Abstract
This literature-based review aims to distinguish studies describing co-infection with tick-borne pathogens from those describing co-detection or co-exposure scenarios. The review analyzed 426 papers and identified only 20 with direct evidence of co-infection in humans and animals, highlighting the need for accurate terminology and proposing definitions for co-infection, co-exposure and co-detection. Current diagnostic methods - including serology and molecular techniques - have limitations in accurately identifying real co-infections, often leading to misinterpretation. The review highlights the importance of developing laboratory models to better understand tick-borne pathogen interactions, and advocates improved diagnostic strategies for tick screening by testing their RNA for co-infections. Moreover, the establishment of additional animal models for pathogen co-infection will help develop our understanding of selection pressures for various traits of tick-borne pathogens (such as virulence and transmissibility) over time. This comprehensive analysis provides insights into the complexity of tick-borne pathogen co-infections and calls for precise diagnostic terms to improve the clarity and effectiveness of future research.
Keywords: Co-infection, Tick-borne pathogens, Tick-borne disease, Animal model, Diagnostic methods, Pathogen interactions
Graphical abstract
Highlights
-
•
Of 426 papers analyzed, only 20 showed direct evidence of co-infection with tick-borne and other vector-borne pathogens.
-
•
There is a need for precise definitions and terminology for co-infection, co-exposure, and co-detection.
-
•
Diagnostic methods like serology and molecular tests have limitations, leading to potential misinterpretation.
-
•
Better laboratory models are crucial to understanding tick-borne pathogen interactions.
-
•
Enhanced diagnostics, including RNA testing and microbiological cultures, are necessary for accurate co-infection detection.
1. Introduction
“It was the best of times, it was the worst of times, it was the age of wisdom, it was the age of foolishness, it was the age of belief, it was the age of incredulity … ". Dickensʼs famous opening words in “A Tale of Two Cities” can be a useful way to think about the current debate on co-infections transmitted by ticks and other pathogens. Just as Dickens captured the complexity of his time, this quote reflects the conflicting views of the scientific community as to whether we are dealing with co-infections or simply cases of co-detection or co-exposure in both ticks and vertebrate hosts.
Nowadays, tick populations are increasing, and their geographical distribution is expanding, creating a more favorable environment for the pathogens they carry. In addition, climate change may facilitate the movement of tick hosts, potentially leading to the spread of ticks and pathogens into previously uncharted territories (Baneth, 2014). Among the various pathogens that cause tick-borne diseases (TBDs) of significant public health concern, several bacterial species have been found, including Borrelia burgdorferi (sensu lato) (s.l.) (responsible for Lyme borreliosis and those phylogenetically related), Borrelia miyamotoi (causing post-tick bite fever), Rickettsia rickettsii (associated with Rocky Mountain spotted fever), Anaplasma phagocytophilum (causing granulocytic anaplasmosis), and Ehrlichia spp. (associated with ehrlichiosis). In addition, protozoans of the genus Babesia (referred to as Ba. spp., causing babesiosis), Theileria, and Hepatozoon, as well as viruses, including tick-borne encephalitis virus (TBEV) and Crimean-Congo hemorrhagic fever virus, contribute to the significant burden of TBDs (Rocha et al., 2022) in humans and animals. In the USA, the Centers for Disease Control and Prevention (CDC) documented 50,865 cases of TBDs in 2019 (CDC, 2024). Interestingly, the most common human co-infection in the USA is Lyme disease (LD) with babesiosis, whereas in Europe, tick-borne encephalitis (TBE) caused by the TBE virus might co-occur with LD. Researchers in both Europe and the USA have extensively studied ixodid ticks for co-infections with important pathogens such as B. burgdorferi (sensu stricto) (s.s.), A. phagocytophilum, Ba. microti, and Rickettsia spp. In Europe, these co-infections mainly consist of combinations such as B. burgdorferi (s.s.), A. phagocytophilum, and Ba. microti, or B. burgdorferi (s.s.) with A. phagocytophilum, or A. phagocytophilum with Rickettsia spp. In the USA, co-infections are mainly with B. burgdorferi (s.s.) together with either A. phagocytophilum or Ba. microti (Rocha et al., 2022).
The co-occurrence of multiple pathogens in ticks was first documented in the 1970s, when researchers observed the coexistence of arboviruses such as TBE and Uukuniemi (UUK) in Norwegian Ixodes ricinus ticks (Rocha et al., 2022). The co-occurrence of TBDs is widespread, and often characterized by overlapping geographical distributions. Several aspects influence the ecology, like reservoir host dynamics (mainly wild rodents), host-pathogen interactions, tick population dynamics, tick feeding patterns (generalists or specialists), vector competence, and pathogen virulence. These factors can lead to differences in the likelihood of ticks acquiring infections when multiple pathogens are present. Co-infections involving both B. burgdorferi (s.s.) and A. phagocytophilum are frequently observed in humans, pets, wildlife, and ticks, but our understanding of the mechanisms and ecological aspects of such co-infections remains limited compared with single-agent infections (Nieto and Foley, 2009).
Accurately estimating the prevalence of multiple infections versus single infections is difficult due to limited case reports. It is unclear whether multiple infections worsen disease compared with single infections such as the TBE virus or Borrelia. This knowledge gap hinders medical care, especially in patients with both TBE and Borrelia infections. There is an urgent need to study how these organisms interact in ticks, and their impact on host health after transmission (Bröker, 2012). Indeed, on the one hand, serological evidence of exposure does not necessarily mean co-infection of vertebrate hosts, which are often reported in the literature as seropositive but not PCR-positive. For this reason, it is important to note that being seropositive does not necessarily mean that the hosts had both diseases at the same time (Nieto and Foley, 2009). On the other hand, it is crucial to understand that the detection of pathogens in ticks found to be co-infected through molecular techniques does not necessarily indicate that the pathogens are alive in the tick. Indeed, recent research using molecular techniques has shown that 50% of infected ticks are co-infected with multiple pathogens (Moutailler et al., 2016), but does not confirm whether these pathogens are alive in the tick at the same time. Nowadays there is a large common misuse of the term “co-infection”, which is often incorrectly applied in cases that are more accurately described as “co-detection” or “co-exposure”. It is therefore important to define the meaning of co-infection, co-exposure, and co-detection. Co-infection specifically refers to the active growth and proliferation of multiple pathogens within a host, potentially worsening the severity or duration of the diseases involved. In contrast, co-exposure indicates that a host has encountered multiple pathogens, as evidenced by the presence of antibodies, without implying an active infection. Co-detection, on the other hand, simply involves the identification of DNA or proteins from different pathogens through molecular techniques, without confirming whether these pathogens are alive or causing an active infection (Table 1). By clearly defining each term, the aim of this literature-based review is not simply to list co-infections, but to distinguish studies that present co-infections with tick-borne pathogens (TBPs) and other pathogens from those that describe co-detection or co-exposure scenarios. This distinction is crucial to ensure clarity and accuracy in discussions about TBPs interactions within hosts. In addition, given the increasing incidence of co-infection in ticks and vertebrate hosts, this review will also assess the need to develop and study laboratory models in which conditions can be precisely controlled. With careful consideration, these models can be instrumental in elucidating the complex interactions between TBPs in both ticks and vertebrate hosts.
Table 1.
Definitions for various interpretations of co-infection, co-exposure, and co-detection.
| Term | Definition |
|---|---|
| Co-infection | Co-infection refers to the simultaneous development and proliferation of multiple pathogens within the host, which may exacerbate the severity and duration of one or both diseases (Segen, 2011). |
| Co-exposure | Co-exposure is defined as the presence of antibodies to multiple pathogens in a host, which may occur as a result of simultaneous or sequential exposure. |
| Co-detection | Co-detection occurs when DNA/proteins of different pathogens are identified or detected by molecular techniques without knowing if the pathogens are alive or dead. |
2. Materials and methods
2.1. Literature search process
To differentiate studies documenting cases of co-infections in ticks and vertebrate hosts from cases of co-detection or co-exposure, we applied the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist (Liberati et al., 2009) (Supplementary Table S1).
The systematic review included all studies that focused on co-infection and TBPs. The search process for our research was carried out using articles that were available between 1981 and December 2022. Specifically, electronic searches on the Scopus and PubMed databases using the search terms “co-infection” AND “tick-borne pathogens” were conducted. The “Article title, Abstract, Keywords” option to collect all papers that contained the search terms in their title, abstract or keywords were used. The collected information was screened and extracted by the same person (SP), and the papers were listed in Mendeley software after removing duplicates.
2.2. Selection process
The selected studies were based on the following inclusion criteria: journal articles written in English and focusing on co-infection in TBPs. Therefore, all studies on co-infection in animals, ticks, humans, and laboratory animals were considered. The exclusion criteria included papers focusing only on serology and/or molecular detection by DNA in ticks and vertebrate hosts (this criterion applies to the final screening to distinguish between direct and indirect evidence, but was not considered during the initial selection process), papers written in a language other than English, review articles, short communications, surveys or reports except those discussing human co-infections, irrelevant papers on the subject, and papers that are currently inaccessible.
The selection process for the studies involved three steps. First, the title and abstract were screened, excluding papers with irrelevant titles and identifying those of legitimate interest. Next, the selected papers were thoroughly read, distinguishing between ‘direct’ and ‘indirect’ evidence of co-infections. Different criteria were used to categorize co-infection papers as providing direct or indirect evidence (Table 2). Direct evidence papers were defined as those identified as co-infection, while those providing indirect evidence were cases of co-detection or co-exposure. Co-infections were considered to be direct evidence in animals and humans when papers used diagnostic tests, such as molecular techniques and/or serological tests, in conjunction with a clinical examination. Conversely, papers on animals and humans that used only diagnostic tests without a clinical examination were classified as providing indirect evidence of co-detection or co-exposure. In contrast, direct observation of pathogens by microscopy or microbiological bioassays cultures, without relying on the above criteria, provides direct evidence and confirms active co-infection. In addition, papers on co-infection in ticks were considered direct evidence if they used RNA or mRNA detection methods, alongside microbiological cultures. Conversely, papers that relied solely on DNA detection in ticks were classified as indirect evidence (Table 3).
Table 2.
Criteria used to classify papers on co-infection in animals and humans as providing either direct or indirect evidence.
| Diagnostic assays |
Clinical findings consistent with TBDs |
Co-infection classification |
|||
|---|---|---|---|---|---|
| Molecular techniques | Serology tests | Yes | No | Direct evidence | Indirect evidence |
| ✔ | ✔ | ✔ | |||
| ✔ | ✔ | ✔ | |||
| ✔ | ✔ | ✔ | ✔ | ||
| ✔ | ✔ | ✔ | ✔ | ||
| ✔ | ✔ | ✔ | |||
| ✔ | ✔ | ✔ | |||
Notes: Direct observation of pathogens by microscopy or microbiological bioassays cultures, without relying on the above criteria, provides direct evidence and confirms active co-infection. The ticks in the table indicate the presence of diagnostic tests, clinical findings, or evidence related to TBDs, with each tick corresponding to specific categories of assays, findings, or evidence types.
Table 3.
Criteria used to classify papers on co-infection in ticks as providing either direct or indirect evidence. Structured.
| Molecular techniques |
Co-infection classification |
||
|---|---|---|---|
| DNA detection | RNA/mRNA detection | Direct evidence | Indirect evidence |
| ✔ | ✔ | ||
| ✔ | ✔ | ||
Both direct and indirect evidence were considered eligible in the review, but only articles considered to report ‘direct’ evidence were analyzed in detail. Articles classified as reporting indirect evidence were consulted to get a general idea of the number of articles available in the literature and to understand the limitations of these studies.
2.3. Data collection process
The different papers were collected in an Excel sheet designed to systematically record the information. The papers were categorized based on their titles, authors, and whether they provided ‘direct’ or ‘indirect’ evidence in different areas, including the section “Co-infection in animals and ticks”, “Co-infection in humans”, and “Co-infection in laboratory animal models and artificial infection of ticks”.
Additional information regarding examples with more details on indirect and direct evidence for each section was compiled into individual tables (Supplementary Table S2).
3. Results
3.1. Study selection
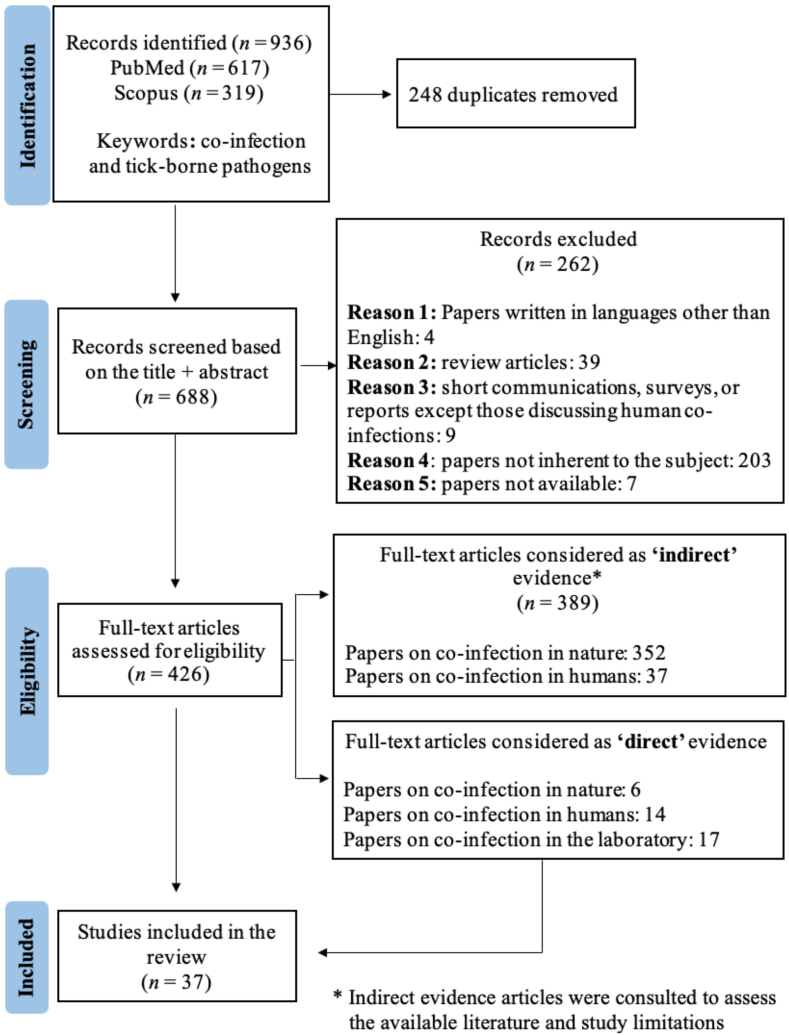
The searches on PubMed and Scopus returned 936 records. At the end of the selection process, 37 remained for the analysis. Of these, 14 papers focus on co-infection in humans, 6 on co-infection in animals, and 17 on co-infection in laboratory animal models (Fig. 1).
Fig. 1.
PRISMA flowchart for selecting articles to review in line with the diagram according to Liberati et al. (2009).
3.2. Co-infection in animals and ticks
Among the 426 papers evaluated for their eligibility, only six provided direct evidence of co-infection in animals, while 207 provided indirect evidence in animals and 145 in ticks in a natural environment. Despite the use of ticks to monitor TBPs, all 145 cases related to ticks were indirect, with no direct evidence of co-infection found. This lack of direct evidence makes it difficult to assess the prevalence of co-infection in ticks in the natural environment. In addition, only two papers in the literature used the term “co-detection” when looking for co-infection of TBPs in ticks (Holden et al., 2006; Beristain-Ruiz et al., 2022), highlighting the frequent inappropriate use of the term “co-infection” to describe cases of co-detection, and further emphasizing the lack of direct evidence of co-infection.
In this section, only the six papers on real co-infection are analyzed to assess the challenges and impact of co-infection for animals in their natural environment (Table 4).
Table 4.
Summary of co-infection studies presenting direct evidence. All reported co-infection studies included clinical examinations.
| Reference | Country | No. of samples evaluated | Animal species | Diagnostic method |
|---|---|---|---|---|
| Abas et al. (2021) | Egypt | 670 | Cattle/buffalo | ‣Molecular: RT-qPCR to confirm FMD and LSD; |
| ‣Examination of blood samples (blood smears) for Babesia spp., Theileria spp., and Anaplasma spp.; | ||||
| ‣Lymph node biopsy examination: Theileria spp. | ||||
| Andersson et al. (2017) | Sweden | 71 | Cattle | ‣Molecular: Real-time PCR |
| Al-Hosary et al. (2020) | Egypt | 394 | Cattle/buffalo | ‣Microscopical examination; |
| ‣Serological tests: ELISA for detection of antibodies of A. marginale; | ||||
| ‣Molecular: Real-time PCR: A. marginale; RLB + PCRs: Theileria spp., Babesia spp., Anaplasma spp., Ehrlichia spp., Rickettsia spp. and Midichloria mitochondrii; | ||||
| ‣Blood smears | ||||
| Attipa et al. (2018) | Cyprus | 50 with clinical leishmaniosis | Dogs | ‣Molecular: qPCR + ELISA: Leishmania infantum; PCR: Ehrlichia/Anaplasma. spp., Babesia spp., Hepatozoon spp. |
| Kordick et al. (1999) | USA | 27 | Dogs | ‣Serological: Microimmunofluorescence test for antibodies against Ehrlichia canis, Ehrlichia chaffeensis Ark, Ehrlichia equi NY, Rickettsia rickettsii Domino, Babesia canis, Bartonella vinsonii subspecies berkhoffii 93-CO-1; |
| ‣Molecular: PCR for the detection of Ehrlichia canis, Babesia canis, Rickettsia spp., Bartonella spp. | ||||
| Bouzouraa et al. (2016) | Mediterranean Basin | 28 | Dogs | ‣Molecular: Multiplex PCR for the detection of Ehrlichia canis, Anaplasma platys, Anaplasma phagocytophilum, Babesia spp., Theileria spp., Hepatozoon spp., and/or Leishmania spp. |
3.2.1. Challenges in diagnosing animal co-infections
The six papers classified as cases of co-infection in animals have highlighted that the main challenge in treating and preventing the possible consequences of co-infection in animals is to establish the correct diagnosis. Yet diagnosis can be complicated by several limitations, including incomplete medical records due to data being collected by different clinicians, leading to gaps such as missing data, and potential oversights during brief skin examinations. The lack of a control group can make comparisons difficult, and not all known pathogens are tested, which may lead to missed co-infections. In addition, other infections or diseases may influence the results, and the inclusion of cases from different regions and time periods may introduce variability in the results (Bouzouraa et al., 2016).
The optimal approach would be to use molecular and serological techniques in addition to clinical examinations for early and accurate diagnosis to facilitate appropriate treatment. Kordick et al. (1999), used serological and molecular techniques to reveal that dogs in the Walker Hound kennel could be co-infected with multiple TBPs. Indeed, dogs sero-reactive to Ehrlichia canis antigen were found to be co-infected with multiple Ehrlichia species by PCR analysis of blood samples. In addition, the study by Al-Hosary et al. (2020) which involved co-infection of cattle and buffalo with piroplasms and other Anaplasmataceae through clinical examination and diagnostic methods (Table 4), highlighted differences in sensitivity between different detection methods, including Giemsa-stained blood smears, PCR-based techniques, ELISA, reverse line blot, and real-time PCR. While ELISA showed high sensitivity but low specificity due to its reliance on antibody detection, the reverse line blot showed high sensitivity and specificity. Real-time PCR proved to be the most sensitive assay for the detection of Anaplasma marginale, especially in carrier states, revealing co-infections of A. marginale and pathogens such as Theileria annulata, Babesia bovis, Babesia bigemina, Babesia occultans, and Anaplasma platys in both cattle and buffaloes. Specifically, co-infection with T. annulata was observed in 49 cattle (15.9%), while co-infections with Ba. bovis, Ba. bigemina, and Ba. occultans were detected in 18 (5.8%), 2 (0.7%), and 1 (0.3%) cattle, respectively. Additionally, co-infection with A. marginale and A. platys was found in 26 cattle (8.4%). Among buffaloes, co-infection with A. marginale and T. annulata was recorded in 1 buffalo (1.18%), with Ba. bigemina - in 2 buffaloes (2.35%), and with A. platys - in 4 buffaloes (4.71%) (Al-Hosary et al., 2020).
3.2.2. Impact on the severity of symptoms in animal co-infection
Finally, given all the diagnostic challenges mentioned above, conflicting results have been reported on the actual impact of co-infection on the severity of symptoms in animals. For instance, one study showed that dogs with clinical leishmaniasis (Table 4) were significantly more susceptible (12 times more) to being co-infected with E. canis than healthy dogs. Moreover, they could exhibit severe clinical and hematological signs beyond those seen with a single infection. Unfortunately, the exact mechanisms underlying the synergistic effect of these co-infections remain poorly understood (Attipa et al., 2018). In addition, viral and bacterial infections can sometimes predispose animals to subsequent infections with TBPs, thus increasing the complexity of clinical presentations of co-infections. For example, one study showed that cattle with lumpy skin disease, along with clinical signs of babesiosis and anaplasmosis, had higher rates of hemoprotozoal infection, resulting in more severe outcomes (Abas et al., 2021). Conversely, another study found that out of 28 dogs that tested positive for A. platys both clinically and via PCR, 13 were co-infected with at least one other vector-borne pathogen. Among these 13 co-infected dogs, one was infected with A. platys, E. canis, and Babesia spp., while the remaining 12 were co-infected with one additional vector-borne pathogen. This included E. canis (2 dogs), Leishmania spp., Babesia vogeli (1 dog), and Hepatozoon spp. (5 dogs) (Bouzouraa et al., 2016).
However, this did not significantly affect the clinical presentation of A. platys, supposing that the severity of A. platys may be influenced by factors such as concurrent diseases, genetic factors, immune status, and the physical condition of individual dogs (Bouzouraa et al., 2016). Furthermore, even in 18% of cattle diagnosed by clinical signs with babebiosis and co-infected with A. phagocytophilum confirmed by PCR, there was no increased mortality, suggesting that the co-infection did not exacerbate disease severity (Andersson et al., 2017). Nevertheless, to effectively manage and prevent the potential impact of co-infection on disease severity, the first task is to establish an appropriate diagnosis (Al-Hosary et al., 2020; Abas et al., 2021).
In conclusion, ticks are important vectors of multiple pathogens, posing risks to both animals and humans (Backus et al., 2022). Although direct evidence of co-infection in animals is limited, and no direct evidence was found in ticks in the reviewed papers due to the detection of DNA rather than RNA/mRNA, numerous cases of indirect evidence highlight the complexity of diagnosing and managing these co-infections. Accurate diagnosis requires a combination of molecular and serological methods, in addition to clinical examination, due to the various diagnostic challenges described above. Moreover, the impact of co-infections on disease severity in animals remains unclear, with studies showing conflicting results. This scenario also applies to human health, where co-infections can similarly complicate diagnosis and treatment.
3.3. Co-infection in humans
A single tick carrying multiple pathogens, several simultaneous tick bites, or two successive tick bites occurring closely in time, each transmitting a different pathogen, can cause co-infection in humans (Belongia, 2002). Among the 426 papers reviewed, a total of 51 papers recorded co-infection in humans, 14 presenting direct evidence and 37 - indirect evidence. In this section, only 14 papers of co-infection (Table 5) are analyzed to assess the challenges and impact of co-infection in humans. In the review of these papers, the criteria given by European guidelines or by the CDC were used to define confirmed cases of TBE, HGA, and LD (Supplementary Table S3). However, most of the 37 indirect papers reviewed described cases of co-detection or co-exposure.
Table 5.
Summary of co-infection studies in humans presenting direct evidence. All reported co-infection studies included clinical examinations.
| Reference | Country | No. of samples evaluated | Diagnostic method |
|---|---|---|---|
| Borawski et al. (2019) | Poland | 704 | ‣Serological: Antibodies against Rickettsia, TBE, Borrelia burgdorferi; |
| ‣Molecular: PCR: Rickettsia spp. Borrelia burgdorferi, Anaplasma phagocytophilum | |||
| Boyer et al. (2018) | France | 1 | ‣Serological: Detection of anti-TBEv IgG in paired sera + presence of anti-TBEv IgM in the first serum (ECDC confirmed TBE case criteria); |
| ‣ELISA + Western Blot: Borrelia burgdorferi (s.l.) | |||
| Diallo et al. (2017) | Senegal | 1 | ‣Malaria rapid diagnostic test for Plasmodium falciparum; |
| ‣Blood smear: Borrelia + Plasmodium falciparum | |||
| Dong et al. (2013) | China | 30 | ‣Serological: IFA for Anaplasma phagocytophilum and Ehrlichia chaffeensis; |
| ‣Molecular: real-time PCR, nested PCR for Anaplasma phagocytophilum and Ehrlichia chaffeensis | |||
| Dumic et al. (2021) | USA | 1 | ‣Serological: Borrelia burgdorferi detection of anti-IgM and anti-IgG in serum; detection of IgM in CSF and plaque reduction neutralization test against POWV; |
| ‣Molecular: PCR for Borrelia burgdorferi in CSF | |||
| Dunaj et al. (2018) | Poland | 118 | ‣Serological: ELISA, Western blot for Borrelia burgdorferi (s.l.) and/or TBEV on serum and CSF; |
| ‣Molecular: PCR for Borrelia burgdorferi (s.l.), Anaplasma phagocytophilum and Babesia spp.; | |||
| ‣Blood smear: Anaplasma phagocytophilum spp. and Babesia spp. | |||
| Gęgotek et al. (2022) | Poland | 21 | ‣TBE diagnosed according the European Academy of Neurology; |
| ‣Lyme disease and Lyme neuroborreliosis diagnosed according to Stanek et al., (2011) | |||
| ‣HGA diagnosed according to the case definition by Dahlgren et al., (2015) (see Supplementary Table S3) | |||
| Groth et al. (2022) | Poland | 22 | ‣TBE diagnosed according the European Academy of Neurology; |
| ‣Lyme disease and Lyme neuroborreliosis diagnosed according to Stanek et al., (2011) (see Supplementary Table S3) | |||
| Liu et al. (2019) | China | 180 | ‣Serological: IFA on serum for TBE, Borrelia burgdorferi, Rickettsia heilongjiangensis, Anaplasma phagocytophilum; |
| ‣Molecular: Nested PCR for Borrelia burgdorferi, Rickettsia spp., Babesia spp.; | |||
| ‣Blood smear: Anaplasma phagocytophilum spp., Babesia spp. | |||
| Moniuszko-Malinowska et al. (2021) | Poland | 120 | ‣Serological: IFA for antibodies detection against Anaplasma phagocytophilum; ELISA for anti-Borrelia burgdorferi (s.l.) antibodies and TBEV antibodies; |
| ‣Molecular: PCR for Anaplasma phagocytophilum, Babesia spp., Borrelia burgdorferi (s.l.), Bartonella spp., Coxiella burnetii, “Candidatus Neoehrlichia mikurensis”; | |||
| ‣Blood smear | |||
| Moniuszko et al. (2014) | Poland | 110 | ‣Serological: ELISA for anti-Borrelia burgdorferi (s.l.) antibodies and TBEV antibodies on serum and CSF; |
| ‣Molecular: PCR for Anaplasma phagocytophilum, Babesia spp., Borrelia burgdorferi (s.l.); | |||
| ‣Blood smear for Anaplasma phagocytophilum, Babesia spp. | |||
| Primus et al. (2018) | USA | 192 | ‣Serological: ELISA for antibodies of B. burgdorferi (s.l.); |
| ‣Molecular: Multiplex qPCR for the detection of Borrelia burgdorferi (s.l.), Babesia microti | |||
| Tijsse-Klasen et al. (2013) | Croatia | 1 | ‣Eritema migrans skin biopsy; ‣Molecular: PCR for Rickettsia spp., duplex qPCR for Borrelia burgdorferi (s.l.) |
| Wormser et al. (2019) | USA | 52 | ‣Serological: IFA for antibodies to Anaplasma phagocytophilum, Babesia microti, Babesia miyamotoi, POWV; |
| ‣Molecular: PCR for the dection of Babesia microti; | |||
| ‣Blood smear for Anaplasma phagocytophilum, Babesia microti |
3.3.1. Overlap of clinical signs
Diagnosing TBDs is challenging enough on its own, but when multiple infections occur together, it becomes even more complicated. The presence of co-infections - whether bacterial, viral, or both - can make the initial clinical presentation following a tick bite more confusing and difficult to identify compared to those in single infections. These overlapping symptoms can lead to delays and difficulties in making an accurate diagnosis based on clinical presentation only and underline the need for laboratory testing for TBPs (Diallo et al., 2017; Boyer et al., 2018; Dunaj et al., 2018; Gęgotek et al., 2022). For example, in Poland infections and co-infections were investigated in patients hospitalized with non-specific symptoms after tick bites. They found no significant differences in symptoms between patients with and without confirmed infections. Co-infections, mainly with B. burgdorferi (s.l.) and A. phagocytophilum, were observed in some cases. The most prevalent co-infection was between B. burgdorferi (s.l.) and A. phagocytophilum, occurring in 4.2% (5/118) of the instances (Dunaj et al., 2018). In Europe, many TBE patients were co-infected with Borrelia spp. (27% or 30/110), A. phagocytophilum (10.9% or 12/110), and Babesia spp. (0.9% or 1/110), with triple co-infections occurring in 2.7% (3/110) of patients (Moniuszko et al., 2014).
A first French case of co-infection with TBEV and B. burgdorferi (s.l.), was a document by Boyer et al. (2018), where the patient exhibited symptoms of both TBE and Lyme borreliosis, making diagnosis challenging due to the overlapping clinical manifestations and highlighting the difficulties physicians face in accurately distinguishing between such illnesses only relying on clinical presentation. Normally, TBE progresses from an initial viral-like phase to a second phase of neurological symptoms, but the patient did not progress to the second phase, making clinical diagnosis difficult. Additionally, a tick bite lesion did not show erythema migrans, a common sign of LD. However, laboratory tests confirmed TBE (meeting the ECDC criteria for a TBE case) with the anti-TBEv IgG in paired sera and the presence of anti-TBEv IgM in the first serum and despite a positive PCR test for B. burgdorferi (s.l.), following initial presentation, the patient remained asymptomatic for six years without antibiotic treatment, suggesting spontaneous recovery and improvement without specific intervention (Boyer et al., 2018).
Another challenge is when a patientʼs symptoms suggest only one infection, but there are actually multiple infections. For example, a patient infected with B. afzelii had skin lesions typical of LD, but no symptoms of a coexisting Rickettsia infection (Tijsse-Klasen et al., 2013). This situation can easily lead to a missed infection. In addition, in regions such as West Africa, the overlap between diseases such as tick-borne relapsing fever (TBRF) and malaria can make it particularly difficult to diagnose co-infections. Often, physicians focus on the more common disease, such as malaria, and overlook the presence of TBRF, which can lead to inappropriate treatment. Even though TBRF is common, it is often overshadowed by malaria, leading to underreporting (Diallo et al., 2017).
All of these studies highlight the need for greater awareness regarding the frequency of co-infections by TBPs and for exploring the presence of multiple infections in patients with TBDs or exposed to TBPs.
3.3.2. Diagnostic approach using serology and molecular methods
Given the challenges of diagnosing co-infections solely on clinical presentation, especially when symptoms are ambiguous, and antibodies are not yet detectable, advanced diagnostic techniques are essential. PCR tests, which identify pathogen DNA, have proven effective in the early detection of diseases such as LD and TBE (Moniuszko et al., 2014). For example, Dong et al. (2013) developed a duplex real-time PCR assay in China to analyze DNA from blood samples of patients with human granulocytic anaplasmosis and human monocytic ehrlichiosis during the acute phase. This assay, confirmed by serological testing showing a 4-fold increase in IgG titer in both acute and convalescent serum samples, demonstrated superior efficiency and sensitivity compared to nested PCR. It excelled in the acute detection and differentiation of these emerging TBPs (A. phagocytophilum and Ehrlichia chaffeensis). This advancement offers significant potential for improving detection in various sample types, particularly for timely screening of clinical samples from patients with undiagnosed febrile illness and field samples from endemic areas (Dong et al., 2013).
Similarly, researchers have developed methods such as multiplex qPCR, which allows the simultaneous detection of multiple infections even before the immune system has responded (Primus et al., 2018). Such techniques are critical for early detection and timely treatment, improving the management of infections.
Newer methods, such as the analysis of blood lipids and proteins, are also showing promise. Research suggests that individuals infected with TBE alone have different blood lipid and protein profiles than those with co-infections (TBE + LD). These differential profiles could pave the way for the development of new, more sensitive diagnostic tools tailored to the complexities of co-infections (Gęgotek et al., 2022; Groth et al., 2022) as well as for personalized treatment strategies for patients with multiple infections.
Despite these advances, the impact of co-infections on symptom severity remains uncertain. Multiple infection studies show contrasted effects on diseases severity (Dunaj et al., 2018; Wormser et al., 2019; Dumic et al., 2021). For example, a study in Poland found high levels of anti-Rickettsia antibodies among local residents. In particular, eight patients were co-infected with B. burgdorferi and TBEV and presented with symptoms such as erythema migrans, neuroborreliosis, and musculoskeletal problems. Interestingly, despite the known presence of rickettsiae in the environment, Rickettsia spp. DNA was not detected in 540 patients hospitalized after tick bites, raising questions about the rarity of reported symptomatic cases. However, despite this high seroprevalence, symptomatic infections with Rickettsia spp. remain rare in the region, highlighting a discrepancy between exposure and clinical manifestation that warrants further investigation (Borawski et al., 2019). In addition, research by Moniuszko-Malinowska et al. (2021) suggests that co-infection with A. phagocytophilum and either B. burgdorferi (s.l.) or TBEV may indicate interactions between these pathogens that could influence clinical presentation. Their findings indicate that no severe cases of the disease were observed, and that symptoms vary between single infections and co-infections, implying that the pathogens might influence each other. Specifically, among 120 patients with A. phagocytophilum infection, 20 (16.7%) were also infected with TBEV, 1 (0.83%) with Babesia spp. and 40 (33.3%) with B. burgdorferi (s.l.) (Moniuszko-Malinowska et al., 2021).
To address these uncertainties, laboratory animal models could offer valuable insights into how co-infections interact to influence disease severity. Such research may be crucial for advancing our understanding of co-infections and improving the diagnosis and treatment of individuals.
3.4. The role of laboratory animals and artificial infection of ticks in studying co-infection
In order to understand the impact of co-infection on the severity of symptoms and the interaction between TBPs in ticks and vertebrate hosts, laboratory animals have been essential. Various animals, from mice to larger species such as dogs, can be infected for research purposes (Table 6).
Table 6.
Summary of animal laboratory models and artificial infection of ticks to investigate TBDs.
| References | Co-infection agents | Infectious dose | Animal model | Objective |
|---|---|---|---|---|
| Akoolo et al. (2021) | B. burgdorferi + Ba. microti | Ba. microti: 104 infected RBCs/mouse i.p.; | C3H/HeN mice; C3H/HeJ mice | Examine TLR4 signaling in B. burgdorferi and Ba. microti infections |
| B. burgdorferi: 103 spirochetes s.c. | ||||
| Djokic et al., 2019 | B. burgdorferi + Ba. microti | Ba. microti: 104 infected RBCs/mouse i.p.; | C3H/HeJ mice | Evaluate spleen responses and Ba. microti effects on B. burgdorferi clearance |
| B. burgdorferi: 103 spirochetes s.c. | ||||
| Djokic et al. (2018) | B. burgdorferi + Ba. microti | Ba. microti: 104 infected RBCs/mouse i.p.; | C3H/HeN mice | Evaluate co-infectionʼs effect on splenic response and parasitemia in C3H mice |
| B. burgdorferi: 103 spirochetes s.c. | ||||
| Gaunt et al. (2010) | A. platys + E. canis | A. platys: blood from a splenectomized dog that had been injected with the A. platys isolate 10 days earlier; | Dogs | Assess co-infectionʼs impact on blood, infection duration, and doxycycline treatment in dogs |
| E. canis isolate: blood from another dog in Louisiana exhibiting symptoms such as fever and thrombocytopenia | ||||
| Genné et al. (2021) | B. afzelii strains NE4049 + Fin-Jyv-A3 | Infection of mice by tick bite using I. ricinus nymphs | BALB/c mice | Investigate B. afzelii strain interactions, tissue abundance, and tick transmission |
| Genné et al. (2018) | B. afzelii strains NE4049 + Fin-Jyv-A3 | Infection of mice by tick bite using I. ricinus nymphs | BALB/c mice | Test B. burgdorferi strain competition in rodents and ticks |
| Hart et al. (2022) | B. burgdorferi + POWV | B. burgdorferi: 3×108 cells/ml; | Adult ticks (capillary feeding) | Model unfed, questing adult ticks after blood digestion and molting to study pathogen interactions at this transmission stage |
| POWV: 2.32×105 FFU/ml | ||||
| Paulsen et al. (2019) | TBEV + A. phagocytophilum | TBEV: 6.5×106 FFU/ml; A. phagocytophilum: 106 infected cells |
Lambs | Study effects of TBEV and A. phagocytophilum co-infection in lambs |
| Rynkiewicz et al. (2017) | B. burgdorferi BL206 + LG734 | Infection of mice by tick bite using I. ricinus nymphs | C3H/HeNCr1 mice | Compare transmission between genotypes, assess host-to-tick transmission, and model persistence |
| Sallay et al. (2017) | R. helvetica + B. afzelii | R. helvetica: 8×104 i.p.; | C3H/N and BALB/c mice | Identify animal model and evaluate bacterial co-infection transmission |
| B. afzelii: 103 spirochetes s.c. | ||||
| Thomas et al. (2001) | B. burgdorferi + A. phagocytophilum | B. burgdorferi: 103 intradermal; | C3H/HeN mice | Investigated B. burgdorferi and A. phagocytophilum agent effects on infection, transmission, and Lyme arthritis |
| A. phagocytophilum: 100 μl of HGE-infected C3H-scid blood i.p. | ||||
| Zafar et al. (2022) | Ba. microti + Ba. rodhaini | Ba. microti: 107 infected RBCs i.p.; | BALB/c mice | Highlighted importance of studying acute co-infections and immune dynamics |
| Ba. rodhaini: 107 infected RBCs i.p. | ||||
| Coleman et al. (2005) | Ba. microti + B. burgdorferi | B. burgdorferi: 103 spirochetes s.c.; | BALB/c and C3H/HeN mice | Compared co-infection effects on parasitemia, splenomegaly, and Lyme disease severity |
| Ba. microti: 105 infected RBCs/0.2 ml i.p. | ||||
| Holden et al. (2005) | B. burgdorferi + A. phagocytophilum | B. burgdorferi: 103 spirochetes s.c.; | C3H/HeN and C3H/Smn.CIcr-scid mice | Determine the effect of A. phagocytophilum on subsequent B. burgdorferi infection |
| A. phagocytophilum: blood from infected SCID mice | ||||
| Moro et al. (2002) | B. burgdorferi + Ba. microti | B. burgdorferi: 104 spirochetes s.c.; | BALB/c and C3H/HeJ mice | Investigate co-infectionʼs synergistic pathogenic effects using a mouse model |
| Ba. microti: 107Ba. microti-infected hamster RBCs | ||||
| Zeidner et al. (2000) | B. burgdorferi + A. phagocytophilum | Infection of mice by tick bite | C3H/HeJ mice | Determine if human granulocytic ehrlichiosis co-infection induces a Th2 cytokine response in mice |
| Maaz et al. (2016) | H. polygyrus + B. afzelii | H. polygyrus: 250 L3 larvae via oral gavage; | C57BL/6JRj, BALB/c/cJRj, and C3H/HeNRj mice | Investigate nematode-tick co-infection effects on immune responses and transmission |
| B. afzelii-infected I. ricinus nymphs |
Abbreviations: RBCs, red blood cells; i.p., intraperitoneal; s.c., subcutaneous.
3.4.1. Dogs as a model for natural co-infection scenarios
Dogs are particularly valuable in co-infection studies because they often encounter multiple pathogens in regions with high vector diversity and are exposed to the same pathogens as their owners.
As described above, co-infected dogs in natural environments may present with unusually severe or atypical clinical signs. This makes them an ideal model for studying complex disease interactions.
In a study by Gaunt et al. (2010), experimental co-infections with A. platys and E. canis demonstrated that co-infection in dogs led to more severe anemia and thrombocytopenia than infection with a single pathogen. The study highlights how co-infection can alter the dynamics of the disease, demonstrating how this results in prolonged infection and more severe clinical outcomes (Gaunt et al., 2010).
3.4.2. Insights on co-infection dynamics from mouse models
Several in vivo laboratory studies have used different strains of mice to investigate the consequences of co-infection with Borrelia spp. and A. phagocytophilum or Babesia spp., to extrapolate these findings to consider explanations for the similar natural reservoirs, vectors and geographical distribution of these pathogens, and for differences in disease severity (Thomas et al., 2001; Moro et al., 2002; Coleman et al., 2005; Holden et al., 2005). Studies in susceptible C3H mice co-infected with combinations of Ba. microti, B. burgdorferi, and A. phagocytophilum have provided important insights into the impact of vertebrate co-infection on important factors such as transmission, pathogenicity, and the influence of pathogen populations on the host immune response, thereby affecting disease outcomes. The most commonly used mouse strains for bacterial-parasite co-infection experiments are BALB/c and C3H/HeN or C3H/HeJ.
In co-infection models, the interaction between B. burgdorferi and Ba. microti in C3H mice demonstrates a noteworthy escalation in spirochete levels across various organs. This simultaneous infection not only prolongs and exacerbates inflammatory LD manifestations due to Ba. microti-induced immunosuppression but also leads to a significant rise in spirochete burden (Djokic et al., 2018, Djokic et al., 2019). Furthermore, Toll-like receptor 4 (TLR4) has been shown to indirectly influence the immune response and pathology during B. burgdorferi infection. In C3H/HeJ mice lacking functional TLR4, there was increased TLR2 signaling due to the abundance of lipoproteins on the surface of spirochetes. This led to a more pronounced inflammatory response in B. burgdorferi-infected and co-infected C3H/HeJ mice compared to C3H/HeN mice. In fact, C3H/HeJ mice co-infected with Ba. microti developed more severe inflammatory arthritis than those infected with either pathogen alone (Akoolo et al., 2021).
Moreover, the dynamics of spirochete-parasite interactions also result in a reduction of splenic B and T cells, lower antibody levels, and impaired humoral immunity, compared with those infected with B. burgdorferi alone, highlighting the role of innate immune receptors in modulating disease severity during co-infection (Djokic et al., 2019; Akoolo et al., 2021).
However, conflicting results exists. For example, Coleman et al. (2005) and Moro et al. (2002) reported that co-infection with babesiosis and LD pathogens did not result in significant changes or increased disease severity in certain mouse strains, such as C3H/HeN and BALB/c.
Indeed, this suggests that the impact of co-infection can vary significantly depending on the hostʼs genetic background and the specific pathogens involved.
3.4.3. Immune responses in co-infected mice with TBPs and parasites
As the impact of co-infection has been described in animals and humans, understanding how pathogens interact with each other and with the host immune system is critical to elucidating disease severity in mouse models. These complex dynamics not only influence the course of individual infections but also significantly alter overall clinical outcomes. For example, co-infection with A. phagocytophilum and B. burgdorferi (s.s.) exacerbate Lyme arthritis and has shown notable immune profile changes in mice. These changes include proliferation of splenic B and CD4 T cells, but a decrease in CD8 T cells, illustrating shifts in immune cell populations. This immune shift is accompanied by increased levels of IL-4 and decreased levels of IFN-γ and IL-2 cytokines. Co-infection also results in increased pathogen loads of both A. phagocytophilum and B. burgdorferi (s.s.), increasing the severity of Lyme arthritis compared to single infections (Thomas et al., 2001). Further, co-infection leads to decreased levels of IL-12, IFN-γ, and TNF-α, increased IL-6 production, and suppressed macrophage activation. Interestingly, while the Lyme spirochete load increased in various tissues of co-infected mice, the A. phagocytophilum load remained unaffected (Holden et al., 2005).
Co-infection not only with TBPs but also with parasites can affect host immunity, which in turn can alter the outcome of infection. In a mouse model involving co-infection with Ba. microti and Ba. rodhaini, it was shown that Ba. microti influenced the immune response against Ba. rodhaini, leading to mouse survival in some cases. Indeed, Ba. rodhaini infects human red blood cells and rodents, being lethal to mice. Babesia microti, on the other hand, while a major cause of human and animal babesiosis, causes a self-limiting disease in mice that resolves over time (Zafar et al., 2022).
Another example involves co-infections with intestinal nematodes, particularly Heligmosomoides polygyrus and I. ricinus ticks in wild Apodemus mice. These mice serve as reservoir hosts for TBDs in Europe, making them a relevant model for studying natural co-infection scenarios. Laboratory experiments showed that while co-infection induced strong systemic Th2 immune responses, it did not alter local immune responses to ticks or affect tick-feeding success (Maaz et al., 2016). This suggests that certain immune responses may be compartmentalized, meaning that changes do not necessarily translate into local effects at the site of infection.
3.5. Strain-specific competition among pathogens and their epidemiological implications
Competitive interactions between different strains of the same pathogen can influence disease transmission and severity. For instance, in experiments with B. afzelii strains Fin-Jyv-A3 and NE4049, it was observed that NE4049 had a greater capacity for tissue infection, suggesting higher invasiveness. This competition led to a significant reduction in the tissue infection prevalence of Fin-Jyv-A3, illustrating how pathogen competition can affect strain-specific transmission (Genné et al., 2018). Further studies emphasize that competition between strains impacts not only their abundance in rodent tissues but also their transmission to ticks, thereby influencing the overall epidemiology of B. burgdorferi strains in natural environments (Genné et al., 2021). These findings underscore the complexity of pathogen interactions within a host and their broader ecological implications.
3.6. Viral-bacterial co-infections in sheep and ticks: Emerging models
Reviewing the literature, it was noted that more studies investigated bacteria-bacteria co-infections (Thomas et al., 2001; Holden et al., 2005; Gaunt et al., 2010; Rynkiewicz et al., 2017; Genné et al., 2018, 2021) or bacteria-parasites interactions (Moro et al., 2002; Coleman et al., 2005; Maaz et al., 2016; Sallay et al., 2017; Djokic et al., 2018; Djokic et al., 2019; Akoolo et al., 2021). Additionally, some models explore viral-bacterial interactions in sheep and ticks but not in mice (Paulsen et al., 2019; Hart et al., 2022). However, no models have been found that investigate virus-parasites interactions.
For instance, co-infection of sheep with A. phagocytophilum and TBEV resulted in significantly higher TBEV titers than in single infections. This suggests that bacterial co-infection can enhance viral replication and immune response, although the mechanisms remain unclear (Paulsen et al., 2019). Similarly, a laboratory model using adult ticks co-infected with B. burgdorferi and POWV (Powassan virus) found that the presence of B. burgdorferi increased POWV replication in the tick midgut (Hart et al., 2022).
To summarize, laboratory animal models provide valuable insights into the complexity of TBDs. Studies of co-infections in laboratory animals reveal multiple interactions between pathogens that affect disease severity and immune response dynamics. While some co-infections are shown to exacerbate symptoms, others show no significant changes. However, there is a need for additional laboratory animal and tick models, especially to understand the complex interaction between TBPs in ticks and vertebrate hosts, to improve our knowledge in this area and to develop effective disease prevention strategies.
4. Discussion
The aim of this review was to distinguish studies that were considered to be real TBP co-infections according to the criteria mentioned above (Table 2, Table 3) from the co-infection or co-exposure studies described, in order to synthesize current knowledge on real TBP co-infections.
According to our criteria, articles on co-infection in vector-borne diseases were divided into direct and indirect evidence. A total of 426 papers were reviewed and 389 cases of indirect evidence documented, divided into 207 papers on animals, 145 on ticks, and 37 cases of indirect evidence for humans. In contrast, 20 papers provided direct evidence: six on co-infection in animals; and 14 on co-infection in humans (Fig. 1). Nevertheless, despite attempting to make the bibliographic search as thorough as possible, some articles may not have been included. At the end of this review, we observed that there were very few papers addressing co-infections in vertebrate hosts, which raises the question of whether these were in fact co-infections or merely cases of co-exposure or co-detection. Moreover, the polysemy of the term “co-infection” made it difficult to accurately interpret studies of co-infection scenarios. Therefore, this review proposes definitions for co-infection, co-exposure, and co-detection (Table 1). Although co-infections of TBPs are now well recognized and routinely considered in various studies (Michelet et al., 2014; Moutailler et al., 2016), co-infection, co-detection and co-exposure should not be used as interchangeable words in order to gain clarity in future studies. It is fundamental to use the appropriate term, “co-detection”, rather than “co-infection”. Co-detection of pathogens by PCR in field-collected ticks and their vertebrate hosts does not always indicate viable co-infection, which may distort the overall understanding of the ecology and evolution co-infection by TBPs (Gomez-Chamorro et al., 2021). Only two papers in the literature used the term “co-detection” when screening for co-infections of TBPs in ticks (Holden et al., 2006; Beristain-Ruiz et al., 2022), highlighting the largely inappropriate use of the term “co-infection” to describe cases of co-detection or co-exposure instead.
There is a potential risk of humans and animals being infected simultaneously with multiple pathogens from a co-infected tick, depending on how common the pathogens are in the ticks and animal reservoirs in the area, how long it takes the tick to transmit the pathogens to the host once it has started feeding, and how effectively the ticks transmit the pathogens (Boyer et al., 2022). Therefore, to improve the detection of real cases of co-infection, the screening for TBPs in ticks could be based on an RNA RT-PCR rather than a DNA one. However, it is important to note that further research is needed to determine if non-replicating pathogens in ticks can become active during feeding and impact detection. While no co-infections have yet been identified in ticks (only cases of co-detection were found in the literature), a few articles have documented co-infections in animals and humans. Indeed, several challenges raised by the diagnosis of these co-infections in vertebrate hosts were described to explain this scarcity.
The use only of serology for surveillance purposes has several limitations, as described in different studies (Bil-Lula et al., 2015; Backus et al., 2022). It is vital to be able to distinguish between a resolved past exposure and an ongoing infection when interpreting serological results. Serological tests often lack specificity and can produce false positives due to cross-reactivity with other pathogens. For example, point-of-care testing such as that performed in veterinary medicine (Snap 4Dx Plus manufactured by IDEXX, for instance) cannot differentiate between certain Anaplasma and Ehrlichia species. This may lead to incorrect assumptions about the presence of certain pathogens (Backus et al., 2022). In addition, active infections may be missed due to the delay in seroconversion. Therefore, serological tests are limited by cross-reactivity, an inability to distinguish between primary and recurrent infections, and ineffectiveness during the “window period” when antibodies are undetectable (Bil-Lula et al., 2015; Rodríguez-Alarcón et al., 2020). In addition to the limitations of using serology alone, there are disadvantages to relying solely on molecular detection (Bil-Lula et al., 2015; Rodríguez-Alarcón et al., 2020). For example, dogs in the subclinical and chronic phases may be asymptomatic, and serological tests may show cross-reactivity and fail to distinguish between current and past infections of E. canis. Low levels of infectious agents in the bloodstream may result in negative PCR results from blood samples despite the presence of the pathogen in other tissues such as the liver, spleen, and bone marrow (Primus et al., 2018; Rodríguez-Alarcón et al., 2020; Backus et al., 2022). Similarly, in borreliosis, testing blood samples by PCR has poor diagnostic sensitivity compared with serological testing, as any circulating spirochetes or bacteria may be present at very low concentrations or there may be a transient spirochetemia. This limited detection of Borrelia spp. in whole blood samples suggests that whole blood may not be the primary site of infection and highlights the fact that alternative tissues or body fluids may provide more suitable sampling options. However, real-time PCR assays have shown potential in identifying Borrelia spp. in various body fluids and tissues, such as cerebrospinal fluid, synovial fluid, skin biopsies, and human urine samples (Bergmann et al., 2002; Ivacic et al., 2007). Due to its high analytical sensitivity and specificity, real-time PCR assays could be particularly useful for detecting B. burgdorferi (s.l.) infections in localized tissues (e.g. skin) or body fluids (e.g. cerebrospinal fluid, synovial fluid) (Bil-Lula et al., 2015).
Conducting both molecular and serological tests simultaneously is particularly important. Negative PCR results associated with positive antibody responses indicate a subclinical or chronic stage of the disease, or previous exposure to the pathogen (de Sousa et al., 2013). Furthermore, the best strategy for reliable diagnosis in animals and humans is to combine molecular and serological techniques with a clinical examination to ensure appropriate treatment, as several studies have shown (Dong et al., 2013; Moniuszko et al., 2014; Diallo et al., 2017; Boyer et al., 2018; Dunaj et al., 2018; Borawski et al., 2019; Liu et al., 2019; Wormser et al., 2019; Grochowska et al., 2020; Dumic et al., 2021; Moniuszko-Malinowska et al., 2021; Gęgotek et al., 2022; Groth et al., 2022).
Finally, the impact of co-infections was analyzed, with conflicting results. It was found that when co-infections occur, the pathogens may either enhance or suppress each other, thereby increasing or decreasing their pathogenic effects or, conversely, they may not have a significant effect.
For example, latent (persistent) TBEV infection may predispose individuals to new infections from bacteria such as Borrelia, and particular attention should be paid to treatment with tetracycline antibiotics, as such treatment has been associated with progression of TBE and sometimes fatal outcomes, particularly due to the immunosuppressive and neurotoxic effects of doxycycline, which may exacerbate the course of TBE health (Kolyasnikova et al., 2022). An integrated approach that includes investigation, diagnosis, and treatment of these different etiologies is essential because the management of bacterial tick-borne infections in addition to TBE remains a major challenge in modern public health (Kolyasnikova et al., 2022).
In order to gain a deeper understanding of the interactions between pathogens during co-infection in animals or humans, it is imperative to develop animal models specifically designed for co-infection studies. Previously, various animal models have been chosen to study the effects of co-infection with B. burgdorferi (s.l.), A. phagocytophilum, and Babesia spp. These studies have used different mouse strains to investigate transmission dynamics, pathogenicity, and the influence of pathogen interactions on the vertebrate hostʼs immune response, which in turn affects disease presentation. However, current models do not include scenarios involving bacterial-viral co-infections. Moreover, while C3H/N and BALB/c mice serve as reliable models for B. afzelii infection, they may not be suitable for other models of infection. Specifically, these mice did not develop rickettsiosis or transmit Rickettsia spp. to ticks during feeding (Sallay et al., 2017). Moreover, there is a scarcity of co-infected tick models, with only one model described in which adult ticks were infected with both B. burgdorferi and POWV by capillary feeding (Hart et al., 2022). Additional laboratory animal and tick co-infection models are required to address the multiple possible co-infections in vertebrate hosts and ticks. However, it is important to interpret these findings cautiously when applying to natural conditions. Additional research in field settings is essential to validate these results.
Ixodes ricinus, the main vector of TBPs in Europe, has a wide geographical distribution and feeds on a variety of vertebrate hosts. Common pathogens transmitted by I. ricinus include B. afzelii, TBEV, and A. phagocytophilum, which infect both vertebrate hosts and ticks (de la Fuente et al., 2017). In Europe, 3817 cases of TBE were reported in 2020 (Hills et al., 2024), while between 65,000 and 85,000 cases of LD were reported annually until 2006 (Lindgren and Jaenson, 2006). In addition, fewer than 300 cases of HGA were reported in Europe between 2004 and 2019 (Matei et al., 2019) due to underreporting and underestimation, possibly due to undetected asymptomatic or mild cases, and lack of awareness among clinicians (Lindgren and Jaenson, 2006).
Therefore, it is essential to establish co-infection models with B. afzelii, TBEV, and A. phagocytophilum in both mice and ticks. In addition, the setting up of new artificial co-infection models in ticks using capillary and microinjection systems would be useful to mimic natural tick infection and compare pathogen distribution pathways in the tick. These studies could represent the first steps toward studying the transmission dynamics of these pathogens from co-infected ticks to uninfected mice and vice versa. Ultimately, such models will improve our understanding of potential synergistic or antagonistic interactions between these pathogens and could improve prevention and TBD management.
5. Conclusions
In conclusion, this review successfully distinguished studies that present co-infections with tick-borne and other pathogens from those describing co-detection or co-exposure. Of the 426 papers analyzed, only 20 provided direct evidence of co-infection, with the remainder documenting indirect evidence. The polysemy of the term “co-infection” has contributed to inconsistencies in research, highlighting the need for clear definitions and appropriate diagnostic criteria, as proposed in this review. Accurate detection methods, such as RNA RT-PCR in addition to microbiological culture, are crucial to avoid misinterpretation and better identify cases of co-infection. Furthermore, the development of laboratory models for animal and tick co-infections will improve our understanding of pathogen interactions and improve diagnostic and treatment strategies for tick-borne diseases. This research highlights the importance of precise terminology and integrated approaches to accurately study and manage co-infections in both humans and animals.
Funding
Research by Stefania Porcelli, Pierre Lucien Deshuillers, Sara Moutailler and Anne-Claire Lagrée was supported by the French Agency for Food, Environmental and Occupational Health & Safety (ANSES), the French National Institute for Agricultural Research (INRAE), and the Ecole Nationale Vétérinaire d’Alfort (EnvA). The BIPAR joint research unit is supported by the French Government’s Investissement d’Avenir program, Laboratoire d’Excellence “Integrative Biology of Emerging Infectious Diseases” (grant No. ANR-10-LABEX-62-IBEID).
Ethical approval
Not applicable.
Data availability
The data supporting the conclusions of this article are included within the article and its supplementary files.
CRediT authorship contribution statement
Stefania Porcelli: Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Pierre Lucien Deshuillers: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition. Sara Moutailler: Conceptualization. Anne-Claire Lagrée: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2024.100219.
Contributor Information
Sara Moutailler, Email: sara.moutailler@anses.fr.
Anne-Claire Lagrée, Email: anne-claire.lagree@vet-alfort.fr.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
Supplementary Table S1PRISMA checklist.
Supplementary Table S2Articles included and excluded from the review.
Supplementary Table S3Case definition of co-infection in humans.
References
- Abas O., Abd-Elrahman A., Saleh A., Bessat M. Prevalence of tick-borne haemoparasites and their perceived co-occurrences with viral outbreaks of FMD and LSD and their associated factors. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoolo L., Djokic V., Rocha S.C., Parveen N. Pathogenesis of Borrelia burgdorferi and Babesia microti in TLR4-competent and TLR4-dysfunctional C3H mice. Cell. Microbiol. 2021;23 doi: 10.1111/cmi.13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hosary A., Rǎileanu C., Tauchmann O., Fischer S., Nijhof A.M., Silaghi C. Epidemiology and genotyping of Anaplasma marginale and co-infection with piroplasms and other Anaplasmataceae in cattle and buffaloes from Egypt. Parasites Vectors. 2020;13:495. doi: 10.1186/s13071-020-04372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M.O., Víchová B., Tolf C., Krzyzanowska S., Waldenström J., Karlsson M.E. Co-infection with Babesia divergens and Anaplasma phagocytophilum in cattle (Bos taurus), Sweden. Ticks Tick Borne Dis. 2017;8:933–935. doi: 10.1016/j.ttbdis.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Attipa C., Solano-Gallego L., Papasouliotis K., Soutter F., Morris D., Helps C., et al. Association between canine leishmaniosis and Ehrlichia canis co-infection: A prospective case-control study. Parasites Vectors. 2018;11:184. doi: 10.1186/s13071-018-2717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus L., Foley J., Chung C., Virata S., Zazueta O.E., López-Pérez A. Tick-borne pathogens detected in sheltered dogs during an epidemic of Rocky Mountain spotted fever, a One Health challenge. J. Am. Vet. Med. Assoc. 2022;261:375–383. doi: 10.2460/javma.22.08.0388. [DOI] [PubMed] [Google Scholar]
- Baneth G. Tick-borne infections of animals and humans: A common ground. Int. J. Parasitol. 2014;44:591–596. doi: 10.1016/j.ijpara.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Belongia E.A. Epidemiology and impact of coinfections acquired from Ixodes ticks. Vector Borne Zoonotic Dis. 2002;2:265–273. doi: 10.1089/153036602321653851. [DOI] [PubMed] [Google Scholar]
- Bergmann A.R., Schmidt B.L., Derler A.M., Aberer E. Importance of sample preparation for molecular diagnosis of Lyme borreliosis from urine. J. Clin. Microbiol. 2002;40:4581–4584. doi: 10.1128/JCM.40.12.4581-4584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beristain-Ruiz D.M., Garza-Hernández J.A., Figueroa-Millán J.V., Lira-Amaya J.J., Quezada-Casasola A., Ordoñez-López S., et al. Possible association between selected tick-borne pathogen prevalence and Rhipicephalus sanguineus sensu lato infestation in dogs from Juarez City (Chihuahua), Northwest Mexico-US Border. Pathogens. 2022;11:552. doi: 10.3390/pathogens11050552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bil-Lula I., Matuszek P., Pfeiffer T., Woźniak M. Lyme borreliosis - the utility of improved real-time PCR assay in the detection of Borrelia burgdorferi infections. Adv. Clin. Exp. Med. 2015;24:663–670. doi: 10.17219/acem/28625. [DOI] [PubMed] [Google Scholar]
- Borawski K., Dunaj J., Czupryna P., Pancewicz S., Świerzbińska R., Żebrowska A., Moniuszko-Malinowska A. Prevalence of spotted fever group Rickettsia in North-Eastern Poland. Inf. Disp. 2019;51:810–814. doi: 10.1080/23744235.2019.1660800. [DOI] [PubMed] [Google Scholar]
- Boyer P.H., Kieffer P., de Martino S.J., Zilliox L., Vogel J.Y., Jaulhac B., Hansmann Y. Borrelia burgdorferi s.l. and tick-borne encephalitis virus coinfection in eastern France. Med. Maladies Infect. 2018;48:218–220. doi: 10.1016/j.medmal.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Boyer P.H., Lenormand C., Jaulhac B., Talagrand-Reboul E. Human co-infections between Borrelia burgdorferi s.l. and other Ixodes-borne microorganisms: A systematic review. Pathogens. 2022;11:282. doi: 10.3390/pathogens11030282/s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzouraa T., René-Martellet M., Chêne J., Attipa C., Lebert I., Chalvet-Monfray K., et al. Clinical and laboratory features of canine Anaplasma platys infection in 32 naturally infected dogs in the Mediterranean basin. Ticks Tick Borne Dis. 2016;7:1256–1264. doi: 10.1016/j.ttbdis.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Bröker M. Following a tick bite: double infections by tick-borne encephalitis virus and the spirochete Borrelia and other potential multiple infections. Zoonoses Public Health. 2012;59:176–180. doi: 10.1111/j.1863-2378.2011.01435.x. [DOI] [PubMed] [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Washington, USA: 2024. Tickborne disease surveillance data summary.https://www.cdc.gov/ticks/data-research/facts-stats/tickborne-disease-surveillance-data-summary.html [Google Scholar]
- Coleman J.L., LeVine D., Thill C., Kuhlow C., Benach J.L. Babesia microti and Borrelia burgdorferi follow independent courses of infection in mice. J. Infect. Dis. 2005;192:1634–1641. doi: 10.1086/496891/2/192-9-1634-TAB001.GIF. [DOI] [PubMed] [Google Scholar]
- Dahlgren F.S., Heitman K.N., Drexler N.A., Massung R.F., Behravesh C.B. Human granulocytic anaplasmosis in the United States from 2008 to 2012: A summary of national surveillance data. Am. J. Trop. Med. Hyg. 2015;93:66–72. doi: 10.4269/ajtmh.15-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J., Antunes S., Bonnet S., Cabezas-Cruz A., Domingos A.G., Estrada-Peña A., et al. Tick-pathogen interactions and vector competence: Identification of molecular drivers for tick-borne diseases. Front. Cell. Infect. Microbiol. 2017;7:114. doi: 10.3389/fcimb.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa K.C., André M.R., Herrera H.M., de Andrade G.B., Jusi M.M., dos Santos L.L., et al. Molecular and serological detection of tick-borne pathogens in dogs from an area endemic for Leishmania infantum in Mato Grosso do Sul, Brazil. Rev. Bras. Parasitol. Vet. 2013;22:525–531. doi: 10.1590/S1984-29612013000400012. (In Portuguese) [DOI] [PubMed] [Google Scholar]
- Diallo M.A., Kane B.S., Ndiaye M., Dieng M., Diongue K., Badiane A.S., et al. Plasmodium falciparum malaria co-infection with tick-borne relapsing fever in Dakar. Malar. J. 2017;16:24. doi: 10.1186/s12936-017-1682-6. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djokic V., Akoolo L., Parveen N. Babesia microti infection changes host spleen architecture and is cleared by a Th1 immune response. Front. Microbiol. 2018;9:85. doi: 10.3389/fmicb.2018.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djokic V., Akoolo L., Primus S., Schlachter S., Kelly K., Bhanot P., Parveen N. Protozoan parasite Babesia microti subverts adaptive immunity and enhances Lyme disease severity. Front. Microbiol. 2019;10:1596. doi: 10.3389/fmicb.2019.01596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong T., Qu Z., Zhang L. Detection of A. phagocytophilum and E. chaffeensis in patient and mouse blood and ticks by a duplex real-time PCR assay. PLoS One. 2013;8 doi: 10.1371/journal.pone.0074796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumic I., Glomski B., Patel J., Nordin T., Nordstrom C.W., Sprecher L.J., et al. “Double trouble”: Severe meningoencephalitis due to Borrelia burgdorferi and Powassan virus co-infection successfully treated with intravenous immunoglobulin. Am. J. Case Rep. 2021;22:1–6. doi: 10.12659/AJCR.929952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaj J., Moniuszko-Malinowska A., Swiecicka I., Andersson M., Czupryna P., Rutkowski K., et al. Tick-borne infections and co-infections in patients with non-specific symptoms in Poland. Adv. Med. Sci. 2018;63:167–172. doi: 10.1016/j.advms.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Gaunt S., Beall M., Stillman B., Lorentzen L., Diniz P., Chandrashekar R., Breitschwerdt E. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: Hematologic, serologic and molecular findings. Parasites Vectors. 2010;3:33. doi: 10.1186/1756-3305-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gęgotek A., Moniuszko-Malinowska A., Groth M., Pancewicz S., Czupryna P., Dunaj J., et al. Plasma proteomic profile of patients with tick-borne encephalitis and co-infections. Int. J. Mol. Sci. 2022;23:4374. doi: 10.3390/ijms23084374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genné D., Sarr A., Gomez-Chamorro A., Durand J., Cayol C., Rais O., Voordow M.J. Competition between strains of Borrelia afzelii inside the rodent host and the tick vector. Proc. Biol. Sci. 2018;285 doi: 10.1098/rspb.2018.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genné D., Rossel M., Sarr A., Battilotti F., Rais O., Rego R., Voordow M.J., et al. Competition between strains of Borrelia afzelii in the host tissues and consequences for transmission to ticks. ISME J. 2021;15:2390–2400. doi: 10.1038/s41396-021-00939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Chamorro A., Hodžić A., King K.C., Cabezas-Cruz A. Ecological and evolutionary perspectives on tick-borne pathogen co-infections. Curr. Res. Parasitol. Vector Borne Dis. 2021;2 doi: 10.1016/j.crpvbd.2022.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grochowska A., Pancewicz S., Czupryna P., Dunaj J., Borawski K., Moniuszko-Malinowska A. Pathogens carried by Ixodes ricinus and Dermacentor reticulatus ticks including coinfections. Przegl. Epidemiol. 2020;74:466–474. doi: 10.32394/pe.74.40. [DOI] [PubMed] [Google Scholar]
- Groth M., Łuczaj W., Dunaj-Małyszko J., Skrzydlewska E., Moniuszko-Malinowska A. Differences in the plasma phospholipid profile of patients infected with tick-borne encephalitis virus and co-infected with bacteria. Sci. Rep. 2022;12:9538. doi: 10.1038/s41598-022-13765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C.E., Middleton F.A., Thangamani S. Infection with Borrelia burgdorferi increases the replication and dissemination of coinfecting Powassan virus in Ixodes scapularis ticks. Viruses. 2022;14:1584. doi: 10.3390/v14071584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills S., Gould C., Cossaboom C. CDC Yellow Book 2024. 2024. https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/tick-borne-encephalitis Accessed. [Google Scholar]
- Holden K., Boothby J.T., Kasten R.W., Chomel B. Co-detection of Bartonella henselae, Borrelia burgdorferi, and Anaplasma phagocytophilum in Ixodes pacificus ticks from California, USA. Counsell. Psychol. Rev. 2006;31:67–76. doi: 10.53841/bpscpr.2016.31.1.67. [DOI] [PubMed] [Google Scholar]
- Holden K., Hodzic E., Feng S., Freet K.J., Lefebvre R.B., Barthold S.W. Population distribution in C3H/HeN mice. Infect. Immun. 2005;73:3440–3444. doi: 10.1128/IAI.73.6.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivacic L., Reed K.D., Mitchell P.D., Ghebranious N. A LightCycler TaqMan assay for detection of Borrelia burgdorferi sensu lato in clinical samples. Diagnostic Microbiol. Inf. Disp. 2007;57:137–143. doi: 10.1016/j.diagmicrobio.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Kolyasnikova N.M., Sanchez-Pimentel J.P., Pestov N.B. Insights from experience in the treatment of tick-borne bacterial coinfections with tick-borne encephalitis. Annu. Rep. Med. Chem. 2022;58:157–241. doi: 10.1016/bs.armc.2022.08.004. [DOI] [Google Scholar]
- Kordick S.K., Breitschwerdt E.B., Hegarty B.C., Southwick K.L., Colitz C.M., Hancock S.I., et al. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J. Clin. Microbiol. 1999;37:2631–2638. doi: 10.1128/jcm.37.8.2631-2638.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–e34. doi: 10.1016/J.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Lindgren E., Jaenson T.G.T. World Health Organization; Geneva, Switzerland: 2006. Lyme borreliosis in Europe: Influences of climate and climate change, epidemiology, ecology and adaptation measures. Situation Report.http://www.euro.who.int/__data/assets/pdf_file/0006/96819/E89522.pdf?ua=1 [Google Scholar]
- Liu H.B., Wei R., Ni X.B., Zheng Y.C., Huo Q.B., Jiang B.G., et al. The prevalence and clinical characteristics of tick-borne diseases at One Sentinel Hospital in Northeastern China. Parasitology. 2019;146:161–167. doi: 10.1017/S0031182018001178. [DOI] [PubMed] [Google Scholar]
- Maaz D., Rausch S., Richter D., Krücken J., Kühl A.A., Demeler J., et al. Susceptibility to ticks and Lyme disease spirochetes is not affected in mice co-infected with nematodes. Infect. Immun. 2016;84:1274–1286. doi: 10.1128/IAI.01309-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei I.A., Estrada-Peña A., Cutler S.J., Vayssier-Taussat M., Varela-Castro L., Potkonjak A., et al. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasites Vectors. 2019;12:599. doi: 10.1186/S13071-019-3852-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelet L., Delannoy S., Devillers E., Umhang G., Aspan A., Juremalm M., et al. High-throughput screening of tick-borne pathogens in Europe. Front. Cell. Infect. Microbiol. 2014;4:103. doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniuszko A., Dunaj J., Święcicka I., Zambrowski G., Chmielewska-Badora J., Żukiewicz-Sobczak W., et al. Co-infections with Borrelia species, Anaplasma phagocytophilum and Babesia spp. in patients with tick-borne encephalitis. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:1835–1841. doi: 10.1007/s10096-014-2134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniuszko-Malinowska A., Dunaj J., Andersson M.O., Chmielewski T., Czupryna P., Groth M., et al. Anaplasmosis in Poland – analysis of 120 patients. Ticks Tick Borne Dis. 2021;12 doi: 10.1016/j.ttbdis.2021.101763. [DOI] [PubMed] [Google Scholar]
- Moro M.H., Zegarra-Moro O.L., Bjornsson J., Hofmeister E.K., Bruinsma E., Germer J.J., Persing D.H. Increased arthritis severity in mice co-infected with Borrelia burgdorferi and Babesia microti. J. Infect. Dis. 2002;186:428–431. doi: 10.1086/341452. [DOI] [PubMed] [Google Scholar]
- Moutailler S., Valiente Moro C., Vaumourin E., Michelet L., Tran F.H., Devillers E., et al. Co-infection of ticks: The rule rather than the exception. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto N.C., Foley J.E. Meta-analysis of coinfection and coexposure with Borrelia burgdorferi and Anaplasma phagocytophilum in humans, domestic animals, wildlife, and Ixodes ricinus-complex ticks. Vector Borne Zoonotic Dis. 2009;9:93–101. doi: 10.1089/vbz.2008.0072. [DOI] [PubMed] [Google Scholar]
- Paulsen K.M., Granquist E.G., Okstad W., Vikse R., Stiasny K., Andreassen A.K., Stuen S. Experimental infection of lambs with tick-borne encephalitis virus and co-infection with Anaplasma phagocytophilum. PLoS One. 2019;14 doi: 10.1371/journal.pone.0226836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primus S., Akoolo L., Schlachter S., Gedroic K., Rojtman A.D., Parveen N. Efficient detection of symptomatic and asymptomatic patient samples for Babesia microti and Borrelia burgdorferi infection by multiplex qPCR. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha S.C., Velásquez C.V., Aquib A., Al-Nazal A., Parveen N. Transmission cycle of tick-borne infections and co-infections, animal models and diseases. Pathogens. 2022;11:1309. doi: 10.3390/pathogens11111309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Alarcón C.A., Beristain-Ruiz D.M., Olivares-Muñoz A., Quezada-Casasola A., Pérez-Casio F., Álvarez-Martínez J.A., et al. Demonstrating the presence of Ehrlichia canis DNA from different tissues of dogs with suspected subclinical ehrlichiosis. Parasites Vectors. 2020;13:518. doi: 10.1186/s13071-020-04363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynkiewicz E.C., Brown J., Tufts D.M., Huang C.-I., Kampen H., Bent S.J., et al. Closely-related Borrelia burgdorferi (sensu stricto) strains exhibit similar fitness in single infections and asymmetric competition in multiple infections. Parasites Vectors. 2017;10:64. doi: 10.1186/s13071-016-1964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallay B., Vaculová T., Derdáková M., Rusňáková Taragelová V., Špitalská E., Škultéty L. Two mice models for transferability of zoonotic bacteria via tick vector. Acta Virol. 2017;61:372–376. doi: 10.4149/av_2017_319. [DOI] [PubMed] [Google Scholar]
- Segen J.C. CRC Press; Boca Raton, Florida, USA: 2011. Concise Dictionary of Modern Medicine. [Google Scholar]
- Stanek G., Fingerle V., Hunfeld K.P., Jaulhac B., Kaiser R., Krause A., et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin. Microbiol. Infect. 2011;17:69–79. doi: 10.1111/j.1469-0691.2010.03175.x. [DOI] [PubMed] [Google Scholar]
- Thomas V., Anguita J., Barthold S.W., Fikrig E. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis alters murine immune responses, pathogen burden, and severity of Lyme arthritis. Infect. Immun. 2001;69:3359–3371. doi: 10.1128/IAI.69.5.3359-3371.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijsse-Klasen E., Sprong H., Pandak N. Co-infection of Borrelia burgdorferi sensu lato and Rickettsia species in ticks and in an erythema migrans patient. Parasites Vectors. 2013;6:347. doi: 10.1186/1756-3305-6-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormser G.P., McKenna D., Scavarda C., Cooper D., El Khoury M.Y., Nowakowski J., et al. Co-infections in persons with early Lyme disease, New York, USA. Emerg. Infect. Dis. 2019;25:748–752. doi: 10.3201/eid2504.181509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar I., Galon E.M., Kondoh D., Efstratiou A., Li J., Ji S., et al. The cross-species immunity during acute Babesia co-infection in mice. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.885985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidner N.S., Dolan M.C., Massung R., Piesman J., Fish D. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis suppresses IL-2 and IFNγ production and promotes an IL-4 response in C3H/HeJ mice. Parasite Immunol. 2000;22:581–588. doi: 10.1046/J.1365-3024.2000.00339.X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the conclusions of this article are included within the article and its supplementary files.