Abstract
The Vaal River catchment drains the largest and most populated industrial and mining region in Southern Africa. Heron, ibis, cormorant, egrets, and darter eggs, representing three habitats and four feeding guilds, were collected at four locations in 2009/10 to identify hotspots and hazards associated with persistent organic pollutants (POPs). The POPs included 21 organochlorine pesticides, five polybrominated diphenyl ether (PBDE) classes, 18 polychlorinated biphenyls (PCBs including six non-dioxin-like PCBs; NDL-PCB), and 12 dioxin-like PCBs (DL-PCBs), 17 polychlorinated dibenzo-p-dioxins and dibenzo-p-furans (PCDD/Fs), and perfluorooctane sulfonate (PFOS). Aquatic predators had higher PFOS and PCDD/F concentrations, while PCBs dominated in terrestrial eggs. Organochlorine pesticides, PBDEs, and PCBs were strongly associated with eggs from the industrial regions, while PCDD/F concentrations were evenly distributed. PCDD/F and PCB toxic equivalency quotient concentrations were low with no adverse effects expected. PFOS peaked at Bloemhof Dam with a maximum of 2300 ng/g wm in an African Darter egg, indicating an unexpected PFOS hotspot, the source of which is unknown. Despite order of differences in compound class concentrations, there was no association with egg size. To the best of our knowledge, this is the only study that analysed all 2010 POPs in bird eggs on a large geographic scale. This study highlighted the importance of multi-species studies sampling from multiple locations to assess the risk that POPs pose to avian populations as hotspots and species at risk may be missed by studies looking at one or few species.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00244-024-01088-4.
Keywords: PCB, PBDE, PCDD/F, DDT, Pesticide, Heron, Ardeidae
Introduction
One of the Southern Africa’s largest rivers, the Vaal River, flows westwards from Mpumalanga province to the Atlantic (Fig. 1). It flows through South Africa’s most industrialised regions before passing through rural and agricultural areas. The Vaal River merges with the Orange-Senqu River near the town of Douglas, forming the Orange-Senqu River Basin (OSRB), that stretches over four countries (Botswana, Lesotho, Namibia, and South Africa) covering approximately 1 000 000 km2 (Lange et al. 2007). The Orange River mouth at the South Atlantic Ocean was once a flourishing wetland with over 20 000 resident water birds and attracting many migrant birds. However, the number of resident birds has drastically decreased (Anderson et al. 2003).
Fig. 1.
Map showing the wild bird egg sampling locations
There are seven Ramsar sites located in the OSRB (Orange River Mouth, Lets'eng-la-Letsie, Barbers Pan, Blesbok Spruit, Kgaswane Mountain Reserve, Seekoeivlei Nature Reserve, and Ingula Nature Reserve; Ramsar Sites Information Service. 2018b). Southern Africa is, however, a water-scarce region; many rural households, agriculture, mining, and industry directly make use of the OSRB’s surface and groundwater. The influx of agricultural and industrial products (including persistent organic pollutants, POPs) is a major cause of concern (Chokwe et al. 2019; Groffen et al. 2021; Gilbert et al. 2016; Quinn et al. 2009). POPs that have been investigated include organochlorine pesticides (OCPs), polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), and metals in bird eggs (Bouwman et al. 2008; Chokwe et al. 2015; Polder et al. 2008; Van der Schyff et al. 2016).
Organochlorine pesticides can bioaccumulate in lipid tissue and are resistant to degradation (Newman 2015). In many African countries, the current and historical use of dichlorodiphenyltrichloroethane (DDT) in controlling diseases and pests led to unintentional consequences such as eggshell thinning in many bird species (Bitman et al. 1970; Holm et al. 2006; Lundholm 1997) and human health effects (Bornman et al. 2010). However, less literature is available on other main groups of POPs in bird eggs from South Africa including perfluorooctane sulfonate (PFOS), polychlorinated dibenzofurans and dibenzo-p-dioxins (PCDD/Fs), and PCBs (non-dioxin –like PCBs (NDL-PCBs) and dioxin-like PCB (DL-PCBs)).
Due to the chemical properties (Wania and Mackay 1995) and the effects of POPs, many of these compounds are banned or severely restricted (Stockholm Convention 2016a). Halogenated compounds, such as the chlorinated and brominated compounds, tend to be lipophilic and bioaccumulate in lipid tissues. The DL-PCBs (those PCBs that have chlorine atoms in the non-ortho position) and PCDD/Fs specifically mediate their toxicity via the aryl-hydrocarbon receptor (AhR; Mandal 2005). However, the fluorinated compounds that are also halogenated have both lipophilic and hydrophilic moieties (Newman 2015) which allow them to be distributed by blood to various organs such as the liver, kidneys, and lungs, among others (Kwiatkowski et al. 2021). These compounds cause peroxisomal proliferation, increased activity of lipid and xenobiotic metabolising enzymes (Newman 2015). Residues in the environment reflect current and historical production and use of these compounds (Orisakwe et al. 2019).
The bird egg is a good matrix for environmental monitoring of pollution (Medvedev and Markove 1995; Lebedev et al. 1998). They have a fairly consistent composition, decompose slowly, are easy to handle, and can be randomly sampled in a cost-effective manner. Furthermore, eggs represent the pollutant uptake by the female bird before the egg is laid, while giving insight into the effects, these compounds have on both the female bird and in the developing egg (Braune 2007; van den Steen et al. 2006). In addition, embryonic and foetal development is more sensitive to POPs than in adults since exposure prior or during organ development may have greater consequences than after (Caralson and Duby 1973). Moreover, many bird species are widely distributed over multiple continents and provide opportunities for continental comparison (Lesch et al. 2023).
Both aquatic and terrestrial birds have been used as pollution indicators (Aurigi et al. 2000; Bouwman et al. 2019, 2021; Eljarrat et al. 2019). Elevated PFOS concentrations can lead to endocrine disruption (Jensen and Leffers., 2008) and organ dysfunction, especialli the liver (Hoff et al. 2005). PCBs can cause reproductive abnormalities and lead to developmental effects (Barron et al. 1995). At elevated concentrations, PBDEs cause behavioural and growth abnormalities in the American kestrel (Falco sparverius; Fernie et al. 2006 and 2008).
Knowledge of POPs of the Stockholm convention on persistent organic pollutants (SCPOPs) in Southern Africa is restricted. The current study was carried out under the auspices of the Orange-Senqu River Commission’s (ORASECOM) 2010 Joint Basin Survey on POPs in the OSRB as part of the transboundary diagnostic analysis of the OSRB that also evaluated all POPs listed at that time. The aims of this study were therefore to investigate the concentrations of 22 POPs, as listed in the SCPOPs in 2010, in wild bird eggs from the Vaal River. Based on the data, we will identify pollution hotspots, assess the hazard that the compound concentrations may have to the developing embryo and compare the concentrations with concentration reported in literature. Additionally, we determined the concentrations between different species, feeding guilds, and habitat preferences. Lastly, we evaluated the relationship between egg size and POPs concentrations. This study, based on 2010 data, serves as a baseline for future work, identify compounds that need no further attention, but specifically highlight compounds and compound classes of concern that would also inform other studies in Southern Africa. As far as we know, this is the only study that analysed all 2010 POPs in bird eggs on a large geographic scale.
Materials and Methods
Bird Egg Sampling
Bird Egg Sampling Locations and Descriptions
The necessary provincial permits and the appropriate ethical approvals (NWU-00055–07-S3 and NWU-00594–19-A9) were obtained. Wild bird eggs were collected from four breeding colonies in the OSRB, a 192 000 km2 catchment during the breeding season (October to February) 2009/10 (Fig. 1). Efforts to date have recorded 154 heronries in South Africa (Harebottle 2019), although this number is an underestimation. The four selected breeding colonies were located during aerial surveys. The Potchefstroom colony location is near the Mooi River and is closed to residential properties and a golf course. The colony at Barbers Pan (a Ramsar site) is in a bird sanctuary with no town or city nearby. The Bloemhof Dam colony was on Snake Island. The Eldorado Park colony is within a suburb in a highly industrialised region of Gauteng province. Eggs from nine species were sampled: Grey Heron (Ardea cinerea), African Darter (Anhinga rufa), Glossy Ibis (Ardea melanocephala), Great White Egret (Ardea alba), Reed Cormorant (Microcarbo africanus), African Sacred Ibis (Threskiornis aethiopicus), Little Egret (Egretta garzetta), Cattle Egret (Bubulcus ibis), and Glossy Ibis (Plegadis falcinellus). General distributions and descriptions, habitat preferences, breeding behaviour, diet, and egg descriptions are summarised in Table S1.
Egg Sampling Effort
Eggs were sampled from nests by either climbing trees using rock-climbing gear or using ladders. Although efforts were made to collect eggs of the same species at all sites, this was not possible. Eggs were wrapped in pre-washed foil, labelled, carefully stored in thick egg cartons, and transported to the laboratory where they were photographed before being frozen at -24ºC until sample preparation. Eggs were analysed within 6 months of collection. On the day of sample preparation, selected eggs were measured and pooled per species and location as presented in Table 1. Egg contents were ultrasonically homogenised. Samples of the 16 pools were sent with the necessary permits to Oëkometric GmbH—The Bayreuth Institute of Environmental Research, in Germany. This is an accredited POPs laboratory. Coordinates of sampling locations, the closest water source, and analytical pool numbers are presented in Table 1.
Table 1.
Summary of the wild bird species from which eggs were collected at each location, along with the GPS coordinates, closest river, pool number, number of eggs per pool (n), habitat and feeding guilds according to Hockey et al. (2005), and mean egg mass (g)
| Location | Longitude | Latitude | River | Pool no | n | Common name | Scientific name | Habitat guild | Feeding guild | Egg mass |
|---|---|---|---|---|---|---|---|---|---|---|
| Barbers Pan | 25,57 | −266 | Harts River | 1 | 6 | Grey Heron | Ardea cinerea | Aquatic | Large aquatic predator | 61 |
| 5 | 5 | African Darter | Anhinga rufa | Aquatic | Large aquatic predator | 37 | ||||
| 14 | 5 | Black-headed Heron | Ardea melanocephala | Terrestrial | Terrestrial insectivore | 60 | ||||
| Bloemhof Dam | 2564 | −277 | Vaal River | 2 | 3 | Great White Egret | Ardea alba | Aquatic | Large aquatic predator | 61 |
| 3 | 5 | Grey Heron | Ardea cinerea | Aquatic | Large aquatic predator | 61 | ||||
| 6 | 3 | African Darter | Anhinga rufa | Aquatic | Large aquatic predator | 37 | ||||
| 8 | 5 | Reed Cormorant | Microcarbo africanus | Aquatic | Large aquatic predator | 21 | ||||
| 10 | 6 | African Sacred Ibis | Threskiornis aethiopicus | Wetland | Scavenger | 62 | ||||
| 11 | 4 | Little Egret | Egretta garzetta | Aquatic | Small aquatic predator | 28 | ||||
| 16 | 6 | Cattle Egret | Bubulcus ibis | Terrestrial | Terrestrial insectivore | 27 | ||||
| Eldorado Park | 2788 | −263 | Klip River | 4 | 5 | African Sacred Ibis | Threskiornis aethiopicus | Wetland | Scavenger | 62 |
| Potchefstroom | 2709 | −2678 | Mooi River | 7 | 5 | Reed Cormorant | Microcarbo africanus | Aquatic | Large aquatic predator | 21 |
| 9 | 5 | Glossy Ibis | Plegadis falcinellus | Wetland | Small aquatic predator | 34 | ||||
| 12 | 5 | Black-headed Heron | Ardea melanocephala | Terrestrial | Terrestrial insectivore | 60 | ||||
| 13 | 4 | Black-headed Heron | Ardea melanocephala | Terrestrial | Terrestrial insectivore | 60 | ||||
| 15 | 5 | Cattle Egret | Bubulcus ibis | Terrestrial | Terrestrial insectivore | 27 |
Chemical Analyses
All samples were analysed within 6 months of collection. Laboratory analysis was undertaken by Oëkometric GmbH—The Bayreuth Institute of Environmental Research, in Germany. All POPs analyses were executed with quality assurance and quality control protocols as per ISO/IEC 17025:2005 accreditation that covered, preparation, calibration, extraction, clean-up, measurement, quantification, quality control, concentration calculations, and reporting. Chemical analysis and compounds analysed are presented in Table 2. Laboratory blanks and internal reference material were routinely analysed for quality assurance and QA/QC procedures. Toxic equivalency quotients (TEQs) were calculated according to the WHO (2005), and all are reported as exclusive (van den Berg et al. 2006).
Table 2.
Summary of the chemical analysis and compounds analysed
| PCDD/F | 18 PCBs | PBDE classes | PFOS | 21 pesticides |
|---|---|---|---|---|
| PCDD/F | DL-PCB | TetraBDE | Perfluorooctanesulfonic acid (PFOS) | α-Hexachlorocyclohexane (α-HCH) |
| 2,3,7,8-TCDD | PCB 77 | PentaBDE | β-Hexachlorocyclohexane (β-HCH) | |
| 1,2,3,7,8-PeCDD | PCB 81 | HexaBDE | γ-Hexachlorocyclohexane (Lindane) | |
| 1,2,3,4,7,8-HxCDD | PCB 126 | HeptaBDE | Hexachlorobenzene (HCB) | |
| 1,2,3,6,7,8-HxCDD | PCB 169 | HexaBB | Heptachlor | |
| 1,2,3,7,8,9-HxCDD | PCB 105 | Aldrin | ||
| 1,2,3,4,6,7,8-HpCDD | PCB 114 | Dieldrin | ||
| OCDD | PCB 118 | Endrin | ||
| 2,3,7,8-TCDF | PCB 123 | Heptachloroepoxide | ||
| 1,2,3,7,8-PeCDF | PCB 156 | Chlordane (trans-) | ||
| 2,3,4,7,8-PeCDF | PCB 157 | Chlordane (cis-) | ||
| 1,2,3,4,7,8-HxCDF | PCB 167 | o,p'-DDE | ||
| 1,2,3,6,7,8-HxCDF | p,p-DDE | |||
| 1,2,3,7,8,9-HxCDF | o,p'-DDD | |||
| 2,3,4,6,7,8-HxCDF | p,p-DDD | |||
| 1,2,3,4,6,7,8-HpCDF | o,p'-DDT | |||
| 1,2,3,4,7,8,9-HpCDF | p,p-DDT | |||
| OCDF | Mirex | |||
| Pentachlorobenzene | ||||
| Chlordecone | ||||
| Toxaphene | ||||
| Regulation EC 1883/2006 and EPA 1613 B | Regulation EC 1883/2006, ASU L 00.00–12 and ASU L 00.00–38 | Proprietary method and EPA 1614 | Proprietary method | Regulation ASU L 00209,.00–12, ASU L 00.00–38 Proprietary method (based on S19 multimethod) |
| High resolution GC/MS | High resolution GC/MS | High resolution HRGC/HRMS. To minimise the degradation of BDE 209, a short column (15 cm) was used (Agilent DB-5 ms 15 m × 0.25 mm × 0.25 µm) instead of the 30 cm column that were used for the other congeners | Using LC/MS–MS A daily internal lab blank was used as the QA/QC. the lab blanks were routinely analysed (once a week) | High resolution GC/MS |
| LOQ TCDD/F–HxCDD/F = 0.00005 ng/g wet mass (wm), HpCDD/F = 0.00015 ng/g wm, OCDD/F = 0.0005 ng/g wm) | LOQ DL-PCB 81, 126, 169 = 0.0005 ng/g wm, DL-PCB 77, 105, 114, 123, 156, 157, 167,169 = 0.005 ng/g wm, DL-PCB 118 = 0.050 ng/g wm, NDL-PCB 28, 52, 101, 138, 180 = 0.1 ng/g wm) | LOQ 0.05–0.1 ng/g wm | LOQ = 1 ng/g wm | LOQ = 0.1–2 ng/g wm |
Statistical Analyses and Measuring Unit Conversions.
Descriptive and comparative statistics were performed using GraphPad Prism version 10.2.0. Concentration unit conversions were performed to compare published and current data. The data were received from the laboratory in wet mass (wm). The values reported in parts per million (ppm), parts per billion (ppb), milligrammes per kilogramme (mg / kg), and microgrammes per kilogramme (µg/kg) by other authors were converted to nanogrammeme per gramme (ng/g). The concentration values reported in lipid mass (lm) by other authors were converted to wet mass (wm) (Clatterbuck et al. 2018). We evaluated and compared wet mass (wm)-based data, given that embryo development affects lipid composition more than water content (Herzke et al. 2002; Romanoff 1932). The current data were converted to data based on lipid mass (lm) and are presented in Table S2. The determination of lipids was done gravimetrically.
The ΣPCB value is the total concentration of both DL-PCBs and NDL-PCBs. The PCB TEQ value consists of only DL-PCBs. The logarithmic transformation of the POP classes was regressed against the egg mass. Firstly, Prism compares whether slopes are parallel, calculating a two-tailed p-value. The null hypothesis is that the slopes are identical and therefore parallel. Second, Prism calculates if the Y-intercepts (elevations) for the regression lines are identical. Low p-values signify that the slopes and intercepts are significantly different.
Results
Summary results are given in Table 3 and presented in several ways in Figs.2, 3, 4, 5, 6, 7. The concentration quantified of individual congeners can be viewed in the supplementary material in Table S2. And the results converted to lipid mass (lm) are given in Table S3.
Table 3.
Summary of all quantified concentrations detected in wild bird eggs. All concentrations are expressed in ng/g wet mass (wm) except for the TEQ values which are expressed in ng/kg wm. Values based on lipid mass (lm) are provided in Table S3
| ng/g wm | ng/kg wm | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Site | Pool no | PFOS | ΣPBDE | β-HCH | HCB | Dieldrin | pp,p'-DDD | p,p'-DDT | p,p'-DDE | ΣDDT | ΣOCP | Dl- PCB* | NDL- PCB** | ΣPCB | ∑PCDD/F | WHO-TE 2005: Exclusive | |
| WHO PCB TEQ | WHO PCDD/F TEQ | |||||||||||||||||
| Grey Heron | Barbers Pan | 1 | 7 | 2 | 3 | 1 | 4 | 38 | 38 | 46 | 1 | 8 | 9 | 1 | 1 | 0.2 | ||
| Great White Egret | Bloemhof Dam | 2 | 350 | 0.2 | 1 | 2 | 8 | 12 | 400 | 420 | 420 | 1 | 5 | 6 | 0.2 | 0.7 | 0.1 | |
| Grey Heron | Bloemhof Dam | 3 | 720 | 0.4 | 1 | 1 | 54 | 54 | 56 | 1 | 9 | 10 | 0.2 | 2 | 0.1 | |||
| African Sacred Ibis | Eldorado Park | 4 | 69 | 20 | 2 | 2 | 9 | 10 | 130 | 140 | 160 | 3 | 33 | 35 | 7 | 2 | 0.4 | |
| African Darter | Barbers Pan | 5 | 850 | 0.3 | 2 | 1 | 2 | 3 | 86 | 86 | 91 | 1 | 6 | 7 | 0.3 | 1 | 0.1 | |
| African Darter | Bloemhof Dam | 6 | 2300 | 0.4 | 2 | 1 | 2 | 90 | 90 | 95 | 14 | 88 | 100 | 5 | 12 | 2 | ||
| Reed Cormorant | Potchefstroom | 7 | 200 | 6 | 1 | 1 | 2 | 150 | 150 | 150 | 9 | 45 | 54 | 1 | 5 | 0.2 | ||
| Reed Cormorant | Bloemhof Dam | 8 | 1100 | 0.2 | 1 | 1 | 180 | 180 | 180 | 4 | 18 | 22 | 1 | 2 | 0.3 | |||
| Glossy Ibis | Potchefstroom | 9 | 5 | 1 | 3 | 1 | 3 | 55 | 58 | 61 | 1 | 6 | 7 | 1 | 1 | 0.2 | ||
| African Sacred Ibis | Bloemhof Dam | 10 | 17 | 0.4 | 1 | 2 | 1 | 70 | 71 | 74 | 0.3 | 2 | 3 | 0,1 | 0.3 | 0.01 | ||
| Little Egret | Bloemhof Dam | 11 | 500 | 1 | 19 | 19 | 19 | 1 | 8 | 9 | 1 | 1 | 0.2 | |||||
| Black-headed Heron | Potchefstroom | 12 | 6 | 1 | 1 | 1 | 4 | 27 | 27 | 33 | 0.2 | 2 | 2 | 0.2 | 0.4 | 0.04 | ||
| Black-headed Heron | Potchefstroom | 13 | 17 | 3 | 1 | 1 | 6 | 24 | 24 | 32 | 4 | 43 | 47 | 6 | 7 | 2 | ||
| Black-headed Heron | Barbers Pan | 14 | 6 | 0.5 | 6 | 1 | 7 | 27 | 27 | 41 | 2 | 14 | 16 | 9 | 3 | 1 | ||
| Cattle Egret | Potchefstroom | 15 | 7 | 0.2 | 1 | 1 | 18 | 18 | 20 | 1 | 6 | 7 | 1 | 1 | 0.2 | |||
| Cattle Egret | Bloemhof Dam | 16 | 580 | 1 | 27 | 28 | 28 | 0.3 | 2 | 2 | 0.3 | 0.3 | 0.03 | |||||
*Dl- Dioxin like
** NDL-Non-dioxin like
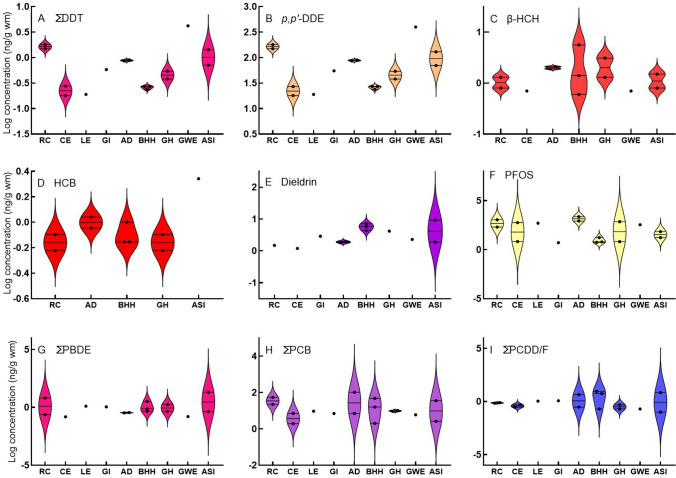
Fig. 2.
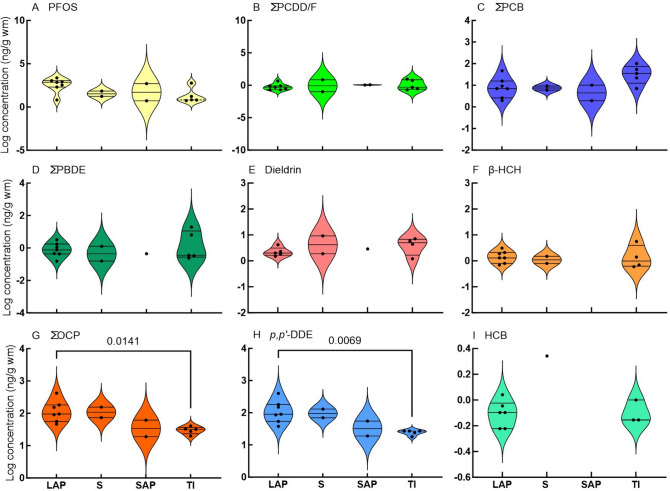
Violin plots (frequency distributions) of log-transformed concentrations of selected compounds quantified in bird eggs regardless of sampling location. Horizontal lines are medians and 25 and 75% quartiles. Species are arranged according to increasing reported mean egg mass. RC = Reed Cormorant, CE = Cattle Egret, LE = Little Egret, GI = Glossy Ibis, AD = African Darter, BHH = Black Headed Heron, GH = Grey Heron, GWE = Great White Egret, and ASI = African Sacred Ibis
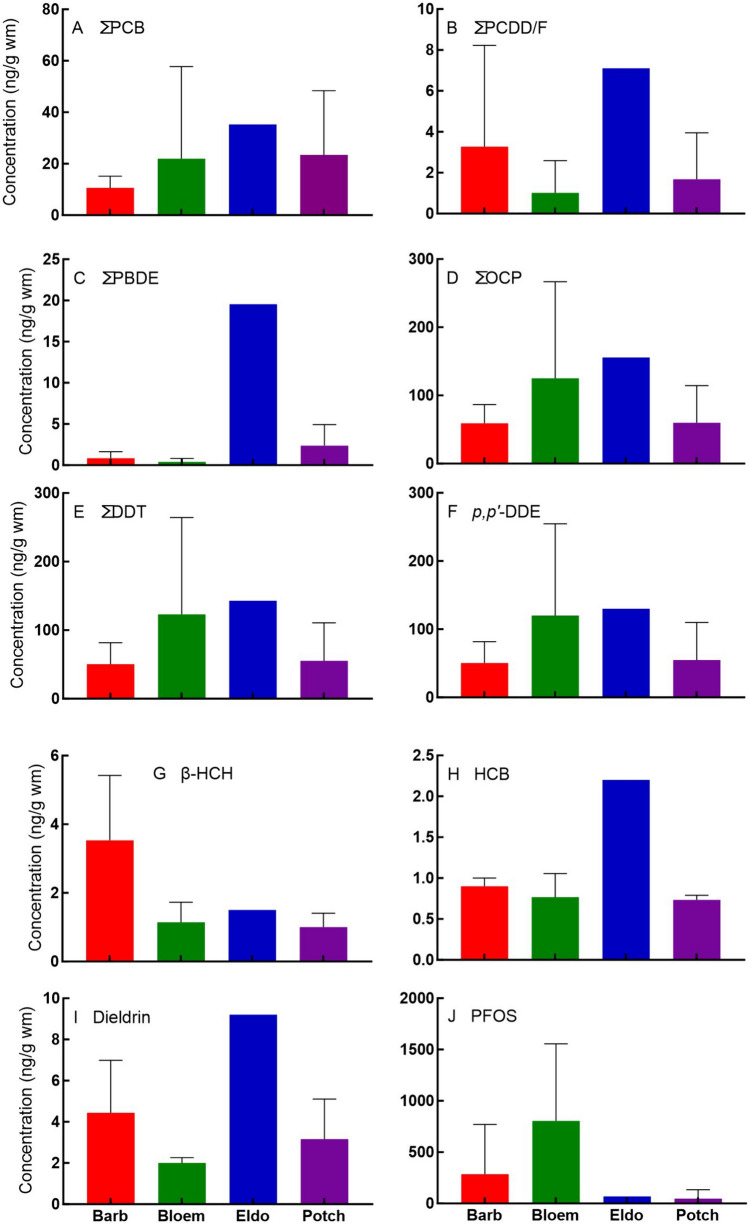
Fig. 3.
Mean concentrations and standard deviations of selected compounds quantified at each sampling location regardless of species. Barb = Barbers Pan, Bloem = Bloemhof Dam, Eldo = Eldorado Park, and Potch = Potchefstroom
Fig. 4.
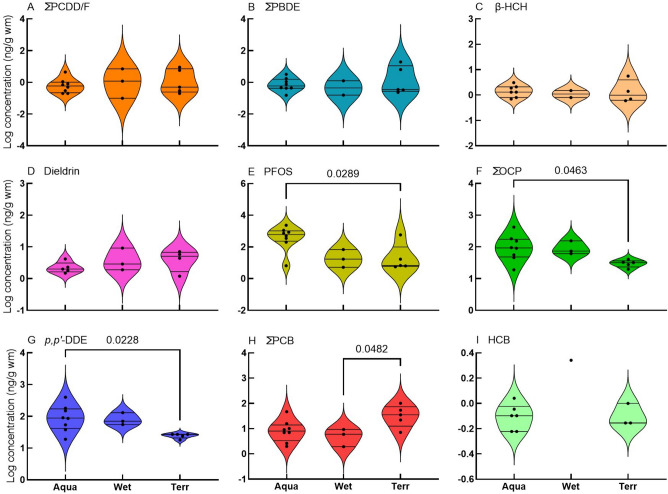
Violin plots (frequency distributions) of log-transformed concentrations of selected compounds quantified in bird eggs according to habitat guilds. Horizontal lines are medians and 25 and 75% quartiles. Habitat guilds are expressed as aquatic, wetland, and terrestrial. Aqua = aquatic, Wet = wetland, and Terr = terrestrial. ANOVA p-values of guilds that were found to be statistically significant different are indicated with brackets. Two-way unpaired t-test was performed for HCB
Fig. 5.
Violin plots (frequency distributions) of concentrations of selected compounds quantified in bird eggs according to feeding guilds. Horizontal lines are medians and 25 and 75% quartiles. Feeding guilds are expressed as LAP = large aquatic predators, S = scavengers, SAP = small aquatic predators, and TI = terrestrial insectivore. ANOVA p-values of guilds that were found to be statistically significant different are indicated with brackets. Two-way unpaired t-test was performed for HCB
Fig. 6.
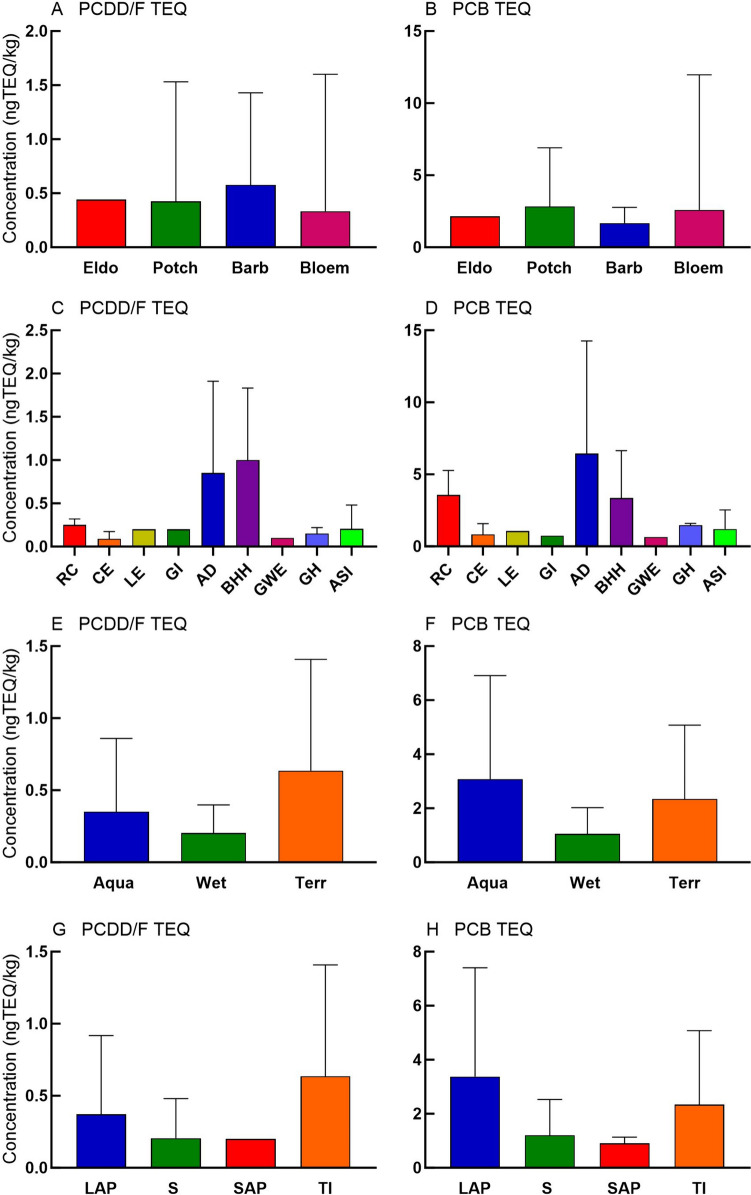
TEQ values in bird eggs. Data are expressed as mean with range. A) PCDD/F TEQ values according to sampling location. B) PCB TEQ values according to sampling location. Sampling locations are expressed as Barb = Barbers Pan, Bloem = Bloemhof Dam, Eldo = Eldorado Park and Potch = Potchefstroom. C) PCDD/F TEQ values according to species. D) PCB TEQ values according to species. Species are arranged according to increasing reported average egg mass. Species are expressed as RC = Reed Cormorant, CE = Cattle Egret, LE = Little Egret, GI = Glossy Ibis, AD = African Darter, BHH = Black Headed Heron, GH = Grey Heron, GWE = Great White Egret, and ASI = African Sacred Ibis. E) PCDD/F TEQ values according to habitat guilds. F) PCB TEQ according to habitat guilds. Habitat guilds as expressed as Aqua = aquatic, Wet = wetland, and Terr = terrestrial. G) PCDD/F TEQ values according to feeding guilds. H) PCB TEQ according to feeding guilds. Feeding guilds are expressed as LAP = large aquatic predators, S = scavengers, SAP = small aquatic predators, and TI = terrestrial insectivore
Fig. 7.
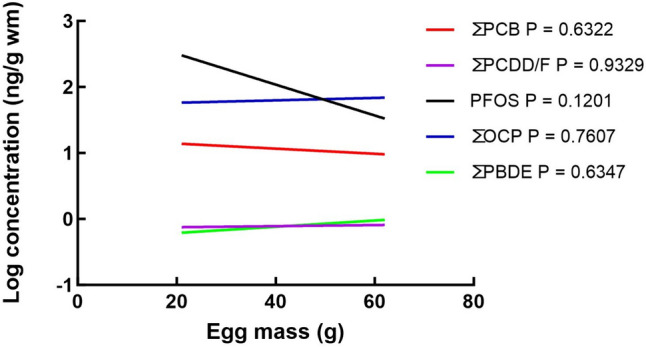
Simple linear regression. Concentrations of compound classes, regardless of species or sample location, regressed against egg mass
Bird Egg Concentrations
Organochlorine compounds such as α-HCH, lindane, heptachlor, aldrin, endrin, heptachloroepoxide, chlordane (trans- and cis -), mirex, pentachlorobenzene, chlordecone, toxaphene, o,p'-DDE, o,p'-DDD, and o,p'-DDT were detected in all eggs, but at concentrations below the LOQ.
The highest ΣOCP concentration was quantified in eggs of Great White Egret eggs (423 ng/g wm) from Bloemhof Dam, primarily as a result of the high p,p’-DDE (400 ng/g wm) (Fig. 2A and B; Table 3). This egg pool (Table 1) had double the ΣOCP concentration than Reed Cormorant eggs from Bloemhof Dam (180 ng/g wm), Potchefstroom (150 ng/g wm), and an order of magnitude greater than the African Sacred Ibis egg pool from Eldorado Park (19 ng/g wm) (Table 3). Most of the ΣOCP concentrations were composed of p,p’-DDE. However, other OCPs were also quantified in some eggs (Table 3). The highest β-HCH concentration was in Black-headed Heron eggs (6 ng/g wm) from Barbers Pan (Fig. 2C; Table 3). The highest HCB (2 ng/g wm) (Fig. 2D) and dieldrin (9 ng/g wm) (Fig. 2E) concentrations were in African Sacred Ibis eggs from Eldorado Park.
The highest PFOS concentrations were quantified in African Darter eggs (2300 ng/g wm) and Reed Cormorant eggs (1100 ng/g wm) from Bloemhof Dam (Fig. 2E). PFOS was also the dominant compound in most species, except Great White Egret, African Sacred Ibis, Black-headed Heron, and Glossy Ibis where ΣOCPs dominated (Table 3). African Sacred Ibis eggs from Eldorado Park had the highest ΣPBDE concentrations (19 ng/g wm), followed by Reed Cormorant eggs from Potchefstroom (Fig. 2G; Table 3). The highest ΣPCB concentration in any pool was in African Darter eggs (100 ng/g wm) from Bloemhof Dam followed by Reed Cormorant eggs (54 ng/g wm) from Potchefstroom (Figure 2H; Table 3). ΣPCDD/F concentrations were highest in Black-headed Heron eggs (9 ng/g wm) from Barbers Pan, followed by African Sacred Ibis eggs from Eldorado Park (7 ng/g wm) (Fig. 2I).
Irrespective of species, the highest mean ΣPCB, ΣPCDD/F, ΣPBDE, ΣOCP, and ΣDDT were found in eggs from Eldorado Park (Fig. 3A–E). In addition, all OCPs were higher at Eldorado Park, except β-HCH which was higher at Barbers Pan (Fig. 3F–I). Bloemhof Dam had the highest mean PFOS concentration, followed by Barbers Pan (Fig. 3J).
Guilds
The species were grouped according to habitat guilds: aquatic, terrestrial, and wetland (Table 1; Fig. 4). There were no significant differences between habitat guilds (one-way ANOVA, Tukey’s multiple comparisons) for ΣPCDD/F, ΣPBDE, β-HCH, and dieldrin (Fig. 4A–D). There were statistically significant differences between aquatic and terrestrial habitat guilds for PFOS, ΣOCP, and p,p’-DDE (Fig. 4E–G), and between terrestrial and wetland habitat guilds for ΣPCB (Fig. 4I). Since only one data point was available for HCB in the wetland guild, we performed a two-way, unpaired t-test between terrestrial and aquatic eggs which was not significantly different (Fig. 4H).
We grouped all species according to their feeding guilds: large aquatic predators (LAP), small aquatic predators (SAP), scavengers (S), and terrestrial insectivores (TI) (Table 1). There were no significant differences between feeding guilds (one-way ANOVA, Tukey’s multiple comparison) for PFOS, ΣPCDD/F, ΣPCB, ΣPBDE, dieldrin, and β-HCH (Fig. 5A-F). A statistically significant difference was found between large aquatic predators and terrestrial insectivores for ΣOCP and p,p’-DDE (Fig. 5G and H). We performed two-way, unpaired, t-tests for HCB and found no statistically significant differences (Fig. 5I).
TEQ
Mean PCDD/F TEQ values of bird eggs were highest at Barbers Pan followed by Potchefstroom (Fig. 6A), although the highest PCDD/F TEQ value was from eggs collected at Bloemhof Dam (1.6 ngTEQ/kg wm) (Table 3). PCB TEQ values in bird eggs were highest at Bloemhof Dam (12 ngTEQ/kg wm) followed by Potchefstroom (Fig. 6B). PCDD/F and PCB TEQ values were highest in African Darter (12 ngTEQ/kg wm) and Black-headed Heron (7 ngTEQ/kg wm) eggs, followed by Reed Cormorant eggs (5 ngTEQ/kg wm) (Fig. 6C and D). PCDD/F TEQ values were highest in terrestrial species (Fig. 6E) while PCB TEQ values dominated in aquatic habitat guild eggs (Fig. 6F). PCDD/F TEQ values were highest in terrestrial insectivores (Fig. 6G) while PCB TEQ values were highest in large aquatic predators (Fig. 6H). The PCB TEQs were higher in all species and at all sites compared to the PCDD/F TEQ values (Table 3).
Influence of Egg Mass
We used linear regression to investigate the association of compound classes with egg mass (Fig. 7). None of the slopes were significantly different from the X-axis. We also tested whether slopes and intercepts (vertical distances between the Y-intercepts of each slope – Y-intercepts indicates where the regression slopes meet the Y-axes) were significantly different from each other (Prism uses a method similar to analysis of covariance (ANCOVA)). The slopes themselves were not significantly different from zero or each other (p = 0.2773). There was, however, a significant difference between Y-intercepts (p < 0.0001, ANOVA; p < 0.0001, Brown-Forsythe test; p < 0.0001, Bartlett’s test).
Discussion
Bird Egg Concentrations
Feeding Guilds
With eggs of nine species of birds collected from four locations and measured for 22 POPs, the current study is a multi-species analyses investigating the pollution load of both aquatic and terrestrial birds. POP concentrations differed greatly between species, sites, habitat groups, and feeding groups (Table 3 and Figs. 2, 3, 4, 5). This was as expected since the sites and species were collected over a large area where breeding colonies were available. The locations of the active breeding colonies at the time of sampling were found via aerial reconnaissance for this specific purpose. However, there are apparent patterns based on guilds and localities close to sources, with some exceptions.
Eggs from species that occupy high-trophic levels had higher concentrations of PFOS and ΣOCP concentrations, while species that feed on insects had lower concentrations (Table 3 and 4 and Fig. 5). This was reflected in habitat guilds where aquatic species had higher PFOS and ΣOCP concentrations (Fig. 4). This pattern was also noted by Eriksson et al. (2016) who found higher concentrations of PFAS in eggs from aquatic species compared to terrestrial species. The ΣOCP concentrations were dominated by p,p’-DDE, which is in agreement with others (Bouwman et al. 2008; Venugopal et al. 2020). Although we did not observe any pattern regarding PBDE concentrations in guilds, She et al. (2008) did find higher ΣPBDE concentrations in eggs of piscivorous birds compared with omnivorous species in the USA.
Table 4.
Mean concentrations of compound groups (ng/g wm) in wild bird eggs reported by various authors. The country, location, and year the samples were collected and are shown. All reported concentrations were converted to ng/g wm
| Species | Country | Year sampled | Location | ΣPCB? | ΣPCB# | ∑PCDD/F | PFOS | ∑BDE? | PBDE | ∑OCP | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grey Heron (Ardea cinerea) | |||||||||||
| RSA | 2009 | Barbers Pan | ΣPCB6 | 9 | 0.5 | 7 | ΣBDE5 | 2 | 46 | This study | |
| RSA | 2009 | Bloemhof Dam | ΣPCB6 | 10 | 0.2 | 725 | ΣBDE5 | 0.5 | 56 | This study | |
| Turkey* | 2009 | SÖKE | ΣPCB7 | 70 | 1100 | Kocagöz et al. 2014 | |||||
| RSA* | 2009 | Nandoni Dam | ΣPCB20 | 38 | 14,276 | Bouwman et al. 2013 | |||||
| Creece | 2004 | Lake Kerkini | ΣPCB7 | 64 | Antoniadou et al. 2007 | ||||||
| Spain | 2010–17 | Castrejón reservoir | ΣBDE8 | 119 | Eljarrat et al. 2019 | ||||||
| Romania* | 1997 | Danube Delta | ΣPCB6 | 620 | 2321 | Aurigi et al. 2000 | |||||
| Greece* | 2004 | Lake Kerkini | 255 | Goutner et al. 2012 | |||||||
| France | 1991 | Reserve Naturelle de Grandlieu | ΣPCB1 | 1280 | 745 | de Cruz et al. 1997 | |||||
| Blue Heron (Ardea herodias) | |||||||||||
| Canada | 1979 | Quebec | ΣPCB? | 7880 | 5266 | Laporte 1982 | |||||
| Canada2,3 | 1995 | Columbia River, Bachelor island | ΣPCB3 | 615 | 0.04 | 0.4 | Thomas and Anthony 1999 | ||||
| Canada (2 and 3) | 1994 | Columbia River, Ross island | ΣPCB3 | 3454 | 2 | Thomas and Anthony 1999 | |||||
| USA | 1993 | Indiana Dunes | 245 | ΣBDE6 | 347 | Custer et al. 2009 | |||||
| Canada (3) | 2002 | Fraser River Estuary | ΣBDE6 | 457 | Miller et al. 2015 | ||||||
| Canada (2) | 1989 | University of Brithish Columbia | 0.4 | Elliott et al. 2001 | |||||||
| Canada (2) | 1991 | Victoria | 0.1 | Elliott et al. 2001 | |||||||
| Canada (2) | 1987 | Crofton | 1 | Elliott et al. 2001 | |||||||
| USA (2) | 1993 | Mississippi river / Pig's Eye | 940 | ΣBDE7 | 142 | Custer et al. 2010 | |||||
| Black-headed Heron (Ardea melanocephala) | |||||||||||
| RSA | 2009 | Potchefstroom | ΣPCB6 | 2 | 0.2 | 6 | ΣBDE5 | 1 | 33 | This study | |
| RSA | 2009 | Potchefstroom | ΣPCB7 | 47 | 6 | 17 | ΣBDE5 | 3 | 32 | This study | |
| RSA | 2009 | Barbers Pan | ΣPCB8 | 16 | 9 | 6 | ΣBDE5 | 0.5 | 41 | This study | |
| Night Heron (Genera: Nycticora, Nyctanass, and Gorsachius) | |||||||||||
| Romania* | 1997 | Danube Delta | ΣPCB6 | 127 | 1689 | Aurigi et al. 2000 | |||||
| Israel | 1975 | Coastal plain | ΣPCB? | 770 | 1620 | Perry et al. 1990 | |||||
| USA | 1996 | Alexander island | ΣPCB18 | 2100 | 0.2 | 460 | Frank et al. 2001 | ||||
| Italy | 1994 | Riserva Naturale Garzaia di Villarasca | ΣPCB? | 40 | 200 | Fasola et al. 1998 | |||||
| Creece | 2004 | Lake Kerkini | ΣPCB7 | 26 | Antoniadou et al. 2007 | ||||||
| Hong Kong | 2000 | A Chau | 0.1 | Wang et al. 2012 | |||||||
| Hong Kong | 2000 | Mai Po Village | ΣPCB? | 230 | 704 | Connell et al. 2003 | |||||
| China | 2004 | Xiamen | 123 | Wang et al. 2008 | |||||||
| Hong Kong | 2006 | A Chau | 115 | Wang et al. 2008 | |||||||
| Spain | 2010–17 | Castrejón reservoir | ΣBDE8 | 15 | Eljarrat et al. 2019 | ||||||
| Greece* | 2004 | Lake Kerkini | 172 | Goutner et al. 2012 | |||||||
| Hong Kong* | 2006 | A Chau | ΣPCB? | 55 | 186 | Wang et al. 2011 | |||||
| Purple Heron (Ardea purpurea) | |||||||||||
| Spain | 2010–17 | Castrejón reservoir | ΣBDE8 | 43 | Eljarrat et al. 2019 | ||||||
| Reed Cormorant (Microcarbo africanus) | |||||||||||
| RSA | 2009 | Potchefstroom | ΣPCB6 | 54 | 1 | 201 | ΣBDE5 | 6 | 154 | This study | |
| RSA | 2009 | Bloemhof Dam | ΣPCB6 | 22 | 1 | 1120 | ΣBDE5 | 0.2 | 181 | This study | |
| RSA | 2004/5 | Vaal River | ΣPCB34 | 110 | 308 | Bouwman et al. 2008 | |||||
| RSA* | 2004/5 | Parys | ΣPCB34 | 165 | ΣBDE8 | 1 | 449 | Polder et al. 2008 | |||
| Great Cormorant (Phalacrocorax carbo) | |||||||||||
| Netherlands | 1988/9 | Rhine and Meuse rivers | ΣPCB6 | 1583 | 5318 | Dirksen et al. 1995 | |||||
| Sweden (1) | 2007–9 | Lake Vänern | 552 | Nordén et al. 2013 | |||||||
| Germany | 2009 | Baltic sea / Heuwiese | 90 | Rüsdel et al., 2011 | |||||||
| Germany | 2009 | Elbe estuary/ Haseldorf | 540 | Rüsdel et al., 2011 | |||||||
| Greece* | 2004 | Lake Kerkini | 355 | Goutner et al. 2012 | |||||||
| Creece | 2004 | Lake Kerkini | ΣPCB7 | 60 | Antoniadou et al. 2007 | ||||||
| Neotropic Cormorant (Nannopterum brasilianum) | |||||||||||
| USA | 1996 | Alexander island | ΣPCB18 | 5720 | 0.1 | 1364 | Frank et al. 2001 | ||||
| USA | 1996 | San Bernard Wildlife refuge | ΣPCB18 | 404 | 493 | Frank et al. 2001 | |||||
| USA | 1996 | Smith Point | ΣPCB18 | 1640 | 0.01 | 213 | Frank et al. 2001 | ||||
| USA | 1996 | Vingt-et-un | ΣPCB18 | 3140 | 0.01 | 423 | Frank et al. 2001 | ||||
| White-breasted Cormorant (Phalacrocorax lucidus) | |||||||||||
| RSA | 2013 | KwaZulu-Natal | 600 | Bouwman et al. 2019 | |||||||
| Double-crested Cormorant (Nannopterum auritum) | |||||||||||
| Canada (5) | 1973 | Mandarte island | 0.1 | Harris et al. 2003 | |||||||
| Canada (5) | 1998 | Mandarte island | 0.04 | Harris et al. 2003 | |||||||
| Canada (5) | 1987 | Crofton | 1 | Harris et al. 2003 | |||||||
| Canada (5) | 1997 | Crofton | 0.1 | Harris et al. 2003 | |||||||
| Canada (3) | 1994 | Mandarte island | ΣBDE9 | 385 | Miller et al. 2015 | ||||||
| African Darter (Anhinga rufa) | |||||||||||
| RSA | 2009 | Barbers Pan | ΣPCB6 | 7 | 0.3 | 846 | ΣBDE5 | 0.3 | 91 | This study | |
| RSA | 2009 | Bloemhof Dam | ΣPCB6 | 102 | 5 | 2330 | ΣBDE5 | 0.4 | 95 | This study | |
| RSA | 2004/5 | Vaal River | ΣPCB34 | 300 | 370 | Bouwman et al. 2008 | |||||
| RSA* | 2004/5 | Parys | ΣPCB34 | 314 | ΣBDE8 | 1 | 398 | Polder et al. 2008 | |||
| RSA | 2008/9 | Gauteng/ Free state | ΣPCB34 | 310 | ΣBFR11 | 8 | 590 | Bouwman et al. 2021 | |||
| RSA* | 2008/9 | Kempton Park/ Parys | ΣBDE9 | 11 | Quinn et al. 2020 | ||||||
| Great White Egret (Ardea alba) | |||||||||||
| RSA | 2009 | Bloemhof Dam | ΣPCB6 | 6 | 0.2 | 352 | ΣBDE5 | 0.2 | 423 | This study | |
| Romania* | 1997 | Danube Delta | ΣPCB6 | 740 | 4658 | Aurigi et al. 2000 | |||||
| Hong Kong* | 2006 | A Chau | ΣPCB? | 126 | 1059 | Wang et al. 2011 | |||||
| USA | 1996 | Alexander island | ΣPCB18 | 1510 | 0.1 | 379 | Frank et al. 2001 | ||||
| Little Egret (Egretta garzetta) | |||||||||||
| RSA | 2009 | Bloemhof Dam | ΣPCB6 | 9 | 1 | 505 | ΣBDE5 | 1 | 19 | This study | |
| Spain | 2006 | Aiguabarreig | ΣPCB7 | 230 | 277 | Huertas et al. 2016 | |||||
| France | 1996 | Rhône delta | ΣPCB12 | 3305 | 123 | Berny et al. 2002 | |||||
| Creece | 2004 | Lake Kerkini | ΣPCB7 | 18 | Antoniadou et al. 2007 | ||||||
| Hong Kong | 2000 | Mai Po Village | ΣPCB? | 960 | 2440 | Connell et al. 2003 | |||||
| Hong Kong* | 2000 | Mai Po Village | ΣPCB? | 288 | 417 | Wang et al. 2011 | |||||
| Italy | 1993/4 | Riserva Naturale Garzaia di Villarasca | ΣPCB? | 77 | 249 | Fasola et al. 1998 | |||||
| China | 2004 | Xiamen | 70 | Wang et al. 2008 | |||||||
| RSA | 2013 | KZN | 500 | Bouwman et al. 2019 | |||||||
| Romania* (4) | 1997 | Danube Delta | ΣPCB6 | 546 | 12,448 | Aurigi et al. 2000 | |||||
| Israel | 1975 | Coastal plain | ΣPCB? | 540 | 1610 | Perry et al. 1990 | |||||
| Greece* | 2004 | Lake Kerkini | 103 | Goutner et al. 2012 | |||||||
| Cattle Egret (Bubulcus ibis) | |||||||||||
| RSA | 2009 | Potchefstroom | ΣPCB6 | 7 | 0.5 | 7 | ΣBDE5 | 0.2 | 20 | This study | |
| RSA | 2009 | Bloemhof Dam | ΣPCB6 | 2 | 0.3 | 579 | ΣBDE5 | 0 | 28 | This study | |
| RSA* | 2009 | Elim | ΣPCB20 | 9 | 26 | Bouwman et al. 2013 | |||||
| RSA* | 2009 | Tshakhuma Dam | ΣPCB20 | 5 | 104 | Bouwman et al. 2013 | |||||
| RSA* | 2009 | Xikundu dam | ΣPCB20 | 6 | 307 | Bouwman et al. 2013 | |||||
| RSA | 2004/5 | Baberspan | ΣPCB34 | 4 | 28 | Bouwman et al. 2008 | |||||
| RSA | 2004/5 | Vaal River | ΣPCB34 | 8 | 28 | Bouwman et al. 2008 | |||||
| RSA* | 2004/5 | Barberspan | ΣPCB34 | 3 | ΣBDE8 | 0.1 | 23 | Polder et al. 2008 | |||
| RSA* | 2004/5 | Parys | ΣPCB34 | 8 | ΣBDE8 | 0.3 | 30 | Polder et al. 2008 | |||
| RSA | 2008/9 | Gauteng/ Free state | ΣPCB34 | 16 | ΣBFR11 | 4 | 21 | Bouwman et al. 2021 | |||
| Spain | 2006 | Aiguabarreig | ΣPCB7 | 51 | 49 | Huertas et al. 2016 | |||||
| China* | 2000 | Tai Lake | 56 | Dong et al. 2004 | |||||||
| Israel | 1975 | Coastal plain | 620 | Perry et al. 1990 | |||||||
| Hong Kong | 2000 | Mai Po Village | 0.04 | Wang et al. 2012 | |||||||
| RSA* | 2008/9 | Soweto/Parys/Sasolburg | ΣBDE9 | 5 | Quinn et al. 2020 | ||||||
| African Sacred Ibis (Threskiornis aethiopicus) | |||||||||||
| RSA | 2009 | Eldorado Park | ΣPCB6 | 35 | 7 | 69 | ΣBDE5 | 20 | 156 | This study | |
| RSA | 2009 | Bloemhof Dam | ΣPCB6 | 3 | 0.1 | 17 | ΣBDE5 | 0.4 | 74 | This study | |
| RSA | 2004/5 | Vaal River | ΣPCB34 | 59 | 94 | Bouwman et al. 2008 | |||||
| RSA* | 2004/5 | Parys | ΣPCB34 | 65 | ΣBDE8 | 14 | 91 | Polder et al. 2008 | |||
| RSA | 2008/9 | Gauteng/ Free state | ΣPCB34 | 59 | ΣBFR11 | 53 | 56 | Bouwman et al. 2021 | |||
| RSA* | 2008/9 | Soweto | ΣBDE9 | 54 | Quinn et al. 2020 | ||||||
| Glossy Ibis (Plegadis falcinellus) | |||||||||||
| RSA | 2009 | Potchefstroom | ΣPCB6 | 7 | 1 | 5 | ΣBDE5 | 1 | 61 | This study | |
| Romania* | 1997 | Danube Delta | ΣPCB6 | 154 | 939 | Aurigi et al. 2000 | |||||
All values reported from studies other than the current study were reported in concentration units other than ng/kg and had to be converted. Values had to be converted from dry mass or lipid mass to wet mass. (1) Concentration was expressed as the median and not the mean. (2) Data from more than one location or site were reported, but only the location or site with the highest concentration was used in this Table (3) Data from more than one yearly sample run were reported, but only the year with the highest concentration was used in this Table (4) Concentration is of the egg yolk only. (5) Data from more than 1 year were reported, only selected data were used. # The sum concentration of DL-PCBs and NDL-PCBs as reported by authors
The ΣPCDD/F and ΣPCB concentrations suggest higher availability in terrestrial environments, which is contrary to the patterns found by Bouwman et al. (2021). Higher ΣPCB and ΣPCDD/F concentrations were found in soil rather than sediment (Quinn et al. 2009) from the same industrialised region sampled in this study. PCBs and PCDD/F tend to adhere to organic particles, and concentrations may be greater in terrestrial environments as a result (Quinn et al. 2009). These patterns were not seen in other compound classes, perhaps due to the low concentrations in the environment and small sample sizes. Another possible explanation for the lack of patterns observed may be due to differences in foraging behaviour of species (Harris et al. 2003); some species spend prolonged time near the nesting grounds while others roam over larger areas. In addition, differences in prior individual life histories among colony members can lead to differences in POP concentrations. It would have been insightful to compare the POP concentrations to those found in eggs of herbivorous, granivorous, and omnivorous species from the same sites (Bouwman et al. 2021).
We found few other studies with which to compare our findings. Lopez-Antia et al. (2017) reported no significant differences in PFOS concentrations between three species investigated. However, the PFOS concentration was greater in the more aquatic Mediterranean Gull (Larus melanocephalus). This pattern was also observed by Bouwman et al. (2021), who reported higher POP concentrations in species that inhabit aquatic habitats and species that are aquatic predators. Another multi-species analysis showed that eggs from omnivore species bioaccumulate a higher ΣOCP concentration than species that feed on only one specific food source (Venugopal et al. 2020).
Locations
The eggs of all species collected at Bloemhof Dam, except African Sacred Ibis, had the highest concentrations of PFOS in this survey (Table 3; Fig. 3). This suggests high concentrations of environmentally available PFOS in this region. The African Sacred Ibis eggs from Eldorado Park, in contrast with the other POPs classes (Fig. 3), had higher PFOS concentrations than those of the same species from Bloemhof Dam. Birds from industrialised areas are likely to be exposed to higher POP concentrations than rural birds (Elliott et al. 2015) which may explain the difference in PFOS concentrations between the Eldorado Park and Bloemhof Dam for the African Sacred Ibis (a scavenger, Table S1). Mean concentrations for all other compound classes, except PFOS and β-HCH, were highest at Eldorado Park, located in the highly industrialised Gauteng. Unfortunately, eggs from other species, apart from African Sacred Ibis, were not available in Eldorado Park at the time of sampling, complicating interpretation. A more detailed discussion on sources follows in Sect. "Hotspot identification".
Egg Mass
It could be argued that larger birds with larger eggs would eat larger prey from higher trophic levels. This would reflect in larger concentrations of POPs in their eggs. However, Bouwman et al. (2021) found no such effect, even when including eggs from a granivore trophic level such as sparrows (small eggs at ca. 2 g) and high-trophic level African Darters and herons with large eggs (ca. 60 g). For POPs classes such as ΣDDTs, ΣPCBs, and ΣBDEs, there were no associations (linear regressions) of any POP class concentrations with egg mass (Fig. 7), despite orders of magnitude differences in compound class concentrations as signified by the y-intercepts (Fig. 7) and Table 3. Although the eggs of the current study were from birds from a generally high-trophic level, we also found no association of POPs class concentrations such as DDTs and PCBs with egg mass, including for the first time PFOS and ΣPCDD/Fs (Fig. 7). This phenomenon remains difficult to explain.
At higher concentrations of DDT and chlordane’s, eggs of Glaucous gulls (Larus hyperboreus) were smaller (Verboven et al. 2009). Verboven et al. (2009) include endocrine disruption, direct toxic effects, poor body condition, and food availability as contributing causes causing smaller eggs. DDT also causes thinner eggshells (Findholt 1984; Peakall 1993), suggesting that eggs with thinner shells weigh less per volume of egg. However, reverse causality should also be considered. During the formation of eggs, those that eventually become lighter may have received proportionally more POPs deposited before the shell is formed. However, arguing this phenomenon across multiple species ranging in egg masses between 21 and 62 g would be difficult. Having observed this phenomenon twice (here, and in Bouwman et al. 2021) with POPs analyses done by two different laboratories invites further investigation.
Comparisons With International Data
Many studies have reported POP concentrations in bird eggs. For the current study, we selected articles that used the same species or similar species for comparison (Table 4). The majority of published literature on POP concentrations in eggs primarily focused on PCBs and OCPs, especially DDT and its metabolites. ΣOCP concentrations in all species were generally lower compared to international studies. Little Egret eggs had ΣOCP concentrations two orders of magnitude lower than eggs from Spain (Huertas et al. 2016), and up to three orders of magnitude lower than eggs from France (Berny et al. 2002) and Romania (Aurigi et al. 2000).
∑PBDE concentrations in eggs of the present study were low compared with international data (Table 4). Concentrations quantified in Grey Heron eggs from Barbers Pan were two orders of magnitude lower than ΣPBDE concentrations in eggs from Spain, Canada, and the USA (Table 4; Custer et al. 2009; Eljarret et al. 2019; Miller et al. 2015). PFOS concentrations in eggs from the current study were generally lower, or of the same order of magnitude, than reported from other regions, except for eggs from Bloemhof Dam (Table 4). Night Heron (Nycticorax nycticorax) eggs from China had lower PFOS concentrations than Grey Heron eggs from Bloemhof Dam, but higher concentrations than eggs from Barbers Pan. Great Cormorant eggs from Sweden and Germany (Nordén et al. 2013; Rüdel et al. 2011) had lower PFOS concentrations than Reed Cormorants eggs from Bloemhof Dam, but higher concentration than those quantified in eggs from Potchefstroom. Only eggs of Blue Herons (Ardea herodias) collected in 1993 in the USA near a PFAS source (Custer et al. 2010) had similar PFOS concentrations than eggs from Bloemhof Dam. PFOS concentrations at Bloemhof Dam were therefore extraordinary high considering the absence of any local source.
ΣPCB concentrations were three orders of magnitude lower in eggs from the OSRB compared with internationally reported data (Table 4), especially when comparing similar species and guilds. Grey Heron eggs from Bloemhof Dam had ΣPCB concentrations two orders of magnitude lower than concentrations quantified in France (de Cruz et al. 1997), and one order of magnitude lower than eggs from Romania (Aurigi et al. 2000). A broad observation suggests a worldwide decline in PCB concentrations. Using the Grey Heron as example, the PCB concentrations from 1970s to late 1990s were up to two orders of magnitude higher compared to post 2000 studies (Table 4). This decline was also observed in double-crested Cormorants eggs in Canada, where there was an order of magnitude decline in PCDD/F concentrations from 1987 to 1997. Long-term monitoring of POPs in eggs can aid in assessing patterns and distribution profiles (Harris et al. 2003). We could not find a study that reported ΣPCB concentrations of similar species in African studies. However, free-range chicken eggs from Tanzania had even lower ΣPCB concentrations than in the current study (Polder et al. 2016).
ΣPCDD/F concentrations reported in eggs from other regions were generally lower or of the same order of magnitude than concentrations quantified in the current study (Table 4). In addition, a number of eggs had one order of magnitude higher ΣPCDD/F concentrations (Black-headed Heron: 9 ng/g wm) than eggs from other studies (Tables 3 and 4). Eggs of double-crested Cormorants and Blue Herons from Canada (Elliott et al. 2001; Harris et al. 2003) measured the highest PCDD/F concentrations (1 ng/g wm) outside South Africa. We did not anticipate that bird eggs from South Africa would have the highest measured PCDD/F concentrations. Furthermore, all PCDD/F concentrations reported from international studies pre-date (1973–2000) the current data (2009). More recently reported PCDD/F concentrations in yellow-legged gull eggs (Larus michahellis) from in Spain (0.01 ng/g wm; Morales et al. 2016) and chicken eggs from Canada were also lower (Rawn et al. 2012). The current study is the first and only to report PCDD/F concentrations in wild bird eggs from South Africa, and to the best of our knowledge, also in Africa. TEQ values will be discussed in Sect. "Possible adverse consequences".
Hotspot Identification
The ΣPCB and ΣOCP concentrations in all species were lower than those previously reported from nearby locations except for Cattle Egret eggs which were of the same order of magnitude (Bouwman et al. 2008 and 2021; Polder et al. 2008). However, African Sacred Ibis from Eldorado Park had lower ΣPCB, but higher ΣOCP concentration than those reported from Gauteng and Northern Free State (Bouwman et al. 2021) (Table 4). In addition, the African Darter concentrations from those studies were an order of magnitude higher than the African Sacred Ibis concentrations. The Gauteng concentrations reported, had among others, eggs from a colony near Eldorado Park. This may at first suggest a decrease in ΣPCB and increase in ΣOCP concentrations. However, eggs from some localities were pooled confounding interpretation. The ΣOCP concentrations were four orders of magnitude higher in Grey Heron eggs, and one order of magnitude higher in Cattle Egret eggs from areas of the country where DDT is still used (Bouwman et al. 2013) compared with the current study’s locations where DDT has been banned since 1976 (Bouwman 2004).
∑PBDE concentrations in the current study were of the same order of magnitude or lower than those reported by other authors (Table 4). African Sacred Ibis eggs collected in Eldorado Park had slightly lower ΣPBDE concentrations than those reported from nearby Soweto (Quinn et al. 2020) and Johannesburg (Bouwman et al. 2021). African Darter eggs from Gauteng (Bouwman et al. 2021; Quinn et al. 2020) had an order of magnitude higher ΣPBDE concentrations than those reported from Bloemhof Dam and Barbers Pan of the current study.
Elevated PFOS concentrations were quantified at high concentrations in bird eggs, especially at Bloemhof Dam (Table 3). To our knowledge, there is no production of PFAS in South Africa, much less in the vicinity of Bloemhof Dam, which has no industries close by. This location appears to be a hotspot for PFOS, since PFOS was found to be the dominant PFAS quantified in adult Odonata from there (median: 16 ng/g wm) (Lesch et al. 2017). Bloemhof Dam is a large impoundment, and it is possible that PFAS residues from upriver sources, accumulate at this location. Furthermore, recent published research suggests that PFAS accumulate at the air–water interface in the subsurface layer of freshwater (Brusseau 2018; Stults et al. 2023). In addition to PFOS, high concentrations of mercury (Hg) were also quantified in Great White Egrets eggs from Bloemhof Dam (van der Schyff et al. 2016). No other studies from South Africa reported on PFOS or PCDD/F concentrations in bird eggs of similar species. Compared with international reports, the high PCDD/F concentrations of the current study points towards a PCDD/F hotspot. Eggs from Barbers Pan had the highest PCDD/F concentration (9 ng/g wm; Table 3), this is concerning since this location is a Ramsar site. The four highest measured PCDD/F concentrations (BBH: 9 ng/g wm, ASI: 7 ng/g wm, BHH: 6 ng/g wm, and AD: 5 ng/g wm; Table 3) were in eggs from all four sites and from three different feeding and habitat guilds making it difficult interpret. It is concerning that concentrations quantified in eggs from all four sites were higher than internationally reported concentrations (Table 4), especially since Barbers Pan is a Ramsar site.
The data reported here represent the most current published insight into the pollution load of heron, ibis, egret, darter, and cormorants that breed in the OSRB. All other published reports were conducted during or prior to the current study. Chlordane and mirex were previously quantified in eggs of similar species (Bouwman et al. 2008; Polder et al. 2008). Studies conducted in the same year as the current collection also found quantifiable concentrations of chlordane and mirex in eggs from Gauteng (Bouwman et al. 2021) and Limpopo (Bouwman et al. 2013). These compounds were also quantified in Little Egret and White-breasted Cormorant eggs collected in 2013 in KwaZulu-Natal that is not in the OSRB. The lack of quantifiable concentrations of these compounds may be due to concentrations below LOQ.
Therefore, POP hotspots identified in this study were Bloemhof Dam for PFOS, and Eldorado Park (Gauteng) for most other POPs. We could not identify PCDD/F hotspots due to similar high concentrations detected at all four sites, nor could we explain these concentrations based on guilds. It would be reasonable to assume that the Vaal River catchment is a hotspot for PCDD/Fs, in general, and that more localised investigations need to be conducted. Bloemhof Dam has no associated industrial activities but is located approximately 450 km downstream of the most industrialised centre in the OSRB where African Sacred Ibis eggs were analysed. Eggs from Eldorado Park (in the industrialised centre) had factors to orders of magnitude higher concentrations of all compound classes at any other site, except for PFOS at Bloemhof Dam. The PFOS and PCDD/Fs results from Bloemhof Dam show that single-species studies cannot represent the picture of total avian exposure and risks as was also found by Bouwman et al. (2021). Also, assumptions about proximity to sources should not be assumed as the only factor when identifying hotspots.
Possible Adverse Consequences
The low TEQ values in the eggs from Eldorado Park were unexpected, considering its proximity to industry. The high PCDD/F TEQ value from Barbers Pan was higher than expected, due to its isolation, remoteness from sources, and protection status as a nature sanctuary. However, the Black-headed Heron eggs from this location did have the highest quantifiable PCDD/F concentration (Table 3). Bloemhof Dam on the other hand had the highest PCB TEQ value, possibly a result of compounds accumulating at this point in the Vaal River. Bird embryos and foetuses are more sensitive to POPs than adults. Furthermore, exposure before organ development results in greater consequences when exposed after organ development (Caralson and Duby 1973). However, the TEQ values reported in this study were low compared to others (Harris et al. 2003; Hart et al. 1991). The TEQ values calculated for double-crested Cormorant eggs close to a pulp mill were up to three orders of magnitude higher than any TEQ value of the current study (Harris et al. 2003). The double-crested Cormorant hatchlings had elevated ethoxyresorufin-O-deethylase (EROD) activity and showed brain asymmetry. In addition, it is suggested that neurological activities in bird eggs are more effected by PCDD/F TEQs (Henshel 1998), which were lower than PCB TEQs in the current study. In Blue Heron eggs from Canada, a no-observed-adverse-effect-level (NOAEL) of 18 ngTEQ/kg wm was reported for developmental defects and reduced fledging (Hart et al. 1991), 10 ngTEQ/kg wm for intercerebral brain asymmetry (Henshel et al. 1995), and 100 ngTEQ/kg wm for gross abnormalities and oedema (Sanderson et al. 1994). A NOAEL of 200 ngTEQ/kg wm of coplanar PCBs were reported for Forster's tern eggs (Sterna forsteri) for reduced hatching success and fledging (Kubiak et al. 1989). These TEQ values were all well above all TEQ values from the current study, and therefore, we do not expect any adverse effects in eggs, hatchlings, or fledglings.
The number of PCB congeners measured affect the reported concentrations in bird eggs. While we investigated 18 congeners of which 12 are DL-PCBs and six NDL-PCBs, far higher ΣPCB residues were quantified in eggs that were investigated for fewer congeners. Field studies found mortality in double-crested Cormorant eggs at ΣPCB? of 30 000 ng/kg wm (Tillitt et al. 1992) and developmental defects in Black-crowned Night herons at ΣPCB? concentrations of 800 ng/g wm (Hoffman et al. 1986).
The probability of adverse effects on birds was investigated by comparing the concentrations quantified in the eggs to the available toxic reference values (TRVs) of POP. Unfortunately, TRVs are not available for all species. However, quantifiable concentrations can be compared to TVRs for other species, although it should be noted that these values are estimations and can differ greatly among species due to behavioural and biological differences. The highest ΣPCB concentrations quantified in any egg from the present study (African Darter: 102 ng/g wm) were much lower than the TRV that was derived by Hoffman et al. (1986) and Tilliet et al. (1992). We, therefore, do not expect adverse effects in birds as a result of PCB exposure for the regions sampled.
The HCB concentrations measured in bird eggs were low compared to other studies (Table 3). This is reassuring, since HCB is known to be very toxic to birds. The HCB concentrations measured in all eggs from the current study were far below the NOAEL of 1500 ng/g wm for herring gull embryos (Larus argentatus) embryos (Boersma and Ellenton 1986). DDT, specifically the metabolite p,p’-DDE, reduces eggshell thickness in eggs and may lead to reproductive failure and population decline (Dirksen et al. 1995; Peakall 1993). In Snowy Egrets (Egretta thula), it was found that DDE residues of 5000 ng/g wm in eggs caused thinner eggshells and lower hatching success (Findholt 1984). The p,p’-DDE residues in Little Egret eggs (19 ng/g wm) from Bloemhof Dam were well below this TRV. Additionally, DDE residues of 8000 ng/g wm were found to increase eggshell breakage in populations of Black-crowned Night Heron (Nycticorax nycticorax) populations (Henny et al. 1984). The p,p’-DDE concentrations of all species in the current study were well below that threshold. However, it should be noted that eggshell thinning can occur at lower exposure concentrations. Eggshell thinning has been observed in Cattle Egret eggs with increased p,p’-DDE and p,p’-DDT concentrations of up to 290 ng/g wm (Bouwman et al. 2013). For insecticide POPs, therefore, we do not expect adverse effects in birds for the region sampled.
Certain factors may influence the residue concentrations quantified in bird eggs such as the diet, habitat preference, age, and health of the female bird, as well as the time and the number of eggs laid in the clutch (Dennis et al. 2021; Mineau 1982). In addition, bioaccumulation of PFOS is 1.8 times greater in eggs when exposed through drinking water compared to food (Dennis et al. 2021). The concentrations of PFOS quantified in all eggs of all species from Bloemhof Dam and Eldorado Park (African Darter: 2330 ng/g wm) were two orders of magnitude above the TRV of PFOS estimated for Bobwhite quail (Colinus virginianus) whole egg (92.4 ng/g wm) (Dennis et al. 2021). The predicted no-effect concentration (PNEC) for PFOS in Bobwhite quail, regrading chick survival is 1700 ng/g wm (Newsted et al. 2005). African Darter eggs from Barbers Pan and Reed Cormorant eggs from Potchefstroom exceeded the TRV and PNEC for Bobwhite quail. PFOS therefore poses a risk for adverse effects in birds for all regions sampled.
The ΣPBDE concentrations from African Sacred Ibis eggs from Eldorado Park were 20 ng/g wm, well below the NOEL of 1000 ng/g wm for the Osprey (Pandion haliaetus; Chen et al. 2010). The Osprey is a high-trophic level species when compared with the African Sacred Ibis. It may be that higher PBDE concentrations will bioaccumulate in higher trophic level species from Eldorado Park. However, PBDE does not pose a risk for adverse effects in bird species sampled.
Conclusions and Recommendations
Concentrations of all compounds detected in eggs were generally lower or of the same magnitude than those reported by most local and international studies, except for PCDD/F and PFOS. Organochlorine compounds and PCB concentrations were lower than previously reported, suggesting a decrease in the environment. Differences in POP concentrations in wild bird eggs were found between species, sites, habitat guilds, and feeding guilds. This was to be expected since species from the same region have different life histories combined with the different sources and chemical and physical characteristics of POPs.
Large aquatic predators had greater OCP and PFOS concentrations compared to species that prey on insects, while PCBs and PCDD/Fs were more prominent in terrestrial species. No patterns were observed for the other compound groups. It is recommended that additional species that occupy other feeding guilds, such as seedeaters and frugivorous, be included in future studies. It would also be instructive to determine trends and patterns over multiple years. DDT residues in bird eggs remain high in malaria endemic regions. However, Gauteng appears to be an OCP (p,p’-DDE) and PCB hotspot. It would be reasonable to assume that, given the lower p,p’-DDT concentrations and or lack of quantifiable concentrations in pooled eggs, that the ΣDDT quantified is of legacy use.
PFOS concentrations were observed to peak towards the west in the area of the Bloemhof Dam. The quantified concentrations are comparable to the concentrations detected near a PFAS source. It appears that Bloemhof Dam acts as a ‘retainer’ or ‘trap’ of some compounds coming from upstream or may reflect a local unknown source of PFOS. These concentrations pose a risk of adverse effects and should be monitored. It would be very informative to sample additional locations such as Upington downstream of Bloemhof Dam, especially with respect to the distribution of PFOS. PCDD/F concentrations quantified were unexpected. Due to the widespread occurrence of high PCDD/F concentrations, it is difficult to pinpoint specific hotspots. However, the high ΣPBDE concentrations of PBDE in Barbers Pan are concerning since it is a Ramsar site. Therefore, we recommend that more samples be collected and tested for PCDD/Fs. Overall, Bloemhof Dam would be a good monitoring site for all POPs, given its remoteness from large sources and high breeding density.
The OCP concentrations detected in bird eggs were below known TRVs. ΣPBDE concentrations in wild bird eggs were also low. However, the higher ΣPBDE concentrations from Eldorado Park are concerning and this site should be regarded as a PBDE hotspot. The combined concentrations of POPs may have greater consequences than individual POPs. Furthermore, since 2010, 13 POPs have been added to the Stockholm Convention on Persistent Organic Pollutants (Stockholm Convention 2016b), and others are in the process of being added. These new POPs need further investigation to determine possible threats and hotspots. PCB and PCDD/F concentrations and TEQ values in wild bird eggs were low, and no adverse effects are expected. Therefore, we conclude that single-site and single-species studies would not effectively represent risks representative of the complexity of avian diversity as environmental, behavioural, and physiological differences of species. Therefore, this study provides a data-rich baseline against which trends since 2010 can be investigated, especially in the hotspots and bird species reported here.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was done under the auspices of ORASECOM in association with the UNDP and funded by the GEF via UNOPS. We thank Christof Mor for his kind assistance throughout the project. Mr Carlos Huisamen for the collection of bird eggs and the whole POPT group for assisting with sample preparation. Horst Rottler from Oekometrics is thanked for his inputs and efforts. We also thank Ian Smith, Claudine Roos, Laura Quinn, Karin Blom, and Lohan Bredenhann.
Author contributions
Velesia Lesch was contributed writing original draft. Henk Bouwman was involved in supervision and review and editing. Rialet Pieters was performed review and editing.
Funding
Open access funding provided by North-West University. UNOPS/ORASECOM/NRF,71598/2001/001,Hindrik Bouwman
Declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anderson MD, Kolberg H, Anderson PC, Dini J, Abrahams A (2003) Waterbird populations at the orange River mouth from 1980–2001: a re-assessment of its Ramsar status. Ostrich-J Afr Ornitho 74:159–172 [Google Scholar]
- Antoniadou V, Konstantinou IK, Goutner V, Sakellarides TM, Albanis TA, Bintoudi E (2007) PCB levels and accumulation patterns in waterbird eggs and in their prey at Lake Kerkini, a north-eastern Mediterranean wetland of international importance. Arch Environ Contam Toxicol 53:249–260 [DOI] [PubMed] [Google Scholar]
- Aurigi S, Focardi S, Hulea D, Renzoni A (2000) Organochlorine contamination in bird’s eggs from the Danube Delta. Environ Pollut 109:61–67 [DOI] [PubMed] [Google Scholar]
- Barron MG, Galbraith H, Beltman D (1995) Comparative reproductive and developmental toxicology of PCBs in birds. Comp Biochem Physiol c: Pharmacol Toxicol Endocrinol 112:1–14 [Google Scholar]
- Berny P, Sadoul N, Dol S, Videman B, Kayser Y, Hafner H (2002) Impact of local agricultural and industrial practices on organic contamination of little egret (Egretta garzetta) eggs in the Rhone Delta, southern France. Environ Toxicol Chem Int J 21:520–526 [DOI] [PubMed] [Google Scholar]
- Bitman J, Cecil HC, Fries GF (1970) DDT-induced inhibition of avian shell gland carbonic anhydrase: a mechanism for thin eggshells. Science 168:594–596 [DOI] [PubMed] [Google Scholar]
- Boersma DC, Ellenton JA, Yagminas A (1986) Investigation of the hepatic mixed-function oxidase system in herring gull embryos in relation to environmental contaminants. Environ Toxicol Chem Int J 5:309–318 [Google Scholar]
- Bornman R, De Jager C, Worku Z, Farias P, Reif S (2010) DDT and urogenital malformations in newborn boys in a malarial area. BJU Int 106:405–411 [DOI] [PubMed] [Google Scholar]
- Bouwman H (2004) South Africa and the Stockholm convention on persistent organic pollutants: science policy. S Afr J Sci 100:323–328 [Google Scholar]
- Bouwman H, Polder A, Venter B, Skaare JU (2008) Organochlorine contaminants in cormorant, darter, egret, and ibis eggs from South Africa. Chemosphere 71:227–241 [DOI] [PubMed] [Google Scholar]
- Bouwman H, Viljoen IM, Quinn LP, Polder A (2013) Halogenated pollutants in terrestrial and aquatic bird eggs: converging patterns of pollutant profiles, and impacts and risks from high levels. Environ Res 126:240–253 [DOI] [PubMed] [Google Scholar]
- Bouwman H, Yohannes YB, Nakayama SMM, Motohira K, Ishizuka M, Humphries MS, Van der Schyff V, Du Preez M, Dinkelmann A, Ikenaka Y (2019) Evidence of impacts from DDT in pelican, cormorant, stork, and egret eggs from KwaZulu-Natal, South Africa. Chemosphere 225:647–658 [DOI] [PubMed] [Google Scholar]
- Bouwman H, Pieters R, Polder A, Quinn L (2021) Ten bird species, six guilds, three habitats, and 59 chlorinated and brominated POPs: what do 64 eggs from the largest economic hub of Southern Africa tell us? Arch Environ Contam Toxicol 81:347–366 [DOI] [PubMed] [Google Scholar]
- Braune BM, Mallory ML, Gilchrist HG, Letcher RJ, Drouillard KG (2007) Levels and trends of organochlorines and brominated flame retardants in Ivory Gull eggs from the Canadian Arctic, 1976 to 2004. Sci Total Environ 378:403–417 [DOI] [PubMed] [Google Scholar]
- Brusseau ML (2018) Assessing the potential contributions of additional retention processes to PFAS retardation in the subsurface. Sci Total Environ 613:176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson RW, Duby RT (1973) Embryotoxic effects of three PCB’s in the chicken. Bull Environ Contam Toxicol 9:261–266 [DOI] [PubMed] [Google Scholar]
- Chen D, Hale RC, Watts BD, La Guardia MJ, Harvey E, Mojica EK (2010) Species-specific accumulation of polybrominated diphenyl ether flame retardants in birds of prey from the Chesapeake Bay region, USA. Environ Pollut 158:1883–1889 [DOI] [PubMed] [Google Scholar]
- Chokwe TB, Okonkwo JO, Sibali LL, Ncube EJ (2015) Alkylphenol ethoxylates and brominated flame retardants in water, fish (carp) and sediment samples from the Vaal River, South Africa. Environ Sci Pollut Res 22:11922–11929 [DOI] [PubMed] [Google Scholar]
- Chokwe TB, Magubane MN, Abafe OA, Okonkwo JO, Sibiya IV (2019) Levels, distributions, and ecological risk assessments of polybrominated diphenyl ethers and alternative flame retardants in river sediments from Vaal River, South Africa. Environ Sci Pollut Res 26:7156–7163 [DOI] [PubMed] [Google Scholar]
- Clatterbuck CA, Lewison RL, Dodder NG, Zeeman C, Schiff K (2018) Seabirds as regional biomonitors of legacy toxicants on an urbanized coastline. Sci Total Environ 619:460–469 [DOI] [PubMed] [Google Scholar]
- Connell DW, Fung CN, Minh TB, Tanabe S, Lam PKS, Wong BSF, Lam MHW, Wong LC, Wu RSS, Richardson BJ (2003) Risk to breeding success of fish-eating Ardeids due to persistent organic contaminants in Hong Kong: evidence from organochlorine compounds in eggs. Water Res 37:459–467 [DOI] [PubMed] [Google Scholar]
- Stockholm Convention. 2016a. All POPs listed in the Stockholm Convention. http://www.pops.int/TheConvention/ThePOPs/AllPOPs/tabid/2509/Default.aspx Date of access: 5 November 2022.
- Custer TW, Kannan K, Tao L, Saxena AR, Route B (2009) Perfluorinated compounds and polybrominated diphenyl ethers in great blue heron eggs from Indiana Dunes National Lakeshore, Indiana. J Great Lakes Res 35:401–405 [Google Scholar]
- Custer TW, Kannan K, Tao L, Yun SH, Trowbridge A (2010) Perfluorinated compounds and polybrominated diphenyl ethers in great blue heron eggs from three colonies on the Mississippi River, Minnesota. Waterbirds 33:86–95 [Google Scholar]
- de Cruz I, Mougin C, Grolleau G (1997) Chlorinated hydrocarbons in eggs of grey heron (Ardea cinerea L.) in France (Lac de Grandlieu). Chemosphere 35:1003–1009 [Google Scholar]
- Dennis NM, Subbiah S, Karnjanapiboonwong A, Dennis ML, McCarthy C, Salice CJ, Anderson TA (2021) Species-and tissue-specific avian chronic toxicity values for perfluorooctane sulfonate (PFOS) and a binary mixture of PFOS and perfluorohexane sulfonate. Environ Toxicol Chem 40:899–909 [DOI] [PubMed] [Google Scholar]
- Dirksen S, Boudewijn TJ, Slager LK, Mes RG, Van Schaick MJM, De Voogt P (1995) Reduced breeding success of cormorants (Phalacrocorax carbo sinensis) in relation to persistent organochlorine pollution of aquatic habitats in the Netherlands. Environ Pollut 88:119–132 [DOI] [PubMed] [Google Scholar]
- Dong YH, Wang H, An Q, Ruiz X, Fasola M, Zhang YM (2004) Residues of organochlorinated pesticides in eggs of water birds from Tai Lake in China. Environ Geochem Health 26:259–268 [DOI] [PubMed] [Google Scholar]
- Eljarrat E, Aznar-Alemany Ò, Sala B, Frías Ó, Blanco G (2019) Decreasing but still high levels of halogenated flame retardants in wetland birds in central Spain. Chemosphere 228:83–92 [DOI] [PubMed] [Google Scholar]
- Elliott JE, Brogan J, Lee SL, Drouillard KG, Elliott KH (2015) PBDEs and other POPs in urban birds of prey partly explained by trophic level and carbon source. Sci Total Environ 524:157–165 [DOI] [PubMed] [Google Scholar]
- Elliott, J.E., Harris, M.L., Wilson, L.K., Whitehead, P.E., Norstrom, R.J. 2001. Monitoring temporal and spatial trends in polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) in eggs of great blue heron (Ardea herodias) on the coast of British Columbia, Canada, 1983–1998. AMBIO: A Journal of the Human Environment. 30(7): 416-428. [PubMed]
- Eriksson U, Roos A, Lind Y, Hope K, Ekblad A, Kärrman A (2016) Comparison of PFASs contamination in the freshwater and terrestrial environments by analysis of eggs from osprey (Pandion haliaetus), tawny owl (Strix aluco), and common kestrel (Falco tinnunculus). Environ Res 149:40–47 [DOI] [PubMed] [Google Scholar]
- Fasola M, Movalli PA, Gandini C (1998) Heavy metal, organochlorine pesticide, and PCB residues in eggs and feathers of herons breeding in northern Italy. Arch Environ Contam Toxicol 34:87–93 [DOI] [PubMed] [Google Scholar]
- Fernie KJ, Laird Shutt J, Ritchie IJ, Letcher RJ, Drouillard K, Bird DM (2006) Changes in the growth, but not the survival, of American kestrels (Falco sparverius) exposed to environmentally relevant polybrominated diphenyl ethers. J Toxicol Environ Health A 69:1541–1554 [DOI] [PubMed] [Google Scholar]
- Fernie KJ, Shutt JL, Letcher RJ, Ritchie JI, Sullivan K, Bird DM (2008) Changes in reproductive courtship behaviors of adult American kestrels (Falco sparverius) exposed to environmentally relevant levels of the polybrominated diphenyl ether mixture, DE-71. Toxicol Sci 102:171–178 [DOI] [PubMed] [Google Scholar]
- Findholt SL (1984) Organochlorine residues, eggshell thickness, and reproductive success of snowy egrets nesting in Idaho. The Condor 86:163–169 [Google Scholar]
- Frank DS, Mora MA, Sericano JL, Blankenship AL, Kannan K, Giesy JP (2001) Persistent organochlorine pollutants in eggs of colonial waterbirds from Galveston Bay and East Texas, USA. Environ Toxicol Chem Int J 20:608–617 [PubMed] [Google Scholar]
- Gilbert BM, Avenant-Oldewage A (2016) Effects of altered water quality and trace elements on the infection variables of Paradiplozoon ichthyoxanthon (Monogenea: Diplozoidae) from two sites in the Vaal River system, South Africa. Acta Parasitol 61:52–62 [DOI] [PubMed] [Google Scholar]
- Goutner, V., Frigis, K., Konstantinou, I.K., Sakellarides, T.M., Albanis, T.A. 2012. Organochlorine pesticide residue concentrations and accumulation patterns in waterbirds and in their prey at Lake Kerkini, a Ramsar wetland, Greece. Journal of Biological Research, 17.
- Groffen T, Rijnders J, van Doorn L, Jorissen C, De Borger SM, Luttikhuis DO, de Deyn L, Covaci A, Bervoets L (2021) Preliminary study on the distribution of metals and persistent organic pollutants (POPs), including perfluoroalkylated acids (PFAS), in the aquatic environment near Morogoro, Tanzania, and the potential health risks for humans. Environ Res 192:110299 [DOI] [PubMed] [Google Scholar]
- Harebottle DM (2019) HeronryMAP: Africa-Mapping the distribution and status of breeding sites of Ardeids and other colonial waterbirds in Africa. J Heron Biol Conserv 4:2 [Google Scholar]
- Harris HJ, Erdman TC, Ankley GT, Lodge KB (1993) Measures of reproductive success and polychlorinated biphenyl residues in eggs and chicks of Forster’s terns on Green Bay, Lake Michigan, Wisconsin—1988. Arch Environ Contam Toxicol 25:304–314 [Google Scholar]
- Harris ML, Wilson LK, Norstrom RJ, Elliott JE (2003) Egg concentrations of polychlorinated dibenzo-p-dioxins and dibenzofurans in double-crested (Phalacrocorax auritus) and Pelagic (P. pelagicus) cormorants from the Strait of Georgia, Canada, 1973–1998. Environ Sci Technol 37:822–831 [DOI] [PubMed] [Google Scholar]
- Hart LE, Cheng KM, Whitehead PE, Shah RM, Lewis RJ, Ruschkowski SR, Blair RW, Bennett DC, Bandiera SM, Norstrom RJ, Bellward GD (1991) Dioxin contamination and growth and development in great blue heron embryos. J Toxicol Environ Health Part A 32:331–344 [DOI] [PubMed] [Google Scholar]
- Henny CJ, Blus LJ, Krynitsky AJ, Bunck CM (1984) Current impact of DDE on black-crowned night-herons in the intermountain west. J Wildlife Manag. 10.2307/3808448 [Google Scholar]
- Henshel DS (1998) Developmental neurotoxic effects of dioxin and dioxin-like compounds on domestic and wild avian species. Environ Toxicol Chem Int J 17:88–98 [Google Scholar]
- Henshel DS, Martin JW, Norstrom R, Whitehead P, Steeves JD, Cheng KM (1995) Morphometric abnormalities in brains of great blue heron hatchlings exposed in the wild to PCDDs. Environ Health Perspect 103:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzke D, Kallenborn R, Nygård T (2002) Organochlorines in egg samples from Norwegian birds of prey: congener-, isomer-and enantiomer specific considerations. Sci Total Environ 291:59–71 [DOI] [PubMed] [Google Scholar]
- Hockey PAR, Dean WRJ, Ryan PG (2005) Roberts: Birds of Southern Africa, 7th edn. The trustees of John Voelcker bird book fund, Cape Town [Google Scholar]
- Hoff PT, Van de Vijver K, Dauwe T, Covaci A, Maervoet J, Eens M, Blust R, De Coen W (2005) Evaluation of biochemical effects related to perfluorooctane sulfonic acid exposure in organohalogen-contaminated great tit (Parus major) and blue tit (Parus caeruleus) nestlings. Chemosphere 61:1558–1569 [DOI] [PubMed] [Google Scholar]
- Hoffman DJ, Rattner BA, Bunck CM, Krynitsky A, Ohlendorf HM, Lowe RW (1986) Association between PCBs and lower embryonic weight in black-crowned night herons in San Francisco Bay. J Toxicol Environ Health Part A 19:383–391 [DOI] [PubMed] [Google Scholar]
- Holm L, Blomqvist A, Brandt I, Brunström B, Ridderstråle Y, Berg C (2006) Embryonic exposure to o, p′-DDT causes eggshell thinning and altered shell gland carbonic anhydrase expression in the domestic hen. Environ Toxicol Chem Int J 25:2787–2793 [DOI] [PubMed] [Google Scholar]
- Huertas D, Grimalt JO, Jover L, Sanpera C (2016) Influence of diet in the accumulation of organochlorine compounds in herons breeding in remote riverine environments. Chemosphere 145:438–444 [DOI] [PubMed] [Google Scholar]
- Jensen AA, Leffers H (2008) Emerging endocrine disrupters: perfluoroalkylated substances. Int J Androl 31:161–169 [DOI] [PubMed] [Google Scholar]
- Kocagöz R, Onmuş O, Onat I, Çağdaş B, Sıkı M, Orhan H (2014) Environmental and biological monitoring of persistent organic pollutants in waterbirds by non-invasive versus invasive sampling. Toxicol Lett 230:208–217 [DOI] [PubMed] [Google Scholar]
- Kubiak TJ, Harris HJ, Smith LM, Schwartz TR, Stalling DL, Trick JA, Sileo L, Docherty DE, Erdman TC (1989) Microcontaminants and reproductive impairment of the Forster’s tern on Green Bay, Lake Michigan-1983. Arch Environ Contam Toxicol 18:706–727 [DOI] [PubMed] [Google Scholar]
- Kwiatkowski CF, Andrews DQ, Birnbaum LS, Bruton TA, DeWitt JC, Knappe DR, Maffini MV, Miller MF, Pelch KE, Reade A, Soehl A (2020) Scientific basis for managing PFAS as a chemical class. Environ Sci Technol Lett 7:532–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange GM, Mungatana E, Hassan R (2007) Water accounting for the Orange River Basin: an economic perspective on managing a transboundary resource. Ecol Econ 61:660–670 [Google Scholar]
- Laporte P (1982) Organochlorine residues and eggshell measurements of great blue heron eggs from quebec. Colon Waterbirds 5:95–103 [Google Scholar]
- Lebedev AT, Poliakova OV, Karakhanova NK, Petrosyan VS, Renzoni A (1998) The contamination of birds with organic pollutants in the Lake Baikal region. Sci Total Environ 212:153–162 [DOI] [PubMed] [Google Scholar]
- Lesch V, Bouwman H, Kinoshita A, Shibata Y (2017) First report of perfluoroalkyl substances in South African Odonata. Chemosphere 175:153–160 [DOI] [PubMed] [Google Scholar]
- Lesch V, Kylin H, Bouwman H (2023) The cattle egret bubulcus ibis as a near-global indicator of terrestrial pollution. Environ Chem Ecotoxicol 6:15–25 [Google Scholar]
- Lopez-Antia A, Dauwe T, Meyer J, Maes K, Bervoets L, Eens M (2017) High levels of PFOS in eggs of three bird species in the neighbourhood of a fluoro-chemical plant. Ecotoxicol Environ Saf 139:165–171 [DOI] [PubMed] [Google Scholar]
- Lundholm CE (1997) DDE-induced eggshell thinning in birds: effects of p, p′-DDE on the calcium and prostaglandin metabolism of the eggshell gland. Comp Biochem Physiol c: Pharmacol Toxicol Endocrinol 118:113–128 [DOI] [PubMed] [Google Scholar]
- Mandal PK (2005) Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J Comp Physiol B 175:221–230 [DOI] [PubMed] [Google Scholar]
- Medvedev N, Markova L (1995) Residues of chlorinated pesticides in the eggs of Karelian birds, 1989–90. Environ Pollut 87:65–70 [DOI] [PubMed] [Google Scholar]
- Miller A, Elliott JE, Elliott KH, Guigueno MF, Wilson LK, Lee S, Idrissi A (2015) Brominated flame retardant trends in aquatic birds from the Salish Sea region of the west coast of North America, including a mini-review of recent trends in marine and estuarine birds. Sci Total Environ 502:60–69 [DOI] [PubMed] [Google Scholar]
- Mineau P (1982) Levels of major organochlorine contaminants in sequentially-laid herring gull eggs. Chemosphere 11:679–685 [Google Scholar]
- Morales L, Martrat MGR, Parera J, Bertolero A, Ábalos M, Santos FJ, Lacorte S, Abad E (2016) Dioxins and dl-PCBs in gull eggs from spanish natural parks (2010–2013). Sci Total Environ 550:114–122 [DOI] [PubMed] [Google Scholar]
- Newman, M.C., 2015. Fundamentals of Ecotoxicology: The Science of Pollution, 4th Ed. CRC Press, New York: 34–78.
- Newsted JL, Jones PD, Coady K, Giesy JP (2005) Avian toxicity reference values for perfluorooctane sulfonate. Environ Sci Technol 39:9357–9362 [DOI] [PubMed] [Google Scholar]
- Nordén M, Berger U, Engwall M (2013) High levels of perfluoroalkyl acids in eggs and embryo livers of great cormorant (Phalacrocorax carbo sinensis) and herring gull (Larus argentatus) from Lake Vänern, Sweden. Environ Sci Pollut Res 20:8021–8030 [DOI] [PubMed] [Google Scholar]
- Orisakwe, O.E., Frazzoli, C., Ilo, C.E., Oritsemuelebi, B. 2019. Public health burden of e-waste in Africa. Journal of Health and Pollution: 9. [DOI] [PMC free article] [PubMed]
- Peakall DB (1993) DDE-induced eggshell thinning: an environmental detective story. Environ Rev 1:13–20 [Google Scholar]
- Perry AS, Sidis I, Zemach A (1990) Organochlorine insecticide residues in birds and bird eggs in the coastal plain of Israel. Bull Environ Contam Toxicol 45:523–530 [DOI] [PubMed] [Google Scholar]
- Polder A, Venter B, Skaare JU, Bouwman H (2008) Polybrominated diphenyl ethers and HBCD in bird eggs of South Africa. Chemosphere 73:148–154 [DOI] [PubMed] [Google Scholar]
- Polder A, Müller MB, Brynildsrud OB, De Boer J, Hamers T, Kamstra JH, Lie E, Mdegela RH, Moberg H, Nonga HE, Sandvik M (2016) Dioxins, PCBs, chlorinated pesticides and brominated flame retardants in free-range chicken eggs from peri-urban areas in Arusha, Tanzania: levels and implications for human health. Sci Total Environ 551:656–667 [DOI] [PubMed] [Google Scholar]
- Quinn L, Pieters R, Nieuwoudt C, Borgen AR, Kylin H, Bouwman H (2009) Distribution profiles of selected organic pollutants in soils and sediments of industrial, residential and agricultural areas of South Africa. J Environ Monit 11:1647–1657 [DOI] [PubMed] [Google Scholar]
- Quinn LP, Roos C, Pieters R, Polder A, Bouwman H (2020) Brominated flame retardants in wild bird eggs from the industrialised heartland of South Africa. African Zoology 55:43–56 [Google Scholar]
- Ramsar Sites Information Service.2018a. https://rsis.ramsar.org/ris-search/?language=en&f%5B0%5D=regionCountry_en_ss%3AAfrica&f%5B1%5D=regionCountry_en_ss%3ASouth%20Africa&f%5B2%5D=montreuxListed_b%3Atrue Date of access: 12 February 2024.
- Ramsar Sites Information Service. 2018b. https://rsis.ramsar.org/ris-search/?language=en&f%5B0%5D=regionCountry_en_ss%3AAfrica&f%5B1%5D=regionCountry_en_ss%3ASouth%20Africa Date of access: 12 February 2024.
- Rawn DF, Sadler AR, Quade SC, Sun WF, Kosarac I, Hayward S, Ryan JJ (2012) The impact of production type and region on polychlorinated biphenyl (PCB), polychlorinated dibenzo-p-dioxin and dibenzofuran (PCDD/F) concentrations in Canadian chicken egg yolks. Chemosphere 89:929–935 [DOI] [PubMed] [Google Scholar]
- Romanoff AL (1932) Fat metabolism of the chick embryo under standard conditions of artificial incubation. Biol Bull 62:54–62 [Google Scholar]
- Rüdel H, Müller J, Jürling H, Bartel-Steinbach M, Koschorreck J (2011) Survey of patterns, levels, and trends of perfluorinated compounds in aquatic organisms and bird eggs from representative German ecosystems. Environ Sci Pollut Res 18:1457–1470 [DOI] [PubMed] [Google Scholar]
- Sanderson JT, Elliott JE, Norstrom RJ, Whitehead PE, Hart LE, Cheng KM, Bellward GD (1994) Monitoring biological effects of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in great blue heron chicks (Ardea herodias) in British Columbia. J Toxicol Environ Health Part A 41:435–450 [DOI] [PubMed] [Google Scholar]
- She J, Holden A, Adelsbach TL, Tanner M, Schwarzbach SE, Yee JL, Hooper K (2008) Concentrations and time trends of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in aquatic bird eggs from San Francisco Bay, CA 2000–2003. Chemosphere 73:201–209 [DOI] [PubMed] [Google Scholar]
- Stockholm convention. 2016b. The new POPs under the Stockholm Convention. http://www.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx Date of access: 5 November 2022.
- Stults JF, Choi YJ, Rockwell C, Schaefer CE, Nguyen DD, Knappe DR, Illangasekare TH, Higgins CP (2023) Predicting concentration-and ionic-strength-dependent air–water interfacial partitioning parameters of PFASs using quantitative structure–property relationships (QSPRs). Environ Sci Technol 57:5203–5215 [DOI] [PubMed] [Google Scholar]
- Warwick Tarboton. 2022. Bird Images. https://www.warwicktarboton.co.za/Bird%20family%20list.html. Date of Access: 09 November 2022.
- Thomas CM, Anthony RG (1999) Environmental contaminants in great blue herons (Ardea herodias) from the lower Columbia and Willamette Rivers. Or Wash, USA Environ Toxicol Chem Int J 18:2804–2816 [Google Scholar]
- Tillitt DE, Ankley GT, Giesy JP, Ludwig JP, Kurita-Matsuba H, Weseloh DV, Ross PS, Bishop CA, Sileo L, Stromborg KL, Larson J (1992) Polychlorinated biphenyl residues and egg mortality in double-crested cormorants from the Great Lakes. Environ Toxicol Chem Int J 11:1281–1288 [Google Scholar]
- Van den Berg M, Birnbaum L, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson E (2006) The 2005 World Health Organization re-evaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 93:223–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Steen E, Dauwe T, Covaci A, Jaspers VLB, Pinxten R, Eens M (2006) Within-and among-clutch variation of organohalogenated contaminants in eggs of great tits (Parus major). Environ Pollut 144:355–359 [DOI] [PubMed] [Google Scholar]
- Van der Schyff V, Pieters R, Bouwman H (2016) The heron that laid the golden egg: metals and metalloids in ibis, darter, cormorant, heron, and egret eggs from the Vaal River catchment, South Africa. Environ Monit Assess 188:1–7 [DOI] [PubMed] [Google Scholar]
- Venugopal D, Subramanian M, Rajamani J, Palaniyappan J, Samidurai J, Arumugam A (2020) Levels and distribution pattern of organochlorine pesticide residues in eggs of 22 terrestrial birds from Tamil Nadu, India. Environ Sci Pollut Res 27:39253–39264 [DOI] [PubMed] [Google Scholar]
- Verboven N, Verreault J, Letcher RJ, Gabrielsen GW, Evans NP (2009) Differential investment in eggs by Arctic-breeding glaucous gulls (larus hyperboreus) exposed to persistent organic pollutants. Auk 126:123–133 [Google Scholar]
- Wang Y, Yeung LW, Taniyasu S, Yamashita N, Lam JC, Lam PK (2008) Perfluorooctane sulfonate and other fluorochemicals in waterbird eggs from South China. Environ Sci Technol 42:8146–8151 [DOI] [PubMed] [Google Scholar]
- Wang Y, Murphy MB, Lam JC, Jiao L, Wong CC, Yeung LW, Lam PK (2011) Polychlorinated biphenyls and organochlorine pesticides in local waterbird eggs from Hong Kong: risk assessment to local waterbirds. Chemosphere 83:891–896 [DOI] [PubMed] [Google Scholar]
- Wang Y, Lam JC, So MK, Yeung LW, Cai Z, Hung CL, Lam PK (2012) Polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), dioxin-like polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in waterbird eggs of Hong Kong, China. Chemosphere 86:242–247 [DOI] [PubMed] [Google Scholar]
- Wania F, Mackay D (1995) A global distribution model for persistent organic chemicals. Sci Total Environ 160:211–232 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.