Am Coll Cardiol 2012; 60: 1192–1201. doi: 10.1016/j.jacc.2012.01.083 [DOI] [PubMed] [Google Scholar]
- 3.Farmakis IT, Vrana E, Mouratoglou SA, et al. . Haemodynamic effects of initial combination therapy in pulmonary arterial hypertension: a systematic review and meta-analysis. ERJ Open Res 2022; 8: 00313-2022. doi: 10.1183/23120541.00313-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tremblay E, Gosselin C, Mai V, et al. . Assessment of clinical worsening end points as a surrogate for mortality in pulmonary arterial hypertension: a systematic review and meta-analysis of randomized controlled trials. Circulation 2022; 146: 597–612. doi: 10.1161/CIRCULATIONAHA.121.058635 [DOI] [PubMed] [Google Scholar]
- 5.Boucly A, Savale L, Jaïs X, et al. . Association between initial treatment strategy and long-term survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 2021; 204: 842–854. doi: 10.1164/rccm.202009-3698OC [DOI] [PubMed] [Google Scholar]
- 6.Ramjug S, Hussain N, Hurdman J, et al. . Idiopathic and systemic sclerosis-associated pulmonary arterial hypertension: a comparison of demographic, hemodynamic, and MRI characteristics and outcomes. Chest 2017; 152: 92–102. doi: 10.1016/j.chest.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 7.Humbert M, McLaughlin V, Gibbs JSR, et al. . Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med 2021; 384: 1204–1215. doi: 10.1056/NEJMoa2024277 [DOI] [PubMed] [Google Scholar]
- 8.Hoeper MM, Badesch DB, Ghofrani HA, et al. . Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med 2023; 388: 1478–1490. doi: 10.1056/NEJMoa2213558 [DOI] [PubMed] [Google Scholar]
- 9.Chin KM, Sitbon O, Doelberg M, et al. . Three- versus two-drug therapy for patients with newly diagnosed pulmonary arterial hypertension. J Am Coll Cardiol 2021; 78: 1393–1403. doi: 10.1016/j.jacc.2021.07.057 [DOI] [PubMed] [Google Scholar]
- 10.Grünig E, Jansa P, Fan F, et al. . Randomized trial of macitentan/tadalafil single-tablet combination therapy for pulmonary arterial hypertension. J Am Coll Cardiol 2024; 83: 473–484. doi: 10.1016/j.jacc.2023.10.045 [DOI] [PubMed] [Google Scholar]
- 11.White RJ, Jerjes-Sanchez C, Bohns Meyer GM, et al. . Combination therapy with oral treprostinil for pulmonary arterial hypertension. A double-blind, placebo-controlled clinical trial. Am J Respir Crit Care Med 2020; 201: 707–717. doi: 10.1164/rccm.201908-1640OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humbert M, McLaughlin V, Gibbs JSR, et al. . Sotatercept for the treatment of pulmonary arterial hypertension: PULSAR open-label extension. Eur Respir J 2023; 61: 2201347. doi: 10.1183/13993003.01347-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sitbon O, Bosch J, Cottreel E, et al. . Macitentan for the treatment of portopulmonary hypertension (PORTICO): a multicentre, randomised, double-blind, placebo-controlled, phase 4 trial. Lancet Respir Med 2019; 7: 594–604. doi: 10.1016/S2213-2600(19)30091-8 [DOI] [PubMed] [Google Scholar]
- 14.Gatzoulis MA, Landzberg M, Beghetti M, et al. . Evaluation of macitentan in patients with Eisenmenger syndrome. Circulation 2019; 139: 51–63. doi: 10.1161/CIRCULATIONAHA.118.033575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeper MM, Al-Hiti H, Benza RL, et al. . Switching to riociguat versus maintenance therapy with phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension (REPLACE): a multicentre, open-label, randomised controlled trial. Lancet Respir Med 2021; 9: 573–584. doi: 10.1016/S2213-2600(20)30532-4 [DOI] [PubMed] [Google Scholar]
- 16.Howard LS, Rosenkranz S, Frantz RP, et al. . Assessing daily life physical activity by actigraphy in pulmonary arterial hypertension: insights from the randomized controlled study with selexipag (TRACE). Chest 2023; 163: 407–418. doi: 10.1016/j.chest.2022.08.2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoeper MM, Ewert R, Jansa P, et al. . Randomized, multicenter study to assess the effects of different doses of sildenafil on mortality in adults with pulmonary arterial hypertension. Circulation 2024; 149: 1949–1959. doi: 10.1161/CIRCULATIONAHA.123.068107 [DOI] [PubMed] [Google Scholar]
- 18.Galiè N, Ghofrani HA, Torbicki A, et al. . Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005; 353: 2148–2157. doi: 10.1056/NEJMoa050010 [DOI] [PubMed] [Google Scholar]
- 19.Simonneau G, Rubin LJ, Galiè N, et al. . Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med 2008; 149: 521–530. doi: 10.7326/0003-4819-149-8-200810210-00004 [DOI] [PubMed] [Google Scholar]
- 20.Galiè N, Brundage BH, Ghofrani HA, et al. . Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009; 119: 2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274 [DOI] [PubMed] [Google Scholar]
- 21.Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. 10.1056/NEJMoa1413687 [DOI] [PubMed] [Google Scholar]
- 22.Ghofrani HA, Galiè N, Grimminger F, et al. . Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013; 369: 330–340. doi: 10.1056/NEJMoa1209655 [DOI] [PubMed] [Google Scholar]
- 23.Pulido T, Adzerikho I, Channick RN, et al. . Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818. doi: 10.1056/NEJMoa1213917 [DOI] [PubMed] [Google Scholar]
- 24.Galiè N, Olschewski H, Oudiz RJ, et al. . Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 2008; 117: 3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510 [DOI] [PubMed] [Google Scholar]
- 25.Channick RN, Simonneau G, Sitbon O, et al. . Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet 2001; 358: 1119–1123. doi: 10.1016/S0140-6736(01)06250-X [DOI] [PubMed] [Google Scholar]
- 26.Rubin LJ, Badesch DB, Barst RJ, et al. . Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002; 346: 896–903. doi: 10.1056/NEJMoa012212 [DOI] [PubMed] [Google Scholar]
- 27.Galiè N, Badesch D, Oudiz R, et al. . Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol 2005; 46: 529–535. doi: 10.1016/j.jacc.2005.04.050 [DOI] [PubMed] [Google Scholar]
- 28.Sitbon O, Channick R, Chin KM, et al. . Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. doi: 10.1056/NEJMoa1503184 [DOI] [PubMed] [Google Scholar]
- 29.Jing ZC, Parikh K, Pulido T, et al. . Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation 2013; 127: 624–633. doi: 10.1161/CIRCULATIONAHA.112.124388 [DOI] [PubMed] [Google Scholar]
- 30.Barst RJ, McGoon M, McLaughlin V, et al. . Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol 2003; 41: 2119–2125. doi: 10.1016/S0735-1097(03)00463-7 [DOI] [PubMed] [Google Scholar]
- 31.Tapson VF, Jing ZC, Xu KF, et al. . Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest 2013; 144: 952–958. doi: 10.1378/chest.12-2875 [DOI] [PubMed] [Google Scholar]
- 32.Tapson VF, Torres F, Kermeen F, et al. . Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest 2012; 142: 1383–1390. doi: 10.1378/chest.11-2212 [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin VV, Benza RL, Rubin LJ, et al. . Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol 2010; 55: 1915–1922. doi: 10.1016/j.jacc.2010.01.027 [DOI] [PubMed] [Google Scholar]
- 34.Olschewski H, Simonneau G, Galiè N, et al. . Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 2002; 347: 322–329. doi: 10.1056/NEJMoa020204 [DOI] [PubMed] [Google Scholar]
- 35.Barst RJ, Rubin LJ, Long WA, et al. . A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996; 334: 296–301. doi: 10.1056/NEJM199602013340504 [DOI] [PubMed] [Google Scholar]
- 36.Simonneau G, Barst RJ, Galie N, et al. . Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2002; 165: 800–804. doi: 10.1164/ajrccm.165.6.2106079 [DOI] [PubMed] [Google Scholar]
- 37.Sitbon O, Nikkho S, Benza R, et al. . Novel composite clinical endpoints and risk scores used in clinical trials in pulmonary arterial hypertension. Pulm Circ 2020; 10: 2045894020962960. doi: 10.1177/2045894020962960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blette BS, Moutchia J, Al-Naamani N, et al. . Is low-risk status a surrogate outcome in pulmonary arterial hypertension? An analysis of three randomised trials. Lancet Respir Med 2023; 11: 873–882. doi: 10.1016/S2213-2600(23)00155-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma A, Verma S, Bhatt DL, et al. . Optimizing foundational therapies in patients with HFrEF: how do we translate these findings into clinical care? JACC Basic Transl Sci 2022; 7: 504–517. doi: 10.1016/j.jacbts.2021.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dardi F, Boucly A, Benza R, et al. . Risk stratification and treatment goals in pulmonary arterial hypertension. Eur Respir J 2024; 64: 2401323. Doi: 10.1183/13993003.01323-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benza RL, Kanwar MK, Raina A, et al. . Development and validation of an abridged version of the REVEAL 2.0 risk score calculator, REVEAL Lite 2, for use in patients with pulmonary arterial hypertension. Chest 2021; 159: 337–346. doi: 10.1016/j.chest.2020.08.2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoeper MM, Kramer T, Pan Z, et al. . Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50: 1700740. doi: 10.1183/13993003.00740-2017 [DOI] [PubMed] [Google Scholar]
- 43.Boucly A, Weatherald J, Savale L, et al. . Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700889. doi: 10.1183/13993003.00889-2017 [DOI] [PubMed] [Google Scholar]
- 44.Kylhammar D, Kjellström B, Hjalmarsson C, et al. . A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J 2018; 39: 4175–4181. doi: 10.1093/eurheartj/ehx257 [DOI] [PubMed] [Google Scholar]
- 45.McLaughlin VV, Gaine SP, Howard LS, et al. . Treatment goals of pulmonary hypertension. J Am Coll Cardiol 2013; 62: Suppl. 25, D73–D81. doi: 10.1016/j.jacc.2013.10.034 [DOI] [PubMed] [Google Scholar]
- 46.Galiè N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 47.Humbert M, Kovacs G, Hoeper MM, et al. . 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023; 61: 2200879. doi: 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 48.Galiè N, Channick RN, Frantz RP, et al. . Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801889. doi: 10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Addo M, Cornely O, Denkinger M, et al. . RSV vaccination strategies for high-risk patients 2023: a collaborative position paper by leading German medical societies and organizations. Infection 2024; 52: 285–288. doi: 10.1007/s15010-023-02141-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grünig E, MacKenzie A, Peacock AJ, et al. . Standardized exercise training is feasible, safe, and effective in pulmonary arterial and chronic thromboembolic pulmonary hypertension: results from a large European multicentre randomized controlled trial. Eur Heart J 2021; 42: 2284–2295. doi: 10.1093/eurheartj/ehaa696 [DOI] [PubMed] [Google Scholar]
- 51.Kramer T, Wissmüller M, Natsina K, et al. . Ferric carboxymaltose in patients with pulmonary arterial hypertension and iron deficiency: a long-term study. J Cachexia Sarcopenia Muscle 2021; 12: 1501–1512. doi: 10.1002/jcsm.12764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiss BM, Zemp L, Seifert B, et al. . Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996. J Am Coll Cardiol 1998; 31: 1650–1657. doi: 10.1016/S0735-1097(98)00162-4 [DOI] [PubMed] [Google Scholar]
- 53.Bédard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J 2009; 30: 256–265. doi: 10.1093/eurheartj/ehn597 [DOI] [PubMed] [Google Scholar]
- 54.Preston IR, Howard LS, Langleben D, et al. Management of pulmonary hypertension in special conditions. Eur Respir J 2024; 64: 2401180. Doi: 10.1183/13993003.01180-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olsson KM, Delcroix M, Ghofrani HA, et al. . Anticoagulation and survival in pulmonary arterial hypertension: results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA). Circulation 2014; 129: 57–65. doi: 10.1161/CIRCULATIONAHA.113.004526 [DOI] [PubMed] [Google Scholar]
- 56.Preston IR, Roberts KE, Miller DP, et al. . Effect of warfarin treatment on survival of patients with pulmonary arterial hypertension (PAH) in the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL). Circulation 2015; 132: 2403–2411. doi: 10.1161/CIRCULATIONAHA.115.018435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan MS, Usman MS, Siddiqi TJ, et al. . Is anticoagulation beneficial in pulmonary arterial hypertension? Circ Cardiovasc Qual Outcomes 2018; 11: e004757. doi: 10.1161/CIRCOUTCOMES.118.004757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang P, Hu L, Yin Y, et al. . Can anticoagulants improve the survival rate for patients with idiopathic pulmonary arterial hypertension? A systematic review and meta-analysis. Thromb Res 2020; 196: 251–256. doi: 10.1016/j.thromres.2020.08.024 [DOI] [PubMed] [Google Scholar]
- 59.Adegunsoye A, Strek ME, Garrity E, et al. . Comprehensive care of the lung transplant patient. Chest 2017; 152: 150–164. doi: 10.1016/j.chest.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerhardt F, Fiessler E, Olsson KM, et al. . Positive vasoreactivity testing in pulmonary arterial hypertension: therapeutic consequences, treatment patterns, and outcomes in the modern management era. Circulation 2024; 149: 1549–1564. doi: 10.1161/CIRCULATIONAHA.122.063821 [DOI] [PubMed] [Google Scholar]
- 61.Simonneau G, Montani D, Celermajer DS, et al. . Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nathan SD, Barnett SD, King CS, et al. . Impact of the new definition for pulmonary hypertension in patients with lung disease: an analysis of the United Network for Organ Sharing database. Pulm Circ 2021; 11: 2045894021999960. doi: 10.1177/2045894021999960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan Z, Marra AM, Benjamin N, et al. . Early treatment with ambrisentan of mildly elevated mean pulmonary arterial pressure associated with systemic sclerosis: a randomized, controlled, double-blind, parallel group study (EDITA study). Arthritis Res Ther 2019; 21: 217. doi: 10.1186/s13075-019-1981-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saggar R, Khanna D, Shapiro S, et al. . Brief report: effect of ambrisentan treatment on exercise-induced pulmonary hypertension in systemic sclerosis: a prospective single-center, open-label pilot study. Arthritis Rheum 2012; 64: 4072–4077. doi: 10.1002/art.34614 [DOI] [PubMed] [Google Scholar]
- 65.Kovacs G, Olschewski A, Berghold A, et al. . Pulmonary vascular resistances during exercise in normal subjects: a systematic review. Eur Respir J 2012; 39: 319–328. doi: 10.1183/09031936.00008611 [DOI] [PubMed] [Google Scholar]
- 66.Kovacs G, Bartolome S, Denton CP, et al. . Definition, classification and diagnosis of pulmonary hypertension. Eur Respir J 2024; 64: 2401324. Doi: 10.1183/13993003.01324-2024 [DOI] [PubMed] [Google Scholar]
- 67.McLaughlin V, Channick RN, Ghofrani HA, et al. . Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur Respir J 2015; 46: 405–413. doi: 10.1183/13993003.02044-2014 [DOI] [PubMed] [Google Scholar]
- 68.Badesch DB, Tapson VF, McGoon MD, et al. . Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med 2000; 132: 425–434. doi: 10.7326/0003-4819-132-6-200003210-00002 [DOI] [PubMed] [Google Scholar]
- 69.Cascino TM, McLaughlin VV. Upfront combination therapy for pulmonary arterial hypertension: time to be more ambitious than AMBITION. Am J Respir Crit Care Med 2021; 204: 756–759. doi: 10.1164/rccm.202107-1625ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Savale L, Benazzo A, Corris P, et al. . Transplantation, bridging, and support technologies in pulmonary hypertension. Eur Respir J 2024; 64: 2401193.Doi: 10.1183/13993003.01193-202410.1183/13993003.01193-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coghlan JG, Gaine S, Channick R, et al. . Early selexipag initiation and long-term outcomes: insights from randomised controlled trials in pulmonary arterial hypertension. ERJ Open Res 2023; 9: 00456-2022. doi: 10.1183/23120541.00456-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Humbert M, Kovacs G, Hoeper MM, et al. . 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 73.Gaine S, Sitbon O, Channick RN, et al. . Relationship between time from diagnosis and morbidity/mortality in pulmonary arterial hypertension: results from the phase III GRIPHON study. Chest 2021; 160: 277–286. doi: 10.1016/j.chest.2021.01.066 [DOI] [PubMed] [Google Scholar]
- 74.Pan HM, McClelland RL, Moutchia J, et al. . Heterogeneity of treatment effects by risk in pulmonary arterial hypertension. Eur Respir J 2023; 62: 2300190. doi: 10.1183/13993003.00190-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim NH, Fisher M, Poch D, et al. . Long-term outcomes in pulmonary arterial hypertension by functional class: a meta-analysis of randomized controlled trials and observational registries. Pulm Circ 2020; 10: 2045894020935291. doi: 10.1177/2045894020935291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benza RL, Gomberg-Maitland M, Elliott CG, et al. . Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019; 156: 232–337. doi: 10.1016/j.chest.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 77.Weatherald J, Boucly A, Chemla D, et al. . Prognostic value of follow-up hemodynamic variables after initial management in pulmonary arterial hypertension. Circulation 2018; 137: 693–704. doi: 10.1161/CIRCULATIONAHA.117.029254 [DOI] [PubMed] [Google Scholar]
- 78.Badagliacca R, D'Alto M, Ghio S, et al. . Risk reduction and hemodynamics with initial combination therapy in pulmonary arterial hypertension. Am J Respir Crit Care Med 2021; 203: 484–492. doi: 10.1164/rccm.202004-1006OC [DOI] [PubMed] [Google Scholar]
- 79.D'Alto M, Badagliacca R, Argiento P, et al. . Risk reduction and right heart reverse remodeling by upfront triple combination therapy in pulmonary arterial hypertension. Chest 2020; 157: 376–383. doi: 10.1016/j.chest.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 80.Boucly A, Beurnier A, Turquier S, et al. . Risk stratification refinements with inclusion of haemodynamic variables at follow-up in patients with pulmonary arterial hypertension. Eur Respir J 2024; 63: 2400197. doi: 10.1183/13993003.00197-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van de Veerdonk MC, Kind T, Marcus JT, et al. . Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011; 58: 2511–2519. doi: 10.1016/j.jacc.2011.06.068 [DOI] [PubMed] [Google Scholar]
- 82.Souza R, Badesch DB, Ghofrani HA, et al. . Effects of sotatercept on haemodynamics and right heart function: analysis of the STELLAR trial. Eur Respir J 2023; 62: 2301107. doi: 10.1183/13993003.01107-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McLaughlin VV, Oudiz RJ, Frost A, et al. . Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med 2006; 174: 1257–1263. doi: 10.1164/rccm.200603-358OC [DOI] [PubMed] [Google Scholar]
- 84.Picken C, Fragkos KC, Eddama M, et al. . Adverse events of prostacyclin mimetics in pulmonary arterial hypertension: a systematic review and meta-analysis. J Clin Med 2019; 8: 481. doi: 10.3390/jcm8040481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoeper MM, Simonneau G, Corris PA, et al. . RESPITE: switching to riociguat in pulmonary arterial hypertension patients with inadequate response to phosphodiesterase-5 inhibitors. Eur Respir J 2017; 50: 1602425. doi: 10.1183/13993003.02425-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harter ZJ, Agarwal S, Dalvi P, et al. . Drug abuse and HIV-related pulmonary hypertension: double hit injury. AIDS 2018; 32: 2651–2667. doi: 10.1097/QAD.0000000000002030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Egan TM, Edwards LB. Effect of the lung allocation score on lung transplantation in the United States. J Heart Lung Transplant 2016; 35: 433–439. doi: 10.1016/j.healun.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 88.Kolaitis NA, Chen H, Calabrese DR, et al. . The lung allocation score remains inequitable for patients with pulmonary arterial hypertension, even after the 2015 revision. Am J Respir Crit Care Med 2023; 207: 300–311. doi: 10.1164/rccm.202201-0217OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Savale L, Le Pavec J, Mercier O, et al. . Impact of high-priority allocation on lung and heart-lung transplantation for pulmonary hypertension. Ann Thorac Surg 2017; 104: 404–411. doi: 10.1016/j.athoracsur.2017.02.034 [DOI] [PubMed] [Google Scholar]
- 90.McLaughlin VV, Vachiery JL, Oudiz RJ, et al. . Patients with pulmonary arterial hypertension with and without cardiovascular risk factors: results from the AMBITION trial. J Heart Lung Transplant 2019; 38: 1286–1295. doi: 10.1016/j.healun.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 91.Harder EM, Divo MJ, Washko GR, et al. . Implications of mean pulmonary arterial wedge pressure trajectories in pulmonary arterial hypertension. Am J Respir Crit Care Med 2024; 209: 316–324. doi: 10.1164/rccm.202306-1072OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kianzad A, van Wezenbeek J, Celant LR, et al. . Idiopathic pulmonary arterial hypertension patients with a high H2FPEF-score: insights from the Amsterdam UMC PAH-cohort. J Heart Lung Transplant 2022; 41: 1075–1085. doi: 10.1016/j.healun.2022.05.007 [DOI] [PubMed] [Google Scholar]
- 93.Rosenkranz S, Channick R, Chin KM, et al. . The impact of comorbidities on selexipag treatment effect in patients with pulmonary arterial hypertension: insights from the GRIPHON study. Eur J Heart Fail 2022; 24: 205–214. doi: 10.1002/ejhf.2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoeper MM, Dwivedi K, Pausch C, et al. . Phenotyping of idiopathic pulmonary arterial hypertension: a registry analysis. Lancet Respir Med 2022; 10: 937–948. doi: 10.1016/S2213-2600(22)00097-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shlobin OA, Adir Y, Barbera JA, et al. . Pulmonary hypertension associated with lung diseases. Eur Respir J 2024; 64: 2401200. Doi: 10.1183/13993003.01200-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vachiéry JL, Delcroix M, Al-Hiti H, et al. . Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J 2018; 51: 1701886. doi: 10.1183/13993003.01886-2017 [DOI] [PubMed] [Google Scholar]
- 97.Hoeper MM, Pausch C, Grünig E, et al. . Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heart Lung Transplant 2020; 39: 1435–1444. doi: 10.1016/j.healun.2020.09.011 [DOI] [PubMed] [Google Scholar]
- 98.Raghu G, Behr J, Brown KK, et al. . Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med 2013; 158: 641–649. doi: 10.7326/0003-4819-158-9-201305070-00003 [DOI] [PubMed] [Google Scholar]
- 99.Raghu G, Million-Rousseau R, Morganti A, et al. . Macitentan for the treatment of idiopathic pulmonary fibrosis: the randomised controlled MUSIC trial. Eur Respir J 2013; 42: 1622–1632. doi: 10.1183/09031936.00104612 [DOI] [PubMed] [Google Scholar]
- 100.Nathan SD, Behr J, Collard HR, et al. . Riociguat for idiopathic interstitial pneumonia-associated pulmonary hypertension (RISE-IIP): a randomised, placebo-controlled phase 2b study. Lancet Respir Med 2019; 7: 780–790. doi: 10.1016/S2213-2600(19)30250-4 [DOI] [PubMed] [Google Scholar]
- 101.Bermejo J, Yotti R, García-Orta R, et al. . Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double-blind, randomized clinical trial. Eur Heart J 2018; 39: 1255–1264. doi: 10.1093/eurheartj/ehx700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nathan SD, Barbera JA, Gaine SP, et al. . Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019; 53: 1801914. doi: 10.1183/13993003.01914-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Packer M, McMurray JJV, Krum H, et al. . Long-term effect of endothelin receptor antagonism with bosentan on the morbidity and mortality of patients with severe chronic heart failure: primary results of the ENABLE trials. JACC Heart Fail 2017; 5: 317–326. doi: 10.1016/j.jchf.2017.02.021 [DOI] [PubMed] [Google Scholar]
- 104.Machado RF, Barst RJ, Yovetich NA, et al. . Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood 2011; 118: 855–864. doi: 10.1182/blood-2010-09-306167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoeper MM, Oerke B, Wissmüller M, et al. . Tadalafil for treatment of combined postcapillary and precapillary pulmonary hypertension in patients with heart failure and preserved ejection fraction: a randomized controlled phase 3 study. Circulation 2024; 150: 600–610. doi: 10.1161/CIRCULATIONAHA.124.069340 [DOI] [PubMed] [Google Scholar]
- 106.Maron BA, Bortman G, De Marco T, et al. . Pulmonary hypertension associated with left heart disease. Eur Respir J 2024; 64: 2401344. Doi: 10.1183/13993003.01344-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rhee RL, Gabler NB, Sangani S, et al. . Comparison of treatment response in idiopathic and connective tissue disease-associated pulmonary arterial hypertension. Am J Respir Crit Care Med 2015; 192: 1111–1117. doi: 10.1164/rccm.201507-1456OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khanna D, Zhao C, Saggar R, et al. . Long-term outcomes in patients with connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era: meta-analyses of randomized, controlled trials and observational registries. Arthritis Rheumatol 2021; 73: 837–847. doi: 10.1002/art.41669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coghlan JG, Denton CP, Grünig E, et al. . Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73: 1340–1349. doi: 10.1136/annrheumdis-2013-203301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xanthouli P, Jordan S, Milde N, et al. . Haemodynamic phenotypes and survival in patients with systemic sclerosis: the impact of the new definition of pulmonary arterial hypertension. Ann Rheum Dis 2020; 79: 370–378. doi: 10.1136/annrheumdis-2019-216476 [DOI] [PubMed] [Google Scholar]
- 111.Chung L, Domsic RT, Lingala B, et al. . Survival and predictors of mortality in systemic sclerosis-associated pulmonary arterial hypertension: outcomes from the pulmonary hypertension assessment and recognition of outcomes in scleroderma registry. Arthritis Care Res 2014; 66: 489–495. doi: 10.1002/acr.22121 [DOI] [PubMed] [Google Scholar]
- 112.Hassan HJ, Naranjo M, Ayoub N, et al. . Improved survival for patients with systemic sclerosis-associated pulmonary arterial hypertension: the Johns Hopkins registry. Am J Respir Crit Care Med 2023; 207: 312–322. doi: 10.1164/rccm.202204-0731OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mathai SC, Puhan MA, Lam D, et al. . The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 428–433. doi: 10.1164/rccm.201203-0480OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dorfmüller P, Humbert M, Perros F, et al. . Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol 2007; 38: 893–902. doi: 10.1016/j.humpath.2006.11.022 [DOI] [PubMed] [Google Scholar]
- 115.Günther S, Jaïs X, Maitre S, et al. . Computed tomography findings of pulmonary venoocclusive disease in scleroderma patients presenting with precapillary pulmonary hypertension. Arthritis Rheum 2012; 64: 2995–3005. doi: 10.1002/art.34501 [DOI] [PubMed] [Google Scholar]
- 116.Kuwana M, Blair C, Takahashi T, et al. . Initial combination therapy of ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH) in the modified intention-to-treat population of the AMBITION study: post hoc analysis. Ann Rheum Dis 2020; 79: 626–634. doi: 10.1136/annrheumdis-2019-216274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mullin CJ, Khair RM, Damico RL, et al. . Validation of the REVEAL prognostic equation and risk score calculator in incident systemic sclerosis-associated pulmonary arterial hypertension. Arthritis Rheumatol 2019; 71: 1691–1700. doi: 10.1002/art.40918 [DOI] [PubMed] [Google Scholar]
- 118.Brida M, Gatzoulis MA. Pulmonary arterial hypertension in adult congenital heart disease. Heart 2018; 104: 1568–1574. doi: 10.1136/heartjnl-2017-312106 [DOI] [PubMed] [Google Scholar]
- 119.Galiè N, Beghetti M, Gatzoulis MA, et al. . Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation 2006; 114: 48–54. doi: 10.1161/CIRCULATIONAHA.106.630715 [DOI] [PubMed] [Google Scholar]
- 120.Mukhopadhyay S, Nathani S, Yusuf J, et al. . Clinical efficacy of phosphodiesterase-5 inhibitor tadalafil in Eisenmenger syndrome – a randomized, placebo-controlled, double-blind crossover study. Congenit Heart Dis 2011; 6: 424–431. doi: 10.1111/j.1747-0803.2011.00561.x [DOI] [PubMed] [Google Scholar]
- 121.D'Alto M, Constantine A, Balint OH, et al. . The effects of parenteral prostacyclin therapy as add-on treatment to oral compounds in Eisenmenger syndrome. Eur Respir J 2019; 54: 1901401. doi: 10.1183/13993003.01401-2019 [DOI] [PubMed] [Google Scholar]
- 122.Zhang ZN, Jiang X, Zhang R, et al. . Oral sildenafil treatment for Eisenmenger syndrome: a prospective, open-label, multicentre study. Heart 2011; 97: 1876–1881. doi: 10.1136/heartjnl-2011-300344 [DOI] [PubMed] [Google Scholar]
- 123.Zuckerman WA, Leaderer D, Rowan CA, et al. . Ambrisentan for pulmonary arterial hypertension due to congenital heart disease. Am J Cardiol 2011; 107: 1381–1385. doi: 10.1016/j.amjcard.2010.12.051 [DOI] [PubMed] [Google Scholar]
- 124.Dimopoulos K, Inuzuka R, Goletto S, et al. . Improved survival among patients with Eisenmenger syndrome receiving advanced therapy for pulmonary arterial hypertension. Circulation 2010; 121: 20–25. doi: 10.1161/CIRCULATIONAHA.109.883876 [DOI] [PubMed] [Google Scholar]
- 125.Luna-Lopez R, Segura de la Cal T, Sarnago Cebada F, et al. . Triple vasodilator therapy in pulmonary arterial hypertension associated with congenital heart disease. Heart 2024; 110: 346–352. doi: 10.1136/heartjnl-2023-323015 [DOI] [PubMed] [Google Scholar]
- 126.Hascoët S, Baruteau AE, Humbert M, et al. . Long-term outcomes of pulmonary arterial hypertension under specific drug therapy in Eisenmenger syndrome. J Heart Lung Transplant 2017; 36: 386–398. doi: 10.1016/j.healun.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 127.Kaemmerer AS, Gorenflo M, Huscher D, et al. . Medical treatment of pulmonary hypertension in adults with congenital heart disease: updated and extended results from the International COMPERA-CHD registry. Cardiovasc Diagn Ther 2021; 11: 1255–1268. doi: 10.21037/cdt-21-351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Savale L, Manes A. Pulmonary arterial hypertension populations of special interest: portopulmonary hypertension and pulmonary arterial hypertension associated with congenital heart disease. Eur Heart J Suppl 2019; 21: Suppl. K, K37–K45. doi: 10.1093/eurheartj/suz221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kempny A, Hjortshøj CS, Gu H, et al. . Predictors of death in contemporary adult patients with Eisenmenger syndrome: a multicenter study. Circulation 2017; 135: 1432–1440. doi: 10.1161/CIRCULATIONAHA.116.023033 [DOI] [PubMed] [Google Scholar]
- 130.Kearney K, Lau EM, Darley D, et al. . Waitlist and post-transplant outcomes for Eisenmenger syndrome: a comparison of transplant strategies. J Heart Lung Transplant 2021; 40: 841–849. doi: 10.1016/j.healun.2021.04.005 [DOI] [PubMed] [Google Scholar]
- 131.Wang Z, Li X, Li M, et al. . The efficacy of the treat-repair-treat strategy for severe pulmonary arterial hypertension associated with congenital heart disease: a meta-analysis. BMC Cardiovasc Disord 2023; 23: 569. doi: 10.1186/s12872-023-03606-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ivy D, Rosenzweig EB, Abman SH, et al. . Embracing the challenges of neonatal and paediatric pulmonary hypertension. Eur Respir J 2024; 64: 2401345. Doi: 10.1183/13993003.01345-202410.1183/13993003.01345-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weatherald J, Chaumais MC, Savale L, et al. . Long-term outcomes of dasatinib-induced pulmonary arterial hypertension: a population-based study. Eur Respir J 2017; 50: 1700217. doi: 10.1183/13993003.00217-2017 [DOI] [PubMed] [Google Scholar]
- 134.Savale L, Chaumais MC, O'Connell C, et al. . Interferon-induced pulmonary hypertension: an update. Curr Opin Pulm Med 2016; 22: 415–420. doi: 10.1097/MCP.0000000000000307 [DOI] [PubMed] [Google Scholar]
- 135.Ranchoux B, Günther S, Quarck R, et al. . Chemotherapy-induced pulmonary hypertension: role of alkylating agents. Am J Pathol 2015; 185: 356–371. doi: 10.1016/j.ajpath.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 136.Kolaitis NA, Zamanian RT, de Jesus Perez VA, et al. . Clinical differences and outcomes between methamphetamine-associated and idiopathic pulmonary arterial hypertension in the pulmonary hypertension association registry. Ann Am Thorac Soc 2021; 18: 613–622. doi: 10.1513/AnnalsATS.202007-774OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zamanian RT, Hedlin H, Greuenwald P, et al. . Features and outcomes of methamphetamine-associated pulmonary arterial hypertension. Am J Respir Crit Care Med 2018; 197: 788–800. doi: 10.1164/rccm.201705-0943OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Salvador ML, Rodríguez-Padial L, Soto Abánades C, et al. . Management and prognosis of HIV-associated pulmonary arterial hypertension: 20 years of evidence from the REHAP registry. J Intern Med 2022; 292: 116–126. doi: 10.1111/joim.13468 [DOI] [PubMed] [Google Scholar]
- 139.Kolaitis NA, Lammi M, Mazimba S, et al. . HIV-associated pulmonary arterial hypertension: a report from the Pulmonary Hypertension Association Registry. Am J Respir Crit Care Med 2022; 205: 1121–1124. doi: 10.1164/rccm.202111-2481LE [DOI] [PubMed] [Google Scholar]
- 140.Sitbon O, Gressin V, Speich R, et al. . Bosentan for the treatment of human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med 2004; 170: 1212–1217. doi: 10.1164/rccm.200404-445OC [DOI] [PubMed] [Google Scholar]
- 141.Degano B, Guillaume M, Savale L, et al. . HIV-associated pulmonary arterial hypertension: survival and prognostic factors in the modern therapeutic era. AIDS 2010; 24: 67–75. doi: 10.1097/QAD.0b013e328331c65e [DOI] [PubMed] [Google Scholar]
- 142.Chinello P, Petrosillo N. Pharmacological treatment of HIV-associated pulmonary hypertension. Expert Rev Clin Pharmacol 2016; 9: 715–725. doi: 10.1586/17512433.2016.1151785 [DOI] [PubMed] [Google Scholar]
- 143.Atsukawa M, Tsubota A, Hatano M, et al. . Prevalence and characteristics of portopulmonary hypertension in cirrhotic patients who underwent both hepatic vein and pulmonary artery catheterization. Hepatol Res 2020; 50: 1244–1254. doi: 10.1111/hepr.13560 [DOI] [PubMed] [Google Scholar]
- 144.Badesch DB, Raskob GE, Elliott CG, et al. . Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest 2010; 137: 376–387. doi: 10.1378/chest.09-1140 [DOI] [PubMed] [Google Scholar]
- 145.Humbert M, Sitbon O, Chaouat A, et al. . Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006; 173: 1023–1030. doi: 10.1164/rccm.200510-1668OC [DOI] [PubMed] [Google Scholar]
- 146.Tamura Y, Kumamaru H, Inami T, et al. . Changes in the characteristics and initial treatments of pulmonary hypertension between 2008 and 2020 in Japan. JACC Asia 2022; 2: 273–284. doi: 10.1016/j.jacasi.2022.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hadengue A, Benhayoun MK, Lebrec D, et al. . Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology 1991; 100: 520–528. doi: 10.1016/0016-5085(91)90225-A [DOI] [PubMed] [Google Scholar]
- 148.Colle IO, Moreau R, Godinho E, et al. . Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology 2003; 37: 401–409. doi: 10.1053/jhep.2003.50060 [DOI] [PubMed] [Google Scholar]
- 149.Castro M, Krowka MJ, Schroeder DR, et al. . Frequency and clinical implications of increased pulmonary artery pressures in liver transplant patients. Mayo Clin Proc 1996; 71: 543–551. doi: 10.4065/71.6.543 [DOI] [PubMed] [Google Scholar]
- 150.Jose A, Kay D, Elwing JD. Treatment of portopulmonary hypertension (PoPH): a review. J Liver Transplant 2022; 6: 100071. doi: 10.1016/j.liver.2022.100071 [DOI] [Google Scholar]
- 151.Savale L, Guimas M, Ebstein N, et al. . Portopulmonary hypertension in the current era of pulmonary hypertension management. J Hepatol 2020; 73: 130–139. doi: 10.1016/j.jhep.2020.02.021 [DOI] [PubMed] [Google Scholar]
- 152.Tamura Y, Tamura Y, Taniguchi Y, et al. . Clinical management and outcomes of patients with portopulmonary hypertension enrolled in the Japanese Multicenter Registry. Circ Rep 2022; 4: 542–549. doi: 10.1253/circrep.CR-22-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Swanson KL, Wiesner RH, Nyberg SL, et al. . Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant 2008; 8: 2445–2453. doi: 10.1111/j.1600-6143.2008.02384.x [DOI] [PubMed] [Google Scholar]
- 154.Jose A, Kopras EJ, Shah SA, et al. . Portopulmonary hypertension practice patterns after liver transplantation. Liver Transpl 2023; 29: 365–376. doi: 10.1002/lt.26575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Deroo R, Trépo E, Holvoet T, et al. . Vasomodulators and liver transplantation for portopulmonary hypertension: evidence from a systematic review and meta-analysis. Hepatology 2020; 72: 1701–1716. doi: 10.1002/hep.31164 [DOI] [PubMed] [Google Scholar]
- 156.Knafl D, Gerges C, King CH, et al. . Schistosomiasis-associated pulmonary arterial hypertension: a systematic review. Eur Respir Rev 2020; 29: 190089. doi: 10.1183/16000617.0089-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Alves JL Jr, Gavilanes F, Jardim C, et al. . Pulmonary arterial hypertension in the southern hemisphere: results from a registry of incident Brazilian cases. Chest 2015; 147: 495–501. doi: 10.1378/chest.14-1036 [DOI] [PubMed] [Google Scholar]
- 158.Graham BB, Hilton JF, Lee MH, et al. . Is pulmonary arterial hypertension associated with schistosomiasis distinct from pulmonary arterial hypertension associated with portal hypertension? JHLT Open 2023; 1: 100007. doi: 10.1016/j.jhlto.2023.100007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sibomana JP, Campeche A, Carvalho-Filho RJ, et al. . Schistosomiasis pulmonary arterial hypertension. Front Immunol 2020; 11: 608883. doi: 10.3389/fimmu.2020.608883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fernandes CJC, Piloto B, Castro M, et al. . Survival of patients with schistosomiasis-associated pulmonary arterial hypertension in the modern management era. Eur Respir J 2018; 51: 1800307. doi: 10.1183/13993003.00307-2018 [DOI] [PubMed] [Google Scholar]
- 161.Barreto AC, Franchi SM, Castro CR, et al. . One-year follow-up of the effects of sildenafil on pulmonary arterial hypertension and veno-occlusive disease. Braz J Med Biol Res 2005; 38: 185–195. doi: 10.1590/S0100-879X2005000200006 [DOI] [PubMed] [Google Scholar]
- 162.Montani D, Achouh L, Dorfmüller P, et al. . Pulmonary veno-occlusive disease: clinical, functional, radiologic, and hemodynamic characteristics and outcome of 24 cases confirmed by histology. Medicine 2008; 87: 220–233. doi: 10.1097/MD.0b013e31818193bb [DOI] [PubMed] [Google Scholar]
- 163.Montani D, Girerd B, Jaïs X, et al. . Clinical phenotypes and outcomes of heritable and sporadic pulmonary veno-occlusive disease: a population-based study. Lancet Respir Med 2017; 5: 125–134. doi: 10.1016/S2213-2600(16)30438-6 [DOI] [PubMed] [Google Scholar]
- 164.Palmer SM, Robinson LJ, Wang A, et al. . Massive pulmonary edema and death after prostacyclin infusion in a patient with pulmonary veno-occlusive disease. Chest 1998; 113: 237–240. doi: 10.1378/chest.113.1.237 [DOI] [PubMed] [Google Scholar]
- 165.Boucly A, Solinas S, Beurnier A, et al. . Outcomes and risk assessment in pulmonary veno-occlusive disease. ERJ Open Res 2024; 10: 00612-2023. doi: 10.1183/23120541.00612-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Montani D, Jaïs X, Price LC, et al. . Cautious epoprostenol therapy is a safe bridge to lung transplantation in pulmonary veno-occlusive disease. Eur Respir J 2009; 34: 1348–1356. doi: 10.1183/09031936.00017809 [DOI] [PubMed] [Google Scholar]
- 167.Ogawa A, Sakao S, Tanabe N, et al. . Use of vasodilators for the treatment of pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis: a systematic review. Respir Investig 2019; 57: 183–190. doi: 10.1016/j.resinv.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 168.Khan MS, Amin E, Memon MM, et al. . Meta-analysis of use of balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Int J Cardiol 2019; 291: 134–139. doi: 10.1016/j.ijcard.2019.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rubin LJ, Groves BM, Reeves JT, et al. . Prostacyclin-induced acute pulmonary vasodilation in primary pulmonary hypertension. Circulation 1982; 66: 334–338. doi: 10.1161/01.CIR.66.2.334 [DOI] [PubMed] [Google Scholar]
- 170.Leuchte HH, Schwaiblmair M, Baumgartner RA, et al. . Hemodynamic response to sildenafil, nitric oxide, and iloprost in primary pulmonary hypertension. Chest 2004; 125: 580–586. doi: 10.1378/chest.125.2.580 [DOI] [PubMed] [Google Scholar]
- 171.McLaughlin VV, Genthner DE, Panella MM, et al. . Reduction in pulmonary vascular resistance with long-term epoprostenol (prostacyclin) therapy in primary pulmonary hypertension. N Engl J Med 1998; 338: 273–277. doi: 10.1056/NEJM199801293380501 [DOI] [PubMed] [Google Scholar]
- 172.Farmakis IT, Baroutidou A, Patsiou V, et al. . Contribution of pressure and flow changes to resistance reduction after pulmonary arterial hypertension treatment: a meta-analysis of 3898 patients. ERJ Open Res 2024; 10: 00706-2023. doi: 10.1183/23120541.00706-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bartolome SD, Sood N, Shah TG, et al. . Mortality in patients with pulmonary arterial hypertension treated with continuous prostanoids. Chest 2018; 154: 532–540. doi: 10.1016/j.chest.2018.03.050 [DOI] [PubMed] [Google Scholar]
- 174.Ghofrani HA, Wiedemann R, Rose F, et al. . Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet 2002; 360: 895–900. doi: 10.1016/S0140-6736(02)11024-5 [DOI] [PubMed] [Google Scholar]
- 175.United States Food and Drug Administration . Sotatercept. Patient Information. www.accessdata.fda.gov/drugsatfda_docs/label/2024/761363s000lbl.pdf. Date last accessed: 2 July 2024. Date last updated: March 2024.
- 176.Sitbon O, Humbert M, Nunes H, et al. . Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 2002; 40: 780–788. doi: 10.1016/S0735-1097(02)02012-0 [DOI] [PubMed] [Google Scholar]
- 177.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation 2002; 106: 1477–1482. doi: 10.1161/01.CIR.0000029100.82385.58 [DOI] [PubMed] [Google Scholar]
- 178.Oudiz RJ, Farber HW. Dosing considerations in the use of intravenous prostanoids in pulmonary arterial hypertension: an experience-based review. Am Heart J 2009; 157: 625–635. doi: 10.1016/j.ahj.2008.10.029 [DOI] [PubMed] [Google Scholar]
- 179.Benza RL, Gomberg-Maitland M, Naeije R, et al. . Prognostic factors associated with increased survival in patients with pulmonary arterial hypertension treated with subcutaneous treprostinil in randomized, placebo-controlled trials. J Heart Lung Transplant 2011; 30: 982–989. doi: 10.1016/j.healun.2011.03.011 [DOI] [PubMed] [Google Scholar]
- 180.Akagi S, Matsubara H, Nakamura K, et al. . Modern treatment to reduce pulmonary arterial pressure in pulmonary arterial hypertension. J Cardiol 2018; 72: 466–472. doi: 10.1016/j.jjcc.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 181.Tokunaga N, Ogawa A, Ito H, et al. . Rapid and high-dose titration of epoprostenol improves pulmonary hemodynamics and clinical outcomes in patients with idiopathic and heritable pulmonary arterial hypertension. J Cardiol 2016; 68: 542–547. doi: 10.1016/j.jjcc.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 182.Rich S, McLaughlin VV. The effects of chronic prostacyclin therapy on cardiac output and symptoms in primary pulmonary hypertension. J Am Coll Cardiol 1999; 34: 1184–1187. doi: 10.1016/S0735-1097(99)00320-4 [DOI] [PubMed] [Google Scholar]
- 183.Holmboe S, Andersen A, Jensen RV, et al. . Prostacyclins have no direct inotropic effect on isolated atrial strips from the normal and pressure-overloaded human right heart. Pulm Circ 2017; 7: 339–347. doi: 10.1177/2045893217691532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.McLaughlin VV, Gaine SP, Barst RJ, et al. . Efficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertension. J Cardiovasc Pharmacol 2003; 41: 293–299. doi: 10.1097/00005344-200302000-00019 [DOI] [PubMed] [Google Scholar]
- 185.Sitbon O, Manes A, Jais X, et al. . Rapid switch from intravenous epoprostenol to intravenous treprostinil in patients with pulmonary arterial hypertension. J Cardiovasc Pharmacol 2007; 49: 1–5. doi: 10.1097/FJC.0b013e31802b3184 [DOI] [PubMed] [Google Scholar]
- 186.Gomberg-Maitland M, Tapson VF, Benza RL, et al. . Transition from intravenous epoprostenol to intravenous treprostinil in pulmonary hypertension. Am J Respir Crit Care Med 2005; 172: 1586–1589. doi: 10.1164/rccm.200505-766OC [DOI] [PubMed] [Google Scholar]
- 187.Rubenfire M, McLaughlin VV, Allen RP, et al. . Transition from IV epoprostenol to subcutaneous treprostinil in pulmonary arterial hypertension: a controlled trial. Chest 2007; 132: 757–763. doi: 10.1378/chest.06-2118 [DOI] [PubMed] [Google Scholar]
- 188.Kikuchi H, Goda A, Takeuchi K, et al. . Transition from intravenous epoprostenol to treprostinil due to intolerable side effects in patients with pulmonary arterial hypertension. Am J Cardiol 2023; 206: 31–34. doi: 10.1016/j.amjcard.2023.08.078 [DOI] [PubMed] [Google Scholar]
- 189.Steringer-Mascherbauer R, Maria L, Reinhold F, et al. . Rapid switch from subcutaneous to intravenous treprostinil in precapillary pulmonary hypertension by pump implantation. J Cardiovasc Pharmacol 2021; 77: 38–42. doi: 10.1097/FJC.0000000000000933 [DOI] [PubMed] [Google Scholar]
- 190.Kumar P, Thudium E, Laliberte K, et al. . A comprehensive review of treprostinil pharmacokinetics via four routes of administration. Clin Pharmacokinet 2016; 55: 1495–1505. doi: 10.1007/s40262-016-0409-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alkukhun L, Bair ND, Dweik RA, et al. . Subcutaneous to intravenous prostacyclin analog transition in pulmonary hypertension. J Cardiovasc Pharmacol 2014; 63: 4–8. doi: 10.1097/FJC.0000000000000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Parikh KS, Doerfler S, Shelburne N, et al. . Experience in transitioning from parenteral prostacyclins to selexipag in pulmonary arterial hypertension. J Cardiovasc Pharmacol 2020; 75: 299–304. doi: 10.1097/FJC.0000000000000800 [DOI] [PubMed] [Google Scholar]
- 193.Hinkamp C, Bartolome S, Mims E, et al. . Transitioning stable patients with pulmonary arterial hypertension from parenteral prostanoids to oral selexipag. Biomed Hub 2022; 7: 115–124. doi: 10.1159/000526190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Frost A, Janmohamed M, Fritz JS, et al. . Safety and tolerability of transition from inhaled treprostinil to oral selexipag in pulmonary arterial hypertension: results from the TRANSIT-1 study. J Heart Lung Transplant 2019; 38: 43–50. doi: 10.1016/j.healun.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 195.Tamura Y, Furukawa A, Tamura Y, et al. . Long-term safety of a structured transition protocol from parenteral prostanoids to selexipag in pulmonary arterial hypertension. Pulm Circ 2022; 12: e12058. doi: 10.1002/pul2.12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chakinala MM, Feldman JP, Rischard F, et al. . Transition from parenteral to oral treprostinil in pulmonary arterial hypertension. J Heart Lung Transplant 2017; 36: 193–201. doi: 10.1016/j.healun.2016.06.019 [DOI] [PubMed] [Google Scholar]
- 197.MacDonald BJ, Turgeon RD. Incorporation of shared decision-making in international cardiovascular guidelines, 2012–2022. JAMA Netw Open 2023; 6: e2332793. doi: 10.1001/jamanetworkopen.2023.32793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tobita K, Sakamoto H, Inami T, et al. . Understanding patient perspectives toward shared decision-making in patients with pulmonary hypertension. Am J Cardiol 2024; 212: 23–29. doi: 10.1016/j.amjcard.2023.11.041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01325-2024.Shareable (479.2KB, pdf)