Abstract
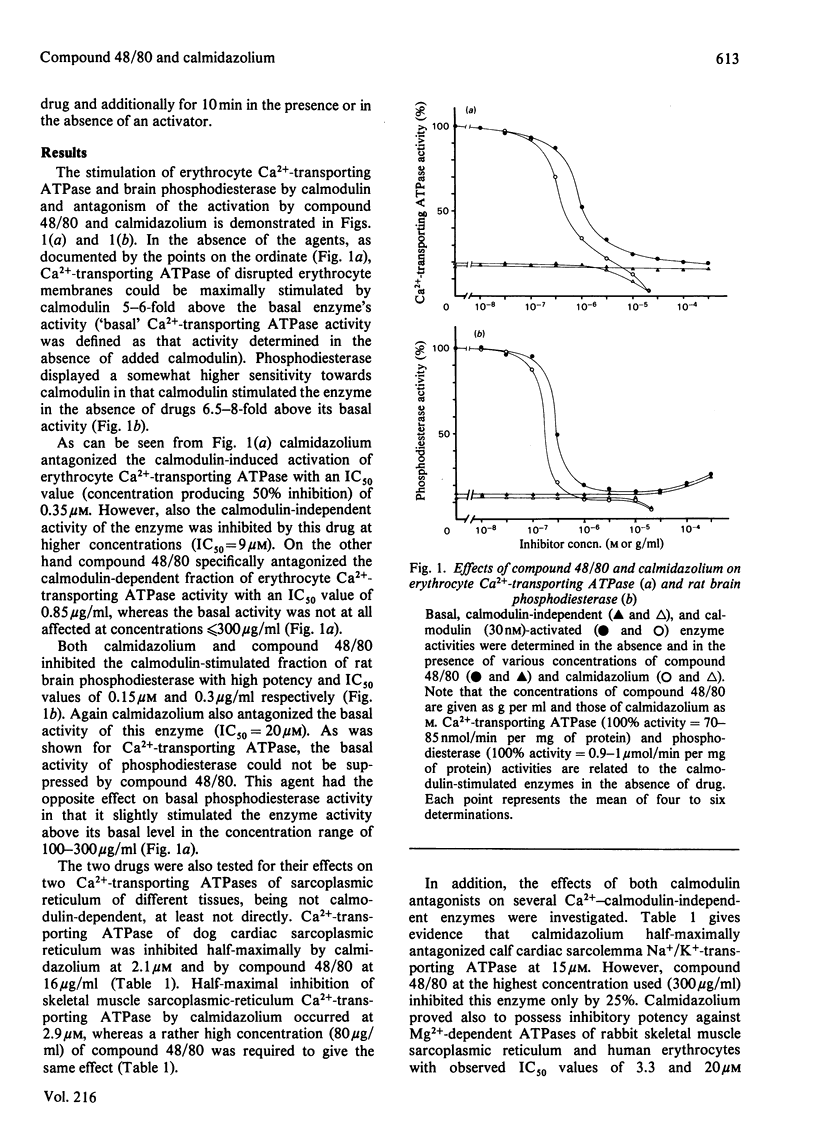
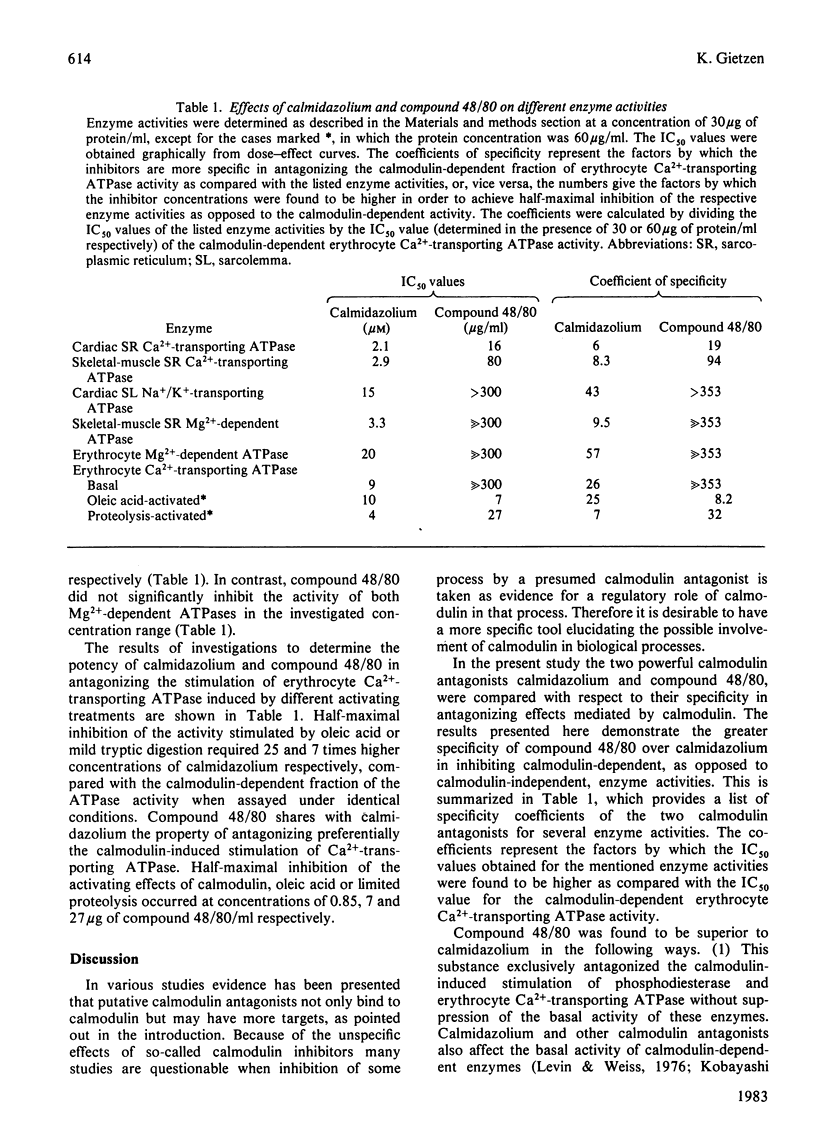
The two presumed calmodulin antagonists calmidazolium and compound 48/80 were compared for their effects on several calmodulin-dependent and calmodulin-independent enzyme systems. Compound 48/80 and calmidazolium were found to be about equipotent in antagonizing the calmodulin-dependent fraction of brain phosphodiesterase and erythrocyte Ca2+-transporting ATPase. Compound 48/80 combines high potency with high specificity in that: (1) the basal, calmodulin-independent, activity of calmodulin-regulated enzymes was not suppressed; (2) calmodulin-independent enzyme activities, such as Ca2+-transporting ATPases of sarcoplasmic reticulum, Mg2+-dependent ATPases of different tissues and Na+/K+-transporting ATPase of cardiac sarcolemma, were far less altered, or not altered at all, by compound 48/80 as compared with calmidazolium; and (3) antagonism of proteolysis-induced stimulation as opposed to calmodulin-induced activation of erythrocyte Ca2+-transporting ATPase required a 32 times higher concentration of compound 48/80. In all these aspects compound 48/80 was found to be a superior antagonist to calmidazolium since inhibition of calmodulin-independent events by the other agent occurred at considerably lower concentrations. Therefore compound 48/80 is proposed to be a much more specific and useful tool for studying the participation of calmodulin in biological processes than the presently used agents.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au K. S. (Ca2+ + Mg2+)-ATPase of chlorpromazine containing rabbit erythrocyte membrane. Gen Pharmacol. 1981;12(4):285–290. doi: 10.1016/0306-3623(81)90060-4. [DOI] [PubMed] [Google Scholar]
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Balzer H., Makinose M., Hasselbach W. The inhibition of the sarcoplasmic calcium pump by prenylamine, reserpine, chlorpromazine and imipramine. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;260(5):444–455. doi: 10.1007/BF00537359. [DOI] [PubMed] [Google Scholar]
- Caroni P., Reinlib L., Carafoli E. Charge movements during the Na+-Ca2+ exchange in heart sarcolemmal vesicles. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6354–6358. doi: 10.1073/pnas.77.11.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Gietzen K., Kolandt J. Large-scale isolation of human erythrocyte Ca2+-transport ATPase. Biochem J. 1982 Oct 1;207(1):155–159. doi: 10.1042/bj2070155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietzen K., Mansard A., Bader H. Inhibition of human erythrocyte Ca++-transport ATPase by phenothiazines and butyrophenones. Biochem Biophys Res Commun. 1980 May 30;94(2):674–681. doi: 10.1016/0006-291x(80)91285-1. [DOI] [PubMed] [Google Scholar]
- Gietzen K., Sadorf I., Bader H. A model for the regulation of the calmodulin-dependent enzymes erythrocyte Ca2+-transport ATPase and brain phosphodiesterase by activators and inhibitors. Biochem J. 1982 Dec 1;207(3):541–548. doi: 10.1042/bj2070541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietzen K., Wüthrich A., Bader H. Effects of microtubular inhibitors on plasma membrane calmodulin-dependent Ca2+-transport ATPase. Mol Pharmacol. 1982 Sep;22(2):413–420. [PubMed] [Google Scholar]
- Gietzen K., Wüthrich A., Bader H. R 24571: a new powerful inhibitor of red blood cell Ca++-transport ATPase and of calmodulin-regulated functions. Biochem Biophys Res Commun. 1981 Jul 30;101(2):418–425. doi: 10.1016/0006-291x(81)91276-6. [DOI] [PubMed] [Google Scholar]
- Jones L. R., Besch H. R., Jr, Fleming J. W., McConnaughey M. M., Watanabe A. M. Separation of vesicles of cardiac sarcolemma from vesicles of cardiac sarcoplasmic reticulum. Comparative biochemical analysis of component activities. J Biol Chem. 1979 Jan 25;254(2):530–539. [PubMed] [Google Scholar]
- Kakiuchi S., Sobue K., Yamazaki R., Kambayashi J., Sakon M., Kosaki G. Lack of tissue specificity of calmodulin: a rapid and high-yield purification method. FEBS Lett. 1981 Apr 20;126(2):203–207. doi: 10.1016/0014-5793(81)80242-6. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Richman P. G. Calmodulin. Annu Rev Biochem. 1980;49:489–515. doi: 10.1146/annurev.bi.49.070180.002421. [DOI] [PubMed] [Google Scholar]
- Kobayashi R., Tawata M., Hidaka H. Ca2+ regulated modulator protein interacting agents: inhibition of Ca2+-Mg2+-ATPase of human erythrocyte ghost. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1037–1045. doi: 10.1016/0006-291x(79)91513-4. [DOI] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Mechanism by which psychotropic drugs inhibit adenosine cyclic 3',5'-monophosphate phosphodiesterase of brain. Mol Pharmacol. 1976 Jul;12(4):581–589. [PubMed] [Google Scholar]
- Luthra M. G. Trifluoperazine inhibition of calmodulin-sensitive Ca2+ -ATPase and calmodulin insensitive (Na+ +K+)- and Mg2+ -ATPase activities of human and rat red blood cells. Biochim Biophys Acta. 1982 Nov 8;692(2):271–277. doi: 10.1016/0005-2736(82)90531-4. [DOI] [PubMed] [Google Scholar]
- Means A. R., Dedman J. R. Calmodulin--an intracellular calcium receptor. Nature. 1980 May 8;285(5760):73–77. doi: 10.1038/285073a0. [DOI] [PubMed] [Google Scholar]
- Meissner G., Conner G. E., Fleischer S. Isolation of sarcoplasmic reticulum by zonal centrifugation and purification of Ca 2+ -pump and Ca 2+ -binding proteins. Biochim Biophys Acta. 1973 Mar 16;298(2):246–269. doi: 10.1016/0005-2736(73)90355-6. [DOI] [PubMed] [Google Scholar]
- Raess B. U., Vincenzi F. F. Calmodulin activation of red blood cell (Ca2+ + Mg2+)-ATPase and its antagonism by phenothiazines. Mol Pharmacol. 1980 Sep;18(2):253–258. [PubMed] [Google Scholar]
- Stewart D. J. Sensitive automated methods for phosphate and (Na+ plus K+)-ATPase. Anal Biochem. 1974 Dec;62(2):349–364. doi: 10.1016/0003-2697(74)90167-5. [DOI] [PubMed] [Google Scholar]
- Suko J., Hasselbach W. Characterization of cardiac sarcoplasmic reticulum ATP-ADP phosphate exchange and phosphorylation of the calcium transport adenosine triphosphatase. Eur J Biochem. 1976 Apr 15;64(1):123–130. doi: 10.1111/j.1432-1033.1976.tb10280.x. [DOI] [PubMed] [Google Scholar]
- Volpi M., Sha'afi R. I., Feinstein M. B. Antagonism of calmodulin by local anesthetics. Inhibition of calmodulin-stimulated calcium transport of erythrocyte inside-out membrane vesicles. Mol Pharmacol. 1981 Sep;20(2):363–370. [PubMed] [Google Scholar]
- Wang J. H., Desai R. Modulator binding protein. Bovine brain protein exhibiting the Ca2+-dependent association with the protein modulator of cyclic nucleotide phosphodiesterase. J Biol Chem. 1977 Jun 25;252(12):4175–4184. [PubMed] [Google Scholar]
- Watanabe K., Williams E. F., Law J. S., West W. L. Specific inhibition of a calcium dependent activation of brain cyclic AMP phosphodiesterase activity by vinblastine. Experientia. 1979 Nov 15;35(11):1487–1489. doi: 10.1007/BF01962801. [DOI] [PubMed] [Google Scholar]
- Weiss B., Prozialeck W., Cimino M., Barnette M. S., Wallace T. L. Pharmacological regulation of calmodulin. Ann N Y Acad Sci. 1980;356:319–345. doi: 10.1111/j.1749-6632.1980.tb29621.x. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Brostrom C. O. Calcium-dependent cyclic nucleotide phosphodiesterase from brain identification of phospholipids as calcium-independent activators. Arch Biochem Biophys. 1976 Apr;173(2):720–731. doi: 10.1016/0003-9861(76)90310-6. [DOI] [PubMed] [Google Scholar]


