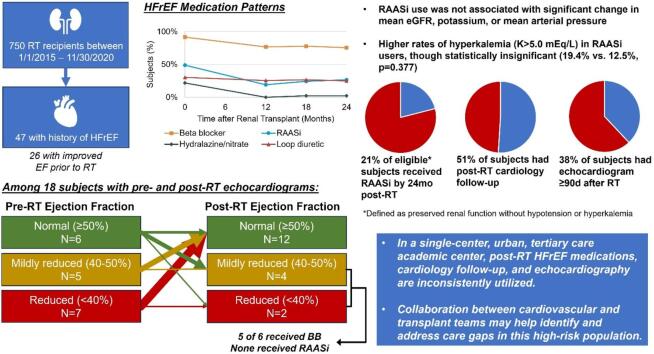
Graphical abstract
Keywords: Heart failure, Renal transplantation, Quality improvement, Medication management
Abstract
Background
The role of medical therapy for heart failure with reduced ejection fraction (HFrEF) in subjects with end-stage renal disease receiving renal transplantation (RT) is understudied. Here, we describe post-RT HFrEF medical management practices at a single urban, academic tertiary care center.
Methods
RT recipients between January 1, 2015 and November 30, 2020 with history of ejection fraction (EF) <40 % prior to RT were included. Medications, renal function, blood pressure, cardiology follow-up, and echocardiograms ≥90d post-RT were retrospectively collected for 2 years post-RT.
Results and conclusions
47/750 (6.3 %) of RT recipients had prior HFrEF diagnosis, of whom 26 experienced improvement in EF prior to RT. Pre-RT medical therapy included beta blocker (BB) in 43 (92 %) of subjects and renin-angiotensin-aldosterone inhibitors (RAASi) in 23 (49 %). By 24 months post-RT, BB were used in 34 (76 %) and RAASi were used in 12 (27 %) of subjects. Rates of post-RT cardiology follow-up (51 %) and echocardiogram (38 %) were lower than expected in this cohort. Of 29 subjects potentially eligible for RAASi based on preserved renal function and no hyperkalemia or hypotension episodes during follow-up, only 6 (21 %) received RAASi. Of 6 subjects with post-RT EF <50 %, 4 were eligible but did not receive RAASi. Multidisciplinary collaboration between cardiology and transplant teams may help improve care for this high-risk patient population.
Heart failure with reduced ejection fraction (HFrEF) is frequently encountered in patients with end stage renal disease (ESRD) and is responsible for up to 16 % of hospitalizations and 78 % of major adverse cardiovascular events following renal transplantation (RT) [1], [2], [3]. Although RT is often associated with improvement in cardiac structure and function [4], [5], evidence informing optimal management of HFrEF after RT remains sparse. Cardiovascular society guidelines strongly recommend continuing HFrEF medications even after left ventricular ejection fraction (EF) improves, as residual structural and functional abnormalities confer high risk of relapse [6]. Pre-RT patients are often on minimal background HFrEF therapy, as advanced renal disease precludes use of guideline-directed medications such as mineralocorticoid receptor antagonists (MRA) and sodium/glucose-cotransporter 2-inhibitors (SGLT2i). Furthermore, renin-angiotensin-aldosterone inhibitors (RAASi) are often discontinued and avoided after RT due to concerns of hyperkalemia or increased serum creatinine. There is a critical need to understand current HFrEF treatment practices after RT in order to identify areas for potential improvement. Here, we describe post-RT HFrEF management practices at an urban, academic tertiary care center and identify factors associated with HFrEF medication use, post-RT cardiology follow-up, and utilization of echocardiography in this cohort.
1. Methods
Individuals were included who underwent RT between January 1, 2015 and November 30, 2020 at our medical center with a diagnosis of HFrEF, defined as ejection fraction (EF) <40 % on echocardiogram or cardiac MRI prior to RT. Multi-organ transplant recipients were excluded. Baseline characteristics were obtained from provider and clinical pharmacist notes in the electronic health record. Medications were recorded at time of transplantation and at 12, 18, and 24 months post-transplantation. Mean arterial pressure (MAP), estimated glomerular filtration rate (eGFR), and potassium (K) were recorded at each follow-up timepoint. Subjects without hyperkalemia (K > 5.0 mEq/L), hypotension (MAP < 70), or severely decreased renal function (eGFR < 30 mL/min) during the follow-up period were defined as potentially eligible to have received RAASi. Echocardiograms at time of initial HFrEF diagnosis, any subsequent echocardiogram performed prior to RT, and first echocardiogram at least 90 days after RT were captured. Continuous variables were compared by unpaired Student’s T-test or analysis of variance (ANOVA). Categorical variables were compared by Fisher exact test, and ordinal variables were compared using the Kruskal-Wallis rank sum test. The McNemar’s Chi-squared test with continuity correction was used for paired comparisons of pre- and post-RT medication use. Analysis was performed in R version 4.2.1.
2. Results
Of 750 RT recipients, 47 (6.3 %) had prior HFrEF diagnosis (Table 1). EF improved to ≥40 % in 26 of 40 subjects who had a subsequent echocardiogram prior to RT (65.0 %). Subjects experiencing pre-RT EF improvement were more commonly female (p = 0.020). Beta blockers (BB) were used in 43 (91.5 %) and RAASi in 23 (48.9 %) of subjects pre-RT, which did not differ based on pre-RT EF improvement (BB: p = 0.311, RAASi: p = 0.244). Of subjects on pre-RT RAASi, angiotensin-converting enzyme inhibitors (ACEi) were used in 15 (65.2 %), angiotensin receptor blockers (ARB) in 7 (30.4 %), and angiotensin receptor neprilysin inhibitors (ARNI) in 1 (4.3 %), and target HFrEF dosing had been achieved in 14 (60.9 %) subjects. No subjects were on SGLT2i or MRA at time of RT. Post-RT, two subjects died during the study period; one death was COVID-19-related and the other cause was unknown. Eight subjects restarted dialysis.
Table 1.
Demographics and clinical characteristics of renal transplant recipients with history of heart failure with reduced ejection fraction.
|
Reduced Pre-RT EF (<40 %) (N = 21) |
Improved Pre-RT EF (≥40 %) (N = 26) |
Total (N = 47) |
P-value | |
|---|---|---|---|---|
| Age at transplant (years) | 0.898 | |||
| Median (Q1, Q3) | 56.0 (49.0, 62.0) | 55.0 (44.5, 65.5) | 56.0 (45.0, 63.5) | |
| Range | 31.0–70.0 | 27.0–73.0 | 27.0–73.0 | |
| Female sex | 2 (9.5 %) | 11 (42.3 %) | 13 (27.7 %) | 0.020 |
| Race/ethnicity | 1.000 | |||
| Hispanic/Latinx | 5 (23.8 %) | 6 (23.1 %) | 11 (23.4 %) | |
| Non-Hispanic Black | 10 (47.6 %) | 11 (42.3 %) | 21 (44.7 %) | |
| Non-Hispanic White | 4 (19.0 %) | 6 (23.1 %) | 10 (21.3 %) | |
| Other | 2 (9.5 %) | 3 (11.5 %) | 5 (10.6 %) | |
| Body mass index (kg/m2) | 0.104 | |||
| Median (Q1, Q3) | 27.8 (25.0, 31.7) | 33.0 (25.4, 42.0) | 30.0 (25.1, 41.4) | |
| Range | 16.6–48.1 | 20.0–52.5 | 16.6–52.5 | |
| Donor type | 0.717 | |||
| Deceased donor | 14 (66.7 %) | 14 (53.8 %) | 28 (59.6 %) | |
| Living related donor | 4 (19.0 %) | 6 (23.1 %) | 10 (21.3 %) | |
| Living unrelated donor | 3 (14.3 %) | 6 (23.1 %) | 9 (19.1 %) | |
| Diabetes | 10 (47.6 %) | 15 (57.7 %) | 25 (53.2 %) | 0.564 |
| Hypertension | 21 (100.0 %) | 26 (100.0 %) | 47 (100.0 %) | 1.000 |
| Coronary artery disease | 8 (38.1 %) | 12 (46.2 %) | 20 (42.6 %) | 0.767 |
| Preoperative cardiology evaluation | 10 (47.6 %) | 18 (69.2 %) | 28 (59.6 %) | 0.151 |
| Pre-RT EF | <0.001 | |||
| 20–30 % | 6 (28.6 %) | 0 (0.0 %) | 6 (12.8 %) | |
| 30–40 % | 15 (71.4 %) | 0 (0.0 %) | 15 (31.9 %) | |
| 40–50 % | 0 (0.0 %) | 7 (26.9 %) | 7 (14.9 %) | |
| ≥50 % | 0 (0.0 %) | 19 (73.1 %) | 19 (40.4 %) | |
| Baseline medications | ||||
| Beta blocker | 18 (85.7 %) | 25 (96.2 %) | 43 (91.5 %) | 0.311 |
| RAASi | 8 (38.1 %) | 15 (57.7 %) | 23 (48.9 %) | 0.244 |
| Hydralazine/nitrate | 5 (23.8 %) | 5 (20.0 %) | 10 (21.7 %) | 1.000 |
| Loop diuretic | 6 (28.6 %) | 8 (32.0 %) | 14 (30.4 %) | 1.000 |
| MRA | 0 (0.0 %) | 0 (0.0 %) | 0 (0.0 %) | − |
| SGLT2i | 0 (0.0 %) | 0 (0.0 %) | 0 (0.0 %) | − |
| Post-RT outpatient cardiology follow-up | 8 (38.1 %) | 16 (61.5 %) | 24 (51.1 %) | 0.147 |
| Post-RT echocardiogram ≥ 90d | ||||
| Echocardiogram performed | 7 (33.3 %) | 11 (42.3 %) | 18 (38.3 %) | 0.562 |
| Post-RT EF | 0.277 | |||
| 20–30 % | 0 (0.0 %) | 0 (0.0 %) | 0 (0.0 %) | |
| 30–40 % | 1 (14.3 %) | 1 (9.1 %) | 2 (11.1 %) | |
| 40–50 % | 0 (0.0 %) | 4 (36.4 %) | 4 (22.2 %) | |
| ≥50 % | 6 (85.7 %) | 6 (54.5 %) | 12 (66.7 %) | |
| Time to echocardiogram (mo) | 0.754 | |||
| Median (Q1, Q3) | 12.7 (5.1, 17.6) | 7.3 (5.8, 13.5) | 8.6 (5.6, 16.8) | |
| Range | 3.3–19.4 | 3.9–24.1 | 3.3–24.1 | |
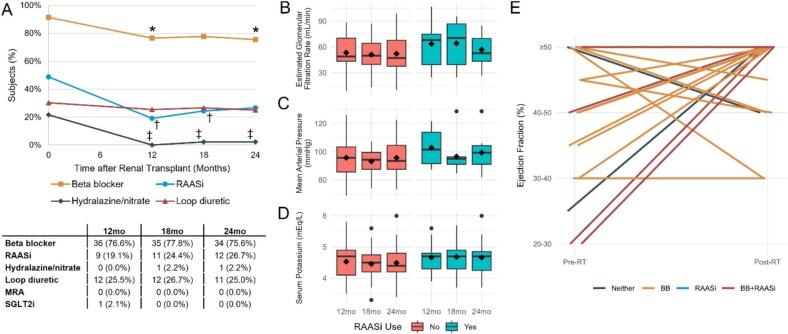
Patterns of post-RT medication use are shown in Fig. 1A. Compared to pre-RT, by 12 months post-RT there was lower usage of BB (p = 0.046), RAASi (p = 0.011), and hydralazine/nitrate (p = 0.004). Loop diuretic use did not significantly change during the follow-up period. No subjects had started an MRA during the follow-up period, and 1 subject started SGLT2i by 12 months for diabetes. Of subjects on RAASi at any point during follow-up, ACEi were used in 13 (86.7 %) and ARB in 2 (13.3 %). Target post-RT RAASi dosing was achieved by the end of the follow-up period in only 2 subjects. Measurements of eGFR, serum K, and MAP stratified by post-RT RAASi use are shown in Fig. 1B–D; there were no significant differences in mean values between RAASi users and non-users at 12, 18, or 24 months. Of 119 serum K measurements captured at the 12, 18, and 24-month timepoints after RT, hyperkalemia (K > 5.0 mEq/L) was detected in 6/31 (19.4 %) measurements among subjects on RAASi, compared to 11/88 (12.5 %) among subjects not on RAASi (p = 0.377). Of 4 individuals with hyperkalemia while on RAASi at either 12 or 18 months, 3 remained on RAASi at the following timepoint. Proportion of eGFR measurements which were <30 mL/min during follow-up were comparably low regardless of RAASi use (3/30 [10.0 %] in RAASi users vs. 10/85 [11.8 %] in non-RAASi users, p = 1.000), and hypotension only occurred once in a non-RAASi user at 12 months (1/87 [1.1 %] vs. 0/29 [0.0 %], p = 1.000). Of 29 subjects deemed eligible for RAASi based on preserved renal function with absence of hyperkalemia or hypotension across the follow-up period, RAASi were prescribed in only 6 (20.7 %).
Fig. 1.
Heart failure medication use and ejection fraction trends after renal transplantation (RT). A) Proportion of subjects receiving heart failure medications across follow-up period. Symbols denote p < 0.05 at indicated timepoint compared to time of RT (* = beta blocker, † = RAASi, ‡ = hydralazine/nitrate). B-D) Box and whisker plots of B) post-RT estimated glomerular filtration rate (eGFR), C), mean arterial pressure (MAP), and D) serum potassium (K) stratified by RAASi use at that timepoint. Diamond denotes mean value. E) Subject-level comparisons of left ventricular ejection fraction (EF) at pre-RT and post-RT assessments.
Overall, 24 (51.1 %) RT recipients had post-RT outpatient cardiology follow-up, and 18 (38.3 %) had post-RT echocardiogram at a median of 8.6 months following RT. Those with post-RT cardiology follow-up were more likely to undergo echocardiogram (14 [58.3 %] vs. 4 [17.4 %], p = 0.006), but were not significantly more likely to receive post-RT BB (22 [91.7 %] vs. 17 [73.9 %], p = 0.137) or RAASi (6 [25.0 %] vs. 9 [39.1 %], p = 0.359). Demographics and comorbidities were otherwise similar between groups who received post-RT cardiology follow-up (Supplemental Table 1) or echocardiogram (Supplemental Table 2). Subject-level comparisons of pre- and post-RT EF are shown in Fig. 1E. EF improved in 10 (55.6 %), remained stable in 3 (16.7 %), and declined in 5 (27.8 %) of subjects. Post-RT EF was <40 % in 2 subjects, both of whom were on BB therapy, had cardiology follow-up, and were eligible but did not receive RAASi during the study period. Post-RT EF was 40–50 % in 4 subjects, of whom 3 were on BB, 3 had cardiology follow-up, and 2 were eligible but did not receive RAASi.
3. Discussion
While pre-transplant coronary disease management and risk stratification has received attention in the literature [7], far less has been given to post-RT cardiovascular care. Individuals with HFrEF prior to RT represent a high-risk cohort that may warrant post-RT follow-up or treatment intensification. In this study of RT recipients with HFrEF, we observed that although BB were started or continued in most subjects, RAASi, were used in a minority of eligible patients. Post-RT cardiology follow-up and echocardiography were additionally underutilized. Finally, in those with ongoing HFrEF on post-RT echocardiography, RAASi were not initiated. Together, these findings highlight important targets for potential improvement in treatment of HFrEF after RT.
Currently available evidence describing medication usage in RT recipients with HFrEF is limited. Among individuals with HFrEF receiving RT between 2000–2013 at an academic center, 64 % received BB, 16 % received RAASi, and 1 % received MRA by 12 months post-RT [5]. In a nationwide registry of RT recipients regardless of HFrEF status, 53 % received BB and 21 % received RAASi for hypertension management [8]. Our rates of BB and RAASi usage exceed the above, but RAASi remained underutilized among subjects without major contraindications.
While our cohort was underpowered to observe smaller changes in kidney function, K, and MAP, there were no clinically significant changes in these values related to RAASi use. We observed a 7 % higher incidence of hyperkalemia in RAASi users among our cohort, but this finding was not statistically significant, requiring replication in a larger cohort to further examine this difference. Importantly, we did not observe reduction in RAASi use at timepoints following a hyperkalemia measurement, suggesting these events were not clinically significant. Other groups have examined these parameters in larger cohorts of RT recipients without HFrEF, and have shown similarly insignificant changes associated with post-RT RAASi use [9].
While many patients in our study experienced EF improvement prior to or after RT, current guidelines strongly recommend continued therapy in patients with improved EF to prevent relapse, even if asymptomatic [6]. Improvement in renal function via RT removes barriers and contraindications for RAASi, MRA, and SGLT2i use, offering an important opportunity for cardiologists to identify appropriate scenarios to start or intensify treatment. As cardiologists may not feel empowered to do so without agreement from the transplant team, we have engaged with transplant surgeons, pharmacists, and nephrologists at our center to implement strategies for coordinating post-RT follow-up, including re-evaluation of EF and initiating guideline-directed therapy in eligible patients. We urge cardiovascular teams at other RT centers to similarly evaluate the burden of post-RT HFrEF in their population and consider multidisciplinary collaboration to improve care delivery. The value of this effort may be enhanced in centers such as ours who do not offer heart transplantation or durable mechanical circulatory support, where the institutional presence of HF-focused clinicians and pharmacists may be proportionally lower as compared to transplant centers.
Limitations of our study include a small sample size and single-center study design. Additionally, data on post-RT functional capacity and reasons for not prescribing medications were limited from retrospective chart review. Substantial improvement in functional capacity due to RT alone could explain a more conservative approach to HFrEF medical management. Our analysis also assumed hypotension, hyperkalemia, and low eGFR as the primary factors determining RAASi eligibility, whereas additional clinical factors may play a role. Finally, our results should also be interpreted in the context of standard HFrEF management during the 2015–2022 study period, and do not reflect recent 2022 guideline updates strongly recommending SGLT2i and angiotensin receptor-neprilysin inhibitors as first line agents.
4. Conclusions
In this study of HFrEF management practices in a contemporary cohort of individuals with history of HFrEF receiving RT at a single tertiary academic medical center, post-RT HFrEF medications, cardiology follow-up, and echocardiography remain inconsistently utilized. Close collaboration between cardiovascular and transplant care teams may help identify and address care gaps in this understudied yet high-risk group of patients.
CRediT authorship contribution statement
Michael C. Hill: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Kaitlyn Legg: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Amer Ardati: Writing – review & editing, Methodology, Conceptualization. Vicki Groo: Writing – review & editing, Supervision, Methodology, Investigation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Michael Hill reports financial support was provided by American Heart Association Inc. Michael Hill reports financial support was provided by National Heart Lung and Blood Institute. Michael Hill reports financial support was provided by National Center for Advancing Translational Sciences. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
MH is supported by NIH T32HL139439 and AHA 23POST1019044. The University of Illinois Chicago, USA Center for Clinical and Translational Science (CCTS) is supported by UL1TR002003. The authors have no conflicts of interest to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101535.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Johansen K.L., Chertow G.M., Foley R.N., et al. US Renal Data System 2020 annual data report: epidemiology of kidney disease in the United States. Am. J. Kidney Dis. 2021;77(4 Suppl. 1):A7–A8. doi: 10.1053/j.ajkd.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rangaswami J., Mathew R.O., Parasuraman R., et al. Cardiovascular disease in the kidney transplant recipient: epidemiology, diagnosis and management strategies. Nephrol. Dial. Transplant. 2019;34(5):760–773. doi: 10.1093/ndt/gfz053. [DOI] [PubMed] [Google Scholar]
- 3.Goyal A., Chatterjee K., Mathew R.O., et al. In-hospital mortality and major adverse cardiovascular events after kidney transplantation in the United States. Cardiorenal Med. 2019;9(1):51–60. doi: 10.1159/000492731. [DOI] [PubMed] [Google Scholar]
- 4.Wali R.K., Wang G.S., Gottlieb S.S., et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J. Am. Coll. Cardiol. 2005;45(7):1051–1060. doi: 10.1016/j.jacc.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 5.Hawwa N., Shrestha K., Hammadah M., Yeo P.S.D., Fatica R., Tang W.H.W. Reverse remodeling and prognosis following kidney transplantation in contemporary patients with cardiac dysfunction. J. Am. Coll. Cardiol. 2015;66(16):1779–1787. doi: 10.1016/j.jacc.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022;145(18):e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 7.Cheng X.S., VanWagner L.B., Costa S.P., et al. Emerging evidence on coronary heart disease screening in kidney and liver transplantation candidates: a scientific statement from the American Heart Association. Circulation. 2022;146(21):e299–e324. doi: 10.1161/CIR.0000000000001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koraishy F.M., Yamout H., Naik A.S., et al. Impacts of center and clinical factors in antihypertensive medication use after kidney transplantation. Clin. Transplant. 2020;34(3):e13803. doi: 10.1111/ctr.13803. [DOI] [PubMed] [Google Scholar]
- 9.Heleniak Z., Kuźmiuk-Glembin I., Adrych D., et al. Management of renin-angiotensin-aldosterone system blockade in kidney transplant recipients. Transpl. Proc. 2018;50(6):1842–1846. doi: 10.1016/j.transproceed.2018.03.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.