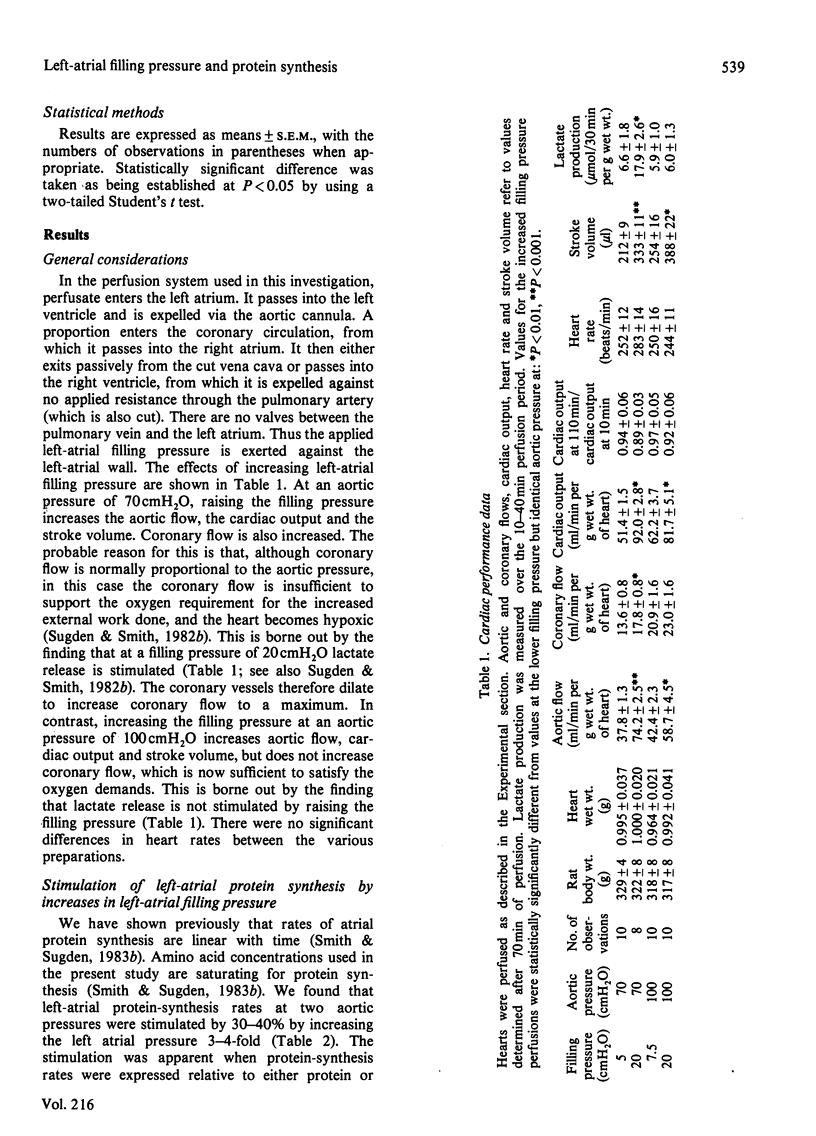
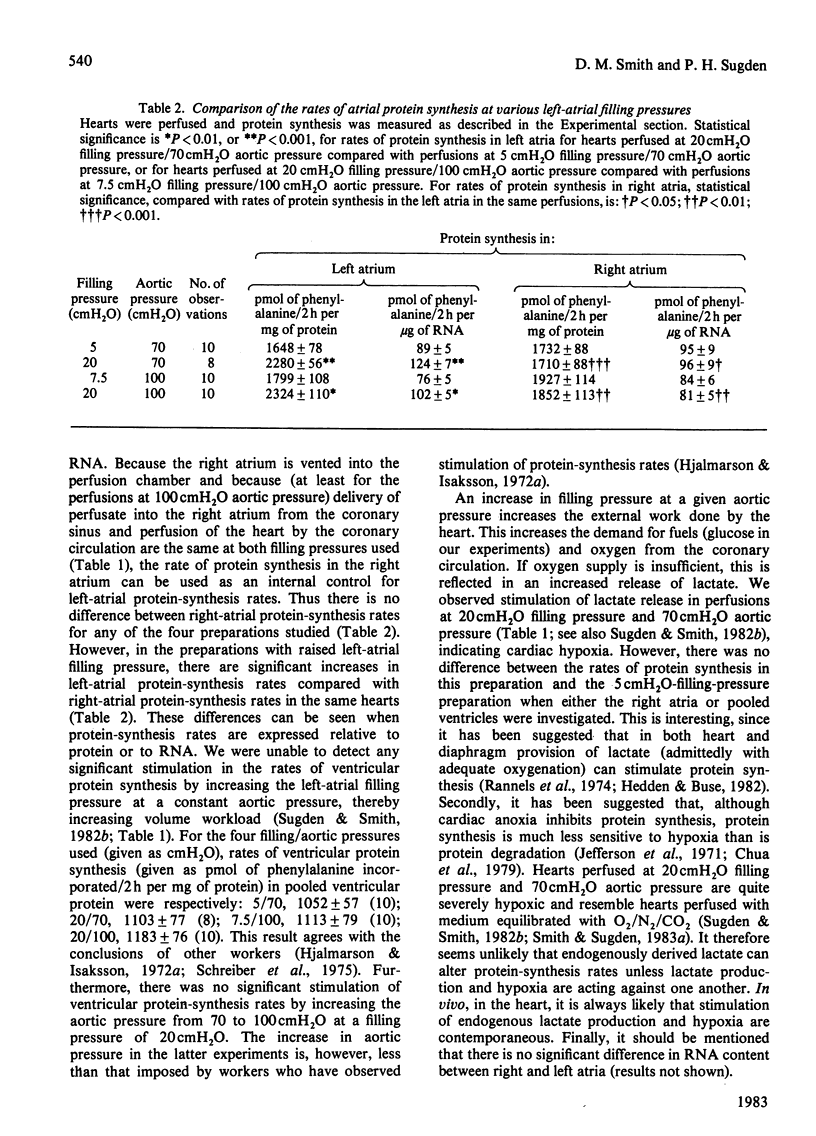
Abstract
We investigated the effect of an increase in the left-atrial filling pressure on the rate of left-atrial protein synthesis in the left-side-perfused working rat heart preparation of Taegtmeyer, Hems & Krebs [(1980) Biochem. J. 186, 701-711]. An increase in filling pressure (preload) at a constant aortic pressure (afterload) increased both the intra-atrial pressure and the atrial stroke volume. The aortic pressure (afterload) was held constant. An increase in filling pressure from 5 to 20 cmH2O at an aortic pressure of 70 cmH2O, or an increase in filling pressure of 7.5 to 20 cmH2O at an aortic pressure of 100 cmH2O, significantly stimulated the rates of left-atrial protein synthesis by 30-40%. The stimulation was observed when the rates of protein synthesis were expressed relative to either protein or RNA content. Since perfusate entering the right atrium from the coronary circulation left that atrium passively, the rate of protein synthesis in this compartment can be used as an internal control. Rates of right-atrial protein synthesis were similar to those in the left atria exposed to the lower filling pressures and were unaffected by the increases in left-atrial filling pressure. We suggest that the acute effects of increased left-atrial filling pressure on protein synthesis in that compartment may be important in the development of left-atrial hypertrophy. This condition is seen in patients who have raised pulmonary venous pressures in, for example, mitral stenosis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrén K., Hjalmarson A., Isaksson O. In vitro work load and rat heart metabolism. II. Effect on amino acid transport. Acta Physiol Scand. 1972 Oct;86(2):257–270. doi: 10.1111/j.1748-1716.1972.tb05331.x. [DOI] [PubMed] [Google Scholar]
- Chua B., Kao R. L., Rannels D. E., Morgan H. E. Inhibition of protein degradation by anoxia and ischemia in perfused rat hearts. J Biol Chem. 1979 Jul 25;254(14):6617–6623. [PubMed] [Google Scholar]
- Cutilletta A. F., Rudnik M., Zak R. Muscle and non-muscle cell RNA polymerase activity during the development of myocardial hypertrophy. J Mol Cell Cardiol. 1978 Aug;10(8):677–687. doi: 10.1016/0022-2828(78)90403-0. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Dankberg F. L. Changes in ribonucleic acid and protein synthesis during induced cardiac hypertrophy. Biochemistry. 1971 Feb 2;10(3):530–535. doi: 10.1021/bi00779a028. [DOI] [PubMed] [Google Scholar]
- Goldspink D. F., Garlick P. J., McNurlan M. A. Protein turnover measured in vivo and in vitro in muscles undergoing compensatory growth and subsequent denervation atrophy. Biochem J. 1983 Jan 15;210(1):89–98. doi: 10.1042/bj2100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden M. P., Buse M. G. Effects of glucose, pyruvate, lactate, and amino acids on muscle protein synthesis. Am J Physiol. 1982 Mar;242(3):E184–E192. doi: 10.1152/ajpendo.1982.242.3.E184. [DOI] [PubMed] [Google Scholar]
- Hjalmarson A., Isaksson O. In vitro work load and rat heart metabolism. 3. Effect on ribosomal aggregation. Acta Physiol Scand. 1972 Nov;86(3):342–352. doi: 10.1111/j.1748-1716.1972.tb05340.x. [DOI] [PubMed] [Google Scholar]
- Hjalmarson A., Isaksson O. In vitro work load and rat heart metabolism. I. Effect on protein synthesis. Acta Physiol Scand. 1972 Sep;86(1):126–144. doi: 10.1111/j.1748-1716.1972.tb00231.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morgan H. E., Chua B. H., Fuller E. O., Siehl D. Regulation of protein synthesis and degradation during in vitro cardiac work. Am J Physiol. 1980 May;238(5):E431–E442. doi: 10.1152/ajpendo.1980.238.5.E431. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Liebermeister H., Morgan H. E. Effect of pressure development on membrane transport of glucose in isolated rat heart. Am J Physiol. 1967 Apr;212(4):815–822. doi: 10.1152/ajplegacy.1967.212.4.815. [DOI] [PubMed] [Google Scholar]
- Peterson M. B., Lesch M. Protein synthesis and amino acid transport in the isolated rabbit right ventricular papillary muscle. Effect of isometric tension development. Circ Res. 1972 Sep;31(3):317–327. doi: 10.1161/01.res.31.3.317. [DOI] [PubMed] [Google Scholar]
- Rabinowitz M., Zak R. Biochemical and cellular changes in cardiac hypertrophy. Annu Rev Med. 1972;23:245–262. doi: 10.1146/annurev.me.23.020172.001333. [DOI] [PubMed] [Google Scholar]
- Rannels D. E., Hjalmarson A. C., Morgan H. E. Effects of noncarbohydrate substrates on protein synthesis in muscle. Am J Physiol. 1974 Mar;226(3):528–539. doi: 10.1152/ajplegacy.1974.226.3.528. [DOI] [PubMed] [Google Scholar]
- Rannels D. E., Kao R., Morgan H. E. Effect of insulin on protein turnover in heart muscle. J Biol Chem. 1975 Mar 10;250(5):1694–1701. [PubMed] [Google Scholar]
- Rubin I. B., Goldstein G. An ultrasensitive isotope dilution method for the determination of L-amino acids. Anal Biochem. 1970 Feb;33(2):244–254. doi: 10.1016/0003-2697(70)90293-9. [DOI] [PubMed] [Google Scholar]
- Schreiber S. S., Evans C. D., Oratz M., Rothschild M. A. Protein synthesis and degradation in cardiac stress. Circ Res. 1981 May;48(5):601–611. doi: 10.1161/01.res.48.5.601. [DOI] [PubMed] [Google Scholar]
- Schreiber S. S., Oratz M., Rothschild M. A. Protein synthesis in the overloaded mammalian heart. Am J Physiol. 1966 Aug;211(2):314–318. doi: 10.1152/ajplegacy.1966.211.2.314. [DOI] [PubMed] [Google Scholar]
- Schreiber S. S., Rothschild M. A., Evans C., Reff F., Oratz M. The effect of pressure or flow stress on right ventricular protein synthesis in the face of constant and restricted coronary perfusion. J Clin Invest. 1975 Jan;55(1):1–11. doi: 10.1172/JCI107899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. M., Sugden P. H. Differential rates of protein synthesis in vitro and RNA contents in rat heart ventricular and atrial muscle. Biochem J. 1983 Aug 15;214(2):497–502. doi: 10.1042/bj2140497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. M., Sugden P. H. Effect of insulin and lack of effect of workload and hypoxia on protein degradation in the perfused working rat heart. Biochem J. 1983 Jan 15;210(1):55–61. doi: 10.1042/bj2100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Smith D. M. The effects of glucose, acetate, lactate and insulin on protein degradation in the perfused rat heart. Biochem J. 1982 Sep 15;206(3):467–472. doi: 10.1042/bj2060467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Smith D. M. The effects of insulin on glucose uptake and lactate release in perfused working rat heart preparations. Biochem J. 1982 Sep 15;206(3):473–479. doi: 10.1042/bj2060473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taegtmeyer H., Hems R., Krebs H. A. Utilization of energy-providing substrates in the isolated working rat heart. Biochem J. 1980 Mar 15;186(3):701–711. doi: 10.1042/bj1860701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K. Studies on myocardial protein metabolism in cardiac hypertrophy. Jpn Heart J. 1966 Nov;7(6):566–589. doi: 10.1536/ihj.7.566. [DOI] [PubMed] [Google Scholar]
- Zak R., Rabinowitz M. Molecular aspects of cardiac hypertrophy. Annu Rev Physiol. 1979;41:539–552. doi: 10.1146/annurev.ph.41.030179.002543. [DOI] [PubMed] [Google Scholar]


