Abstract
Neuromuscular scoliosis can be caused by muscular or nervous system dysfunction resulting from genetic variants. Variation in MYH7 may cause hypertrophic or dilated cardiomyopathy, skeletal myopathies, or a combination of both; however, scoliosis has rarely been reported. We analyzed a Chinese pedigree with two members suffering from scoliosis. Whole-exome sequencing identified a variant (NM_000257.4:c.2011C > T) of MYH7 that cosegregated with the scoliosis phenotype. The variant resulted in a change in the evolutionarily conserved amino acid residue 671 from arginine to cystine (p.R671C), which was predicted to disrupt the structure and function of the motor domain of the slow/β-cardiac myosin heavy chain encoded by MYH7. To date, 913 MYH7 variants were associated with cardiomyopathy and/or skeletal myopathies according to the Human Gene Mutation Database. However, only 15 cases of scoliosis have been reported. In our case, the c.2011C > T variant caused scoliosis with 100 % penetrance and hypertrophic cardiomyopathy with partial penetrance.
Keywords: Neuromuscular scoliosis, Skeletal myopathy, Whole-exome sequencing, MYH7
Highlights
-
•
A Chinese family with two members suffering from neuromuscular scoliosis.
-
•
The MYH7 gene variant, c.2011C > T (p.R671C) cosegregated with the scoliosis phenotype.
-
•
Most of MYH7 variants that cause scoliosis located in the distal region of the C-terminal rod tail domain of Myosin-7.
1. Introduction
Neuromuscular scoliosis is a common spinal deformity caused by abnormal and asymmetric muscle forces owing to muscular or nervous system dysfunction, accounting for approximately 90 % of all scoliosis cases [1]. It typically presents at an early age, progresses continually, and has a massive impact on daily life. Skeletal myopathy caused by genetic variants is a major etiology [2]. To promote the diagnosis and classification of neuromuscular scoliosis and to undertake appropriate treatment, it is essential to identify more pathogenic gene variants and establish genotype–phenotype correlations.
Myosin, which consists of two myosin heavy chains and two pairs of light chain subunits, is the major motor protein that provides mechanical forces during muscle contraction [3]. The slow/β-cardiac myosin heavy chain, Myosin-7, is encoded by MYH7 gene and is primarily expressed in slow-twitch type I fibers and cardiac muscle. Myosin-7 comprises an amino-terminal globular head domain and a carboxyl-terminal rod tail domain. The former can bind to actin and generate mechanical force to drive muscle contraction through ATP-hydrolysis. The latter is responsible for assembly into myosin filaments [4,5]. Variation in MYH7 may cause cardiac and/or skeletal myopathies; however, scoliosis has rarely been reported [6,7]. According to the Human Gene Mutation Database, at least 913 variants in MYH7 have been identified to cause hypertrophic or dilated cardiomyopathy, skeletal myopathies, or a combination of both, including hypertrophic and dilated cardiomyopathy (MIM 192600), non-compaction and restrictive cardiomyopathy (MIM 613426), Laing distal myopathy (MIM 160500), myosin storage myopathy (MIM 608358) and Ebstein anomaly (MIM 224700) [8]. However, only 15 MYH7 variants have been reported to cause scoliosis to date [7,[9], [10], [11], [12], [13]].
In the present study, we identified a de novo heterozygous c.2011C > T variant in MYH7 in a Chinese family with scoliosis via whole-exome sequencing, which was predicted to disrupt the structure and function of Myosin-7. In contrast to previous reports that patients with variants in MYH7 primarily presented cardiomyopathy and/or skeletal myopathies, and rarely scoliosis, the c.2011C > T caused scoliosis with 100 % penetrance, while hypertrophic cardiomyopathy with partial penetrance in our case. Our findings expanded the mutational spectrum of MYH7-related neuromuscular scoliosis, which would be helpful for screening and genetic diagnosis of the disease.
2. Material and methods
2.1. Subjects
A Chinese family with hereditary scoliosis was recruited at Guangzhou Women and Children's Medical Center, including 2 affected and 4 unaffected individuals. Clinical and radiographic examinations were performed. The diagnosis of scoliosis was based on at least a 10° curvature in the coronal plane of spine [14].
2.2. Whole-exome sequencing and genetic variant analysis
Genomic DNA (gDNA) was extracted from peripheral blood cells with the Blood DNA Kit (Omega, USA) according to the manufacturer's instructions. The concentration of gDNA was determined by a Qubit Fluorometer. The integrity and purity were detected with agarose gel electrophoresis. Then, whole-exome sequencing was outsourced to Novogene (Beijing, China). Data analysis was performed as previously described [15]. The detected variants were further filtered following a pipeline: 1) exclusion of variants with a frequency greater than 1 % in any of the four databases (1000g_all, esp6500si_all, gnomAD_ALL and gnomAD_EAS), 2) exclusion of variants that were not in the coding (exonic) region or splicing region (splicing site ± 10 bp), 3) exclusion of synonymous SNPs that were predicted not to affect splicing, 4) retention of variants that were predicted by at least two of four prediction tools (SIFT, PolyPhen, MutationTaster, and CADD) to be deleterious and variants that were predicted to affect splicing. The variant's nomenclature was validated with https://variantvalidator.org/.
2.3. Sanger sequencing
gDNA extracted from peripheral blood was used as template. The following primers were used: 5′-TCTCTTCCCGTCATCTCCTGG-3’ (forward) and 5′- CACACTGCAAGTGCAAGGTAGC-3’ (reverse). PCR reaction was performed with High fidelity PrimeSTAR Max DNA Polymerase (Takara, Beijing, China). The qualified products were sent to Shanghai Sangon Biotech (Shanghai, China) for sequencing.
2.4. Evolutionary conservation analysis
Protein coding sequences of Myosin-7 from seven different animal species including human (ENSP00000347507), horse (ENSECAP00000019439), pig (ENSSSCP00000002219), dog (ENSCAFP00845005623), mouse (ENSMUSP00000099867), chicken (ENSGALP00000048124), and zebrafish (ENSDARP00000146011) were aligned using MultAlin (https://mutalin.toulouse.inra.fr/multalin) to evaluate the evolutionary conservation of the mutated site.
2.5. Structural bioinformatics analysis
The AlphaFold structure prediction PDB file of Myosin-7 was downloaded from http://alphafold.ebi.ac.uk/entry/P12883. To evaluate the structural changes introduced by the amino acid substitution (p.R671C), the PDB file was uploaded to Missense3D website (http://missense3d.bc.ic.ac.uk/∼missense3d/).
3. Results
3.1. Clinical features
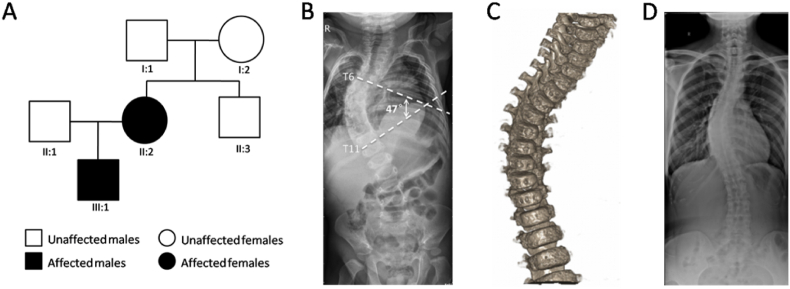
The family pedigree was shown in Fig. 1A. The proband (III:1), an approximately 6-month-old boy, was diagnosed with early onset scoliosis through a series of examinations. Adam's test result was positive. Both X-ray and computed tomography analyses (CT) revealed that the thoracic segment of the spine was convex to the right side with a Cobb angle of 47° (Fig. 1B and C). The shape and bone masses of the thoracic and lumbar vertebrae were normal. The proband also presented clinical features of skeletal myopathy, particularly muscular hypertonia of the extremities and abdomen. In addition, echocardiography revealed patent foramen ovale, mitral regurgitation, and decreased ventricular diastolic function (Fig. 1S). His mother (II:2), 33 years old, presented with mild scoliosis and had no any other corresponding symptoms (Fig. 1D). His father and other family members were healthy.
Fig. 1.
A Chinese family with hereditary scoliosis. (A) The pedigree. (B, C) X-radiographs and Coronal CT scanning of the proband (III:1) demonstrated the thoracic segment of his spine is obviously convex to the right side with a Cobb angel 47°. (D) The X-ray of the proband's mother showed mild scoliosis.
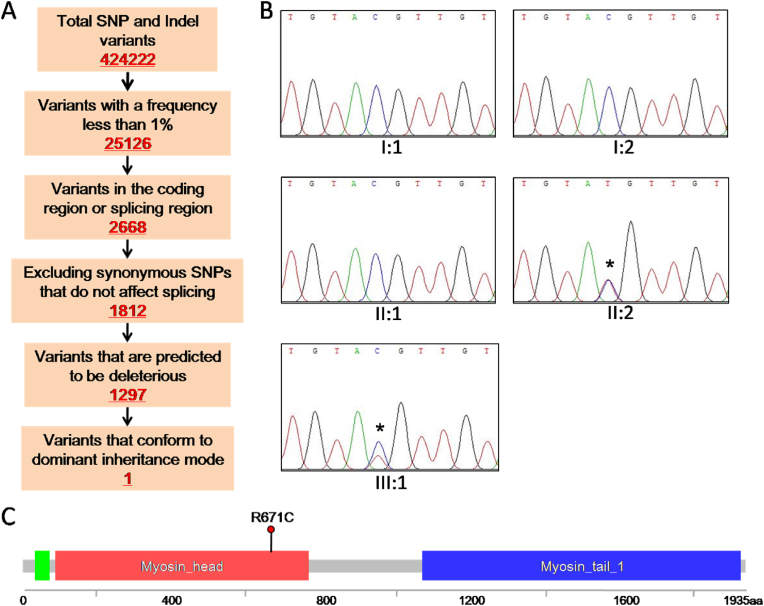
3.2. The MYH7 c.2011C > T variant co-segregated with scoliosis phenotype
As both the proband and his mother have scoliosis, the cause was likely genetic variation. To identify the causative genetic variant, we performed whole-exome sequencing with gDNAs from patients and their relatives. A total of 424,222 genetic variants, including 363,205 SNPs (single nucleotide polymorphisms) and 61,017 Indels (small insertions or deletions, <50 bp), were initially detected. After variant filtering, 960 SNPs and 237 Indels were retained (Fig. 2A). Then, we screened for variants that conformed to dominant inheritance mode. Finally, a heterozygous variant in exon 18 of MYH7, c.2011C > T, was detected in the proband and his mother but not in unaffected family members, suggesting that the variant co-segregated with the scoliosis phenotype and inherited in an autosomal dominant manner. This finding was further verified by Sanger sequencing (Fig. 2B and C). To evaluate the pathogenicity of the missense variant, we employed three common prediction tools: SIFT (Sorting Intolerant From Tolerant, http://pvovean.jcvi.org), Polyphen2 (http://genetics.bwh.harvard.edu/pph2), and MutationTaster (http://www.mutationtaster.org). The variant was predicted to be deleterious by all three tools.
Fig. 2.
Identification of a variant in the MYH7 gene cosegregated with the scoliosis symptom. (A) Schematic representation of the filtering process of WES data. A variant of c.2011C > T in MYH7 gene was identified. (B) Sanger sequencing results from blood genomic DNA. All affected individuals (II:2 and III:1) carry the heterozygous variant. The unaffected (I:1, I:2, and II:1) are as control. The asterisks indicate the variant. (C) The location of the variant (c.2011C > T:p.R671C) was schematically demonstrated in Myosin-7 protein. The website https://www.cbioportal.org/mutation_mapper was used.
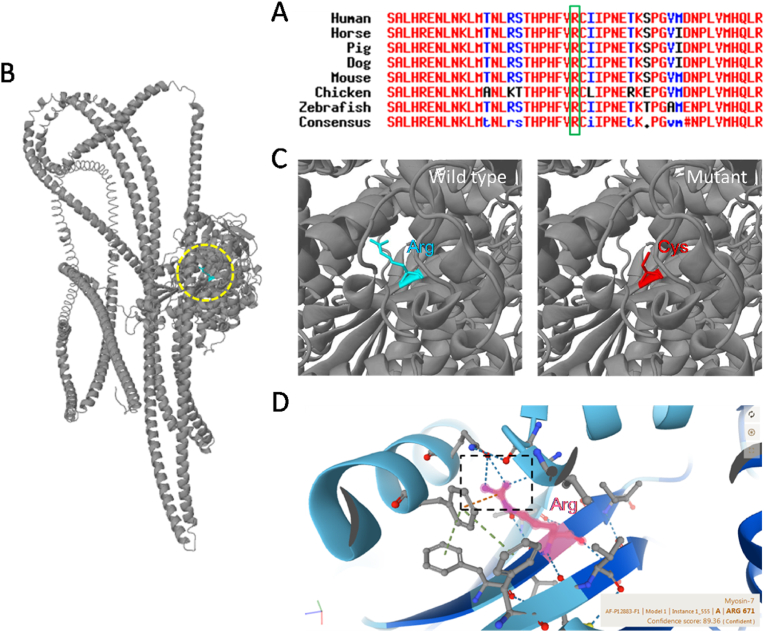
3.3. The structural changes introduced by the amino acid substitution (p.R671C)
The variant resulted in a change in the evolutionarily conserved amino acid residue 671 from arginine to cystine (p.R671C) in one strand of the central β-sheet of the Myosin-7 motor domain (Fig. 3A and B). Further structural bioinformatics analysis with Missense3D indicated that the substitution replaced a buried charged residue with an uncharged residue, which led to the loss of cation-Pi interaction with Phe 489 and 3 hydrogen bonds with neighboring residues (Fig. 3C and D). Consequently, the cavity volume was expanded by 150.768 Å^3. Thus, this variant may disrupt the normal structure and function of the Myosin-7 amino-terminal motor domain/head domain.
Fig. 3.
Evolutionary conservation analysis and structural bioinformatics analysis. A. Alignment of the amino acid sequences of Myosin-7 indicated that the arginine residue replaced owing to mutation is evolutionarily conserved across species. The green square marks the arginine residue. B, C. Structural bioinformatics analysis with Missense3D. The spatial position of the mutation site in the motor/head domain of Myosin-7 protein was circled by yellow dashed line in (B). The analysis results indicated that the substitution leads to the loss of cation-Pi interaction with Phe 489 and hydrogen bonds with neighboring residues (WT: Arg671-NH2 … OG1-Thr177, Arg671-NH1 … OE1-Gln486, Arg671-NH2 … OE1-Gln486, Arg671-NH2 … OD1-Asn486 vs. Mutant: Asn696-ND2 … SG-Cys671). D. The wild type 3D structure from AlphaFold Protein Structure Database to show the interaction of Arg671 with neighboring amino acids. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
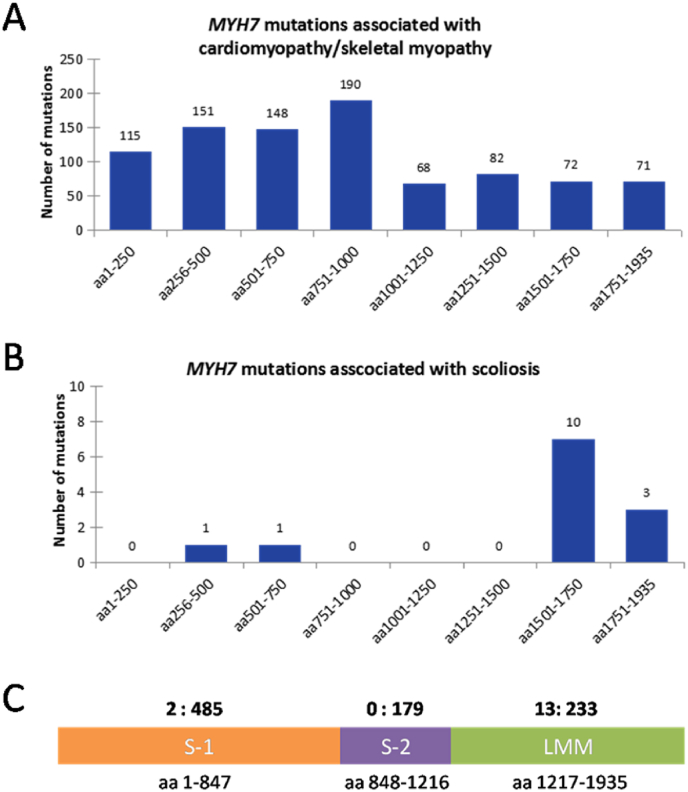
3.4. Analysis of variant distribution in Myosin-7
We further analyzed the distribution of the variants of MYH7. Most MYH7 variants associated with cardiomyopathy/skeletal myopathy are found in the N-terminal region of Myosin-7, corresponding to the motor domain/head domain (Fig. 4A–C). In contrast, 13 of the total 15 variants associated with scoliosis are found in the distal region of the C-terminal rod tail domain, which can form a coiled-coil structure to integrate into thick filaments in muscle sarcomeres (Fig. 4B and C). Nonetheless, the variant p.R671C associated with scoliosis in our case was located in the motor domain of Myosin-7.
Fig. 4.
Variant distribution in Myosin-7. A. Most MYH7 variants associated with cardiomyopathy/skeletal myopathy (M-myo) were distributed in the first half part of Myosin-7. The number of unique variants in every 250 amino acids was shown on the top of each column. B. 13 of total 15 variants associated with scoliosis (M-sco) were located in the C-terminal region composed of last 435 amino acids. C. The ratio of the number of two types of variation (M-myo: M-sco) in each functional domain of Myosin-7 were shown on the top of the schematic. S-1 (subfragment 1), corresponding to head domain, is mainly responsible for ATP-hydrolysis and binding to actin. S-2 (subfragment 2) and LMM (light meromyosin) make up the filament-forming tail domain. Note: 897 of total 913 MYH7 variants were counted here, including 862 missense/nonsense mutation, 29 small deletions, and 4 small indels.
4. Discussion
In this study, we reported the case of a Chinese pedigree with hereditary scoliosis. As the proband also manifested skeletal myopathies and cardiomyopathy, we attributed this to neuromuscular scoliosis. Whole-exome sequencing identified a de novo MYH7 c.2011C > T (p.R671C) heterozygous variant co-segregated with the scoliosis phenotype, which was predicted to disrupt the normal structure and function of the Myosin-7 amino-terminal motor domain/head domain.
According to the Human Gene Mutation Database, at least 913 MYH7 variants are associated with cardiomyopathy and/or skeletal myopathies. The MYH7 c.2011C > T mutation identified in our case has been previously reported to cause hypertrophic cardiomyopathy [6]. However, scoliosis caused by variants in MYH7 was rarely reported. Through a literature review, we found that only 15 MYH7 variants have been related to scoliosis to date (Table 1) [7,[9], [10], [11], [12], [13]]. In our case, the c.2011C > T variant caused scoliosis with 100 % penetrance, with partial penetrance of hypertrophic cardiomyopathy.
Table 1.
MYH7 variants associated with scoliosis based on a literature review.
| Country of Origin | Genotype |
Phenotypes |
Reference | |||
|---|---|---|---|---|---|---|
| Nucleotide change | Amino acid change | Scoliosis | Cardiomyopathy | Skeletal myopathy | ||
| N/A | c.1489_1500del | p.Glu500del | Kyphoscoliosis | N/A | Y | 12 |
| China | c.2011C > T | p.Arg671Cys | Scoliosis | Y | Y | Present study |
| USA | c.4522_4524del | p.Glu1508del | Scoliosis | Y | Y | 7 |
| UK | c.4664A > G | p.Glu1555Gly | Scoliosis | N | Y | 13 |
| UK | c.4699C > T | p.Gln1567∗ | Scoliosis | N | Y | 13 |
| UK | c.4823G > C | p.Arg1608Pro | Thoracic scoliosis | Y | Y | 7 |
| UK | c.4835T > C | p.Leu1612Pro | Spinal rigidity and scoliosis | N | Y | 7 |
| China | c.4849_4851 | p.Lys1617del | Scoliosis | Y | Y | 10 |
| Spain | c.4906G > C | p.Ala1636Pro | Thoracic scoliosis | Y | Y | 7 |
| USA | c.4937T > C | p.Leu1646Pro | Thoracic scoliosis, lumbar scoliosis | N | Y | 7 |
| China | c.5059_5061del | p.Glu1687del | Scoliosis | N | Y | 11 |
| AUS | c.5134C > T | p.Arg1712Trp | Scoliosis | N | Y | 13 |
| N/A | c.5352_5354del | p.Lys1784del | Scoliosis | Y | Y | 9 |
| UK | c.5378_5380del | p.Leu1793del | Kyphoscoliosis | Y | Y | 7 |
| Israel | c.5401G > A | p.Glu1801Lys | Lumbar lordosis | Y | Y | 7 |
Note: MYH7 transcript used = NM_000257.4. Y, yes; N, no.
Myosin-7 comprises an amino-terminal globular head domain and carboxyl-terminal rod tail domain. The former can bind to actin and generate mechanical force to drive muscle contraction through ATP-hydrolysis. The latter can form a coiled-coil structure to integrate into thick filaments in muscle sarcomeres. We found that most MYH7 variants associated with cardiomyopathy/skeletal myopathy were located in the N-terminal region of Myosin-7, corresponding to the motor domain/head domain. In contrast, 13 of the total 15 variants associated with scoliosis were found in the distal region of the C-terminal rod tail domain. These findings suggested a genotype–phenotype correlation in diseases caused by MYH7 variation.
Variants in MYH7 typically cause cardiomyopathy and/or skeletal myopathy. However, it has been rarely reported to be associated with scoliosis. In our case, the MYH7 c.2011C > T variant co-segregated with the scoliosis phenotype. Our findings expand the mutational spectrum of MYH7-related neuromuscular scoliosis, which may be helpful for screening and genetic diagnosis of the disease.
CRediT authorship contribution statement
Ping Wei: Methodology, Investigation. Fulong Xu: Visualization, Validation, Resources. Caixia Xian: Validation, Investigation. Yanhan Liu: Validation, Investigation. Yibo Xu: Investigation. Ting Zhang: Resources. Weizhe Shi: Resources, Funding acquisition. Sihong Huang: Resources. Xiang Zhou: Funding acquisition. Mingwei Zhu: Writing – original draft, Supervision, Project administration, Funding acquisition, Conceptualization. Hongwen Xu: Writing – review & editing, Validation, Supervision, Conceptualization.
Ethics approval and consent to participate
This study was conducted in accordance with the declaration of Helsinki. All study protocols were approved by the Human Ethics Committee of the Guangzhou Women and Children's Medical Center. The written informed consent was obtained from each participant or their legal custodians.
Funding
This work was supported by Natural Science Foundation of Guangdong Province in China (No. 2023A1515010281 to M.Z. and No. 2021A1515010597 to X. Z.) and National Youth Natural Science Foundation of China (No. 82302106 to W.S.).
Declaration of competing interest
The authors declare no conflict of interests.
Acknowledgements
We thank all family members for their kind cooperation and enthusiastic participation in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2024.101845.
Contributor Information
Mingwei Zhu, Email: mweizh@gzhmu.edu.cn.
Hongwen Xu, Email: xuhongwen@gwcmc.org.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Halawi M.J., Lark R.K., Fitch R.D. Neuromuscular scoliosis: current concepts. Orthopedics. 2015;38(6):e452–e456. doi: 10.3928/01477447-20150603-50. [DOI] [PubMed] [Google Scholar]
- 2.Wishart B.D., Kivlehan E. Neuromuscular scoliosis: when, who, why and outcomes. Phys. Med. Rehabil. Clin. 2021;32(3):547–556. doi: 10.1016/j.pmr.2021.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Homburger J.R., Green E.M., Caleshu C., et al. Multidimensional structure-function relationships in human β-cardiac myosin from population-scale genetic variation. Proc. Natl. Acad. Sci. U.S.A. 2016;113(24):6701–6706. doi: 10.1073/pnas.1606950113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colegrave M., Peckham M. Structrual implications of β-cardiac myosin heavy chain mutations in human disease. Anat. Rec. 2014;297(9):1670–1680. doi: 10.1002/ar.22973. [DOI] [PubMed] [Google Scholar]
- 5.Wolny M., Colegrave M., Colman L., et al. Cardiomyopathy mutations in the tail of β-cardiac myosin modify the coiled-coil structure and affect integration into thick filaments in muscle sarcomeres in adult cardiomyocytes. J. Biol. Chem. 2013;288(44):31952–31962. doi: 10.1074/jbc.M113.513291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richard P., Charron P., Carrier L., et al. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107(17):2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- 7.Lamont P.J., Wallefeld W., Hilton-Jones D., et al. Novel mutations widen the phenotypic spectrum of slow skeletal/β-cardiac myosin (MYH7) distal myopathy. Hum. Mutat. 2014;35(7):868–879. doi: 10.1002/humu.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feinstein-Linial M., Buvoli M., Buvoli A., et al. Two novel MYH7 proline substitutions cause Laing distal myopathy-like phenotypes with variable expressivity and neck extensor contracture. BMC Med. Genet. 2016;17(1):57. doi: 10.1186/s12881-016-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stalpers X., Verrips A., Braakhekke J., et al. Scoliosis surgery in a patient with “de novo” myosin storage myopathy. Neuromuscul. Disord. 2011;21(11):812–815. doi: 10.1016/j.nmd.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Oda T., Xiong H., Kobayashi K., et al. A de novo mutation of the MYH7 gene in a large Chinese family with autosomal dominant myopathy. Hum Genome Var. 2015;2 doi: 10.1038/hgv.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li N., Zhao Z., Shen H., et al. MYH7 mutation associated with two phenotypes of myopathy. Neurol. Sci. 2017;39(2):333–339. doi: 10.1007/s10072-017-3192-2. [DOI] [PubMed] [Google Scholar]
- 12.Ko J.Y., Lee M., Jang J.H., et al. A novel de novo mutation in MYH7 gene in a patient with early onset muscular weakness and severe kyphoscoliosis: a case report. Medicine (Baltim.) 2019;98(28) doi: 10.1097/MD.0000000000016389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beecroft S.J., van de Locht M., de Winter J.M., et al. Recessive MYH7-related myopathy in two families. Neuromuscul. Disord. 2019;29(6):456–467. doi: 10.1016/j.nmd.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Yang S., Andras L.M., Redding G.J., et al. Early-onset scoliosis: a review of history, current treatment, and future directions. Pediatrics. 2016;137(1) doi: 10.1542/peds.2015-0709. [DOI] [PubMed] [Google Scholar]
- 15.Li X., Shi W., Ding X., et al. Identification of a novel TBX5 mutation in a Chinese family with rare symptoms of Holt-Oram syndrome. Heliyon. 2022;8(11) doi: 10.1016/j.heliyon.2022.e11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.