Abstract
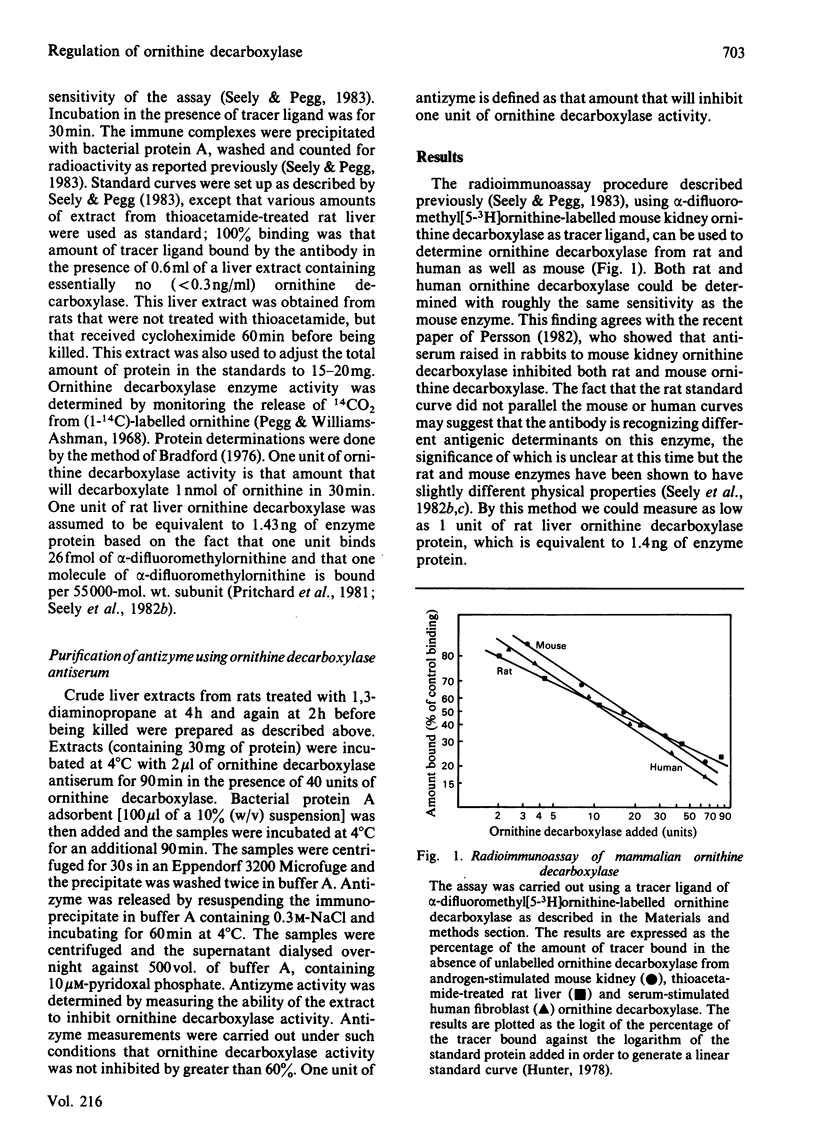
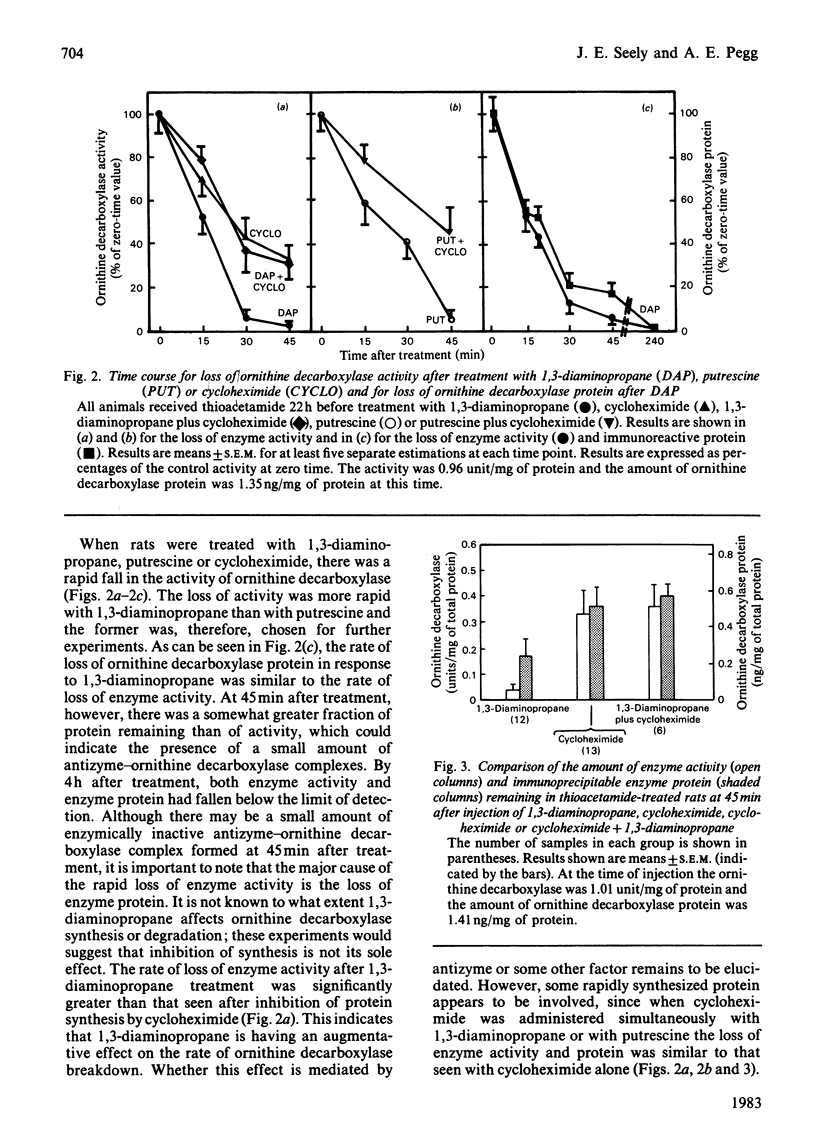
A radioimmunoassay for ornithine decarboxylase was used to study the regulation of this enzyme in rat liver. The antiserum used reacts with ornithine decarboxylase from mouse, human or rat cells. Rat liver ornithine decarboxylase enzyme activity and enzyme protein (as determined by radioimmunoassay) were measured in thioacetamide-treated rats at various times after administration of 1,3-diaminopropane. Enzyme activity declined rapidly after 1,3-diaminopropane treatment as did the amount of enzyme protein, although the disappearance of enzyme activity slightly preceded the loss of immunoreactive protein. The loss of enzyme protein after cycloheximide treatment also occurred rapidly, but was significantly slower than that seen with 1,3-diaminopropane. When 1,3-diaminopropane and cycloheximide were injected simultaneously, the rate of disappearance of enzyme activity and enzyme protein was the same as that seen with cycloheximide alone. These results show that the rapid loss in enzyme activity after 1,3-diaminopropane treatment is primarily due to a loss in enzyme protein and that protein synthesis is needed in order for 1,3-diaminopropane to exert its full effect. A macromolecular inhibitor of ornithine decarboxylase that has been termed antizyme is induced in response to 1,3-diaminopropane, but our results indicate that the loss of enzyme activity is not due to the accumulation of inactive ornithine decarboxylase-antizyme complexes. It is possible that the antizyme enhances the degradation of the enzyme protein. Control experiments demonstrated that the antiserum used would have detected any inactive antizyme-ornithine decarboxylase complexes present in liver since addition of antizyme to ornithine decarboxylase in vitro did not affect the amount of ornithine decarboxylase detected in our radioimmunoassay. Anti-(ornithine decarboxylase) antibodies may be useful in the purification of antizyme since the antizyme-ornithine decarboxylase complex can be immunoprecipitated, and antizyme released from the precipitate with 0.3 M-NaCl.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atmar V. J., Kuehn G. D. Phosphorylation of ornithine decarboxylase by a polyamine-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5518–5522. doi: 10.1073/pnas.78.9.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Canellakis E. S., Viceps-Madore D., Kyriakidis D. A., Heller J. S. The regulation and function of ornithine decarboxylase and of the polyamines. Curr Top Cell Regul. 1979;15:155–202. [PubMed] [Google Scholar]
- Etlinger J. D., Goldberg A. L. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K., Murakami Y., Hayashi S. A macromolecular inhibitor of the antizyme to ornithine decarboxylase. Biochem J. 1982 Jun 15;204(3):647–652. doi: 10.1042/bj2040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E. Immunochemical demonstration of increased accumulation of ornithine carboxylase in rat liver after partial hepatectomy and growth hormone induction. Biochim Biophys Acta. 1975 Aug 13;399(2):420–427. doi: 10.1016/0304-4165(75)90270-6. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Pegg A. E. Studies on ornithine decarboxylase activity in the isolated perfused rat liver. Biochim Biophys Acta. 1977 Sep 15;484(1):177–187. doi: 10.1016/0005-2744(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Kallio A., Löfman M., Pösö H., Jänne J. Diamine-induced inhibition of liver ornithine decarboxylase. Biochem J. 1979 Jan 1;177(1):63–69. doi: 10.1042/bj1770063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio A., Löfman M., Pösö H., Jänne J. Inhibition of ornithine decarboxylase by diamines in regenerating rat liver. Evidence for direct action on the accumulation of the enzyme protein. FEBS Lett. 1977 Jul 1;79(1):195–199. [PubMed] [Google Scholar]
- Kameji T., Murakami Y., Fujita K., Hayashi S. Purification and some properties of ornithine decarboxylase from rat liver. Biochim Biophys Acta. 1982 Jul 16;717(1):111–117. doi: 10.1016/0304-4165(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Benzie C. R. Direct effects of 1,3-diaminopropane on reticulocyte lysate protein synthesis. FEBS Lett. 1980 Dec 1;121(2):309–312. doi: 10.1016/0014-5793(80)80370-x. [DOI] [PubMed] [Google Scholar]
- McCann P. P., Tardif C., Mamont P. S. Regulation of ornithine decarboxylase by ODC-antizyme in HTC cells. Biochem Biophys Res Commun. 1977 Apr 25;75(4):948–954. doi: 10.1016/0006-291x(77)91474-7. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Campbell H. A., Carter D. D. Multiple ornithine decarboxylase forms in Physarum polycephalum: interconversion induced by cycloheximide. FEBS Lett. 1976 Feb 1;62(1):33–37. doi: 10.1016/0014-5793(76)80010-5. [DOI] [PubMed] [Google Scholar]
- Mitchell J. L., Carter D. D., Rybski J. A. Control of ornithine decarboxylase activity in Physarum by polyamines. Eur J Biochem. 1978 Dec;92(2):325–331. doi: 10.1111/j.1432-1033.1978.tb12751.x. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Conover C., Wrona A. Effects of aliphatic diamines on rat liver ornithine decarboxylase activity. Biochem J. 1978 Mar 15;170(3):651–660. doi: 10.1042/bj1700651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. Biosynthesis of putrescine in the prostate gland of the rat. Biochem J. 1968 Jul;108(4):533–539. doi: 10.1042/bj1080533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson L. Antibodies to ornithine decarboxylase. Immunochemical cross-reactivity. Acta Chem Scand B. 1982;36(10):685–688. doi: 10.3891/acta.chem.scand.36b-0685. [DOI] [PubMed] [Google Scholar]
- Persson L. Decarboxylation of ornithine and lysine by ornithine decarboxylase from kidneys of testosterone treated mice. Acta Chem Scand B. 1981;35(6):451–459. doi: 10.3891/acta.chem.scand.35b-0451. [DOI] [PubMed] [Google Scholar]
- Pritchard M. L., Seely J. E., Pösö H., Jefferson L. S., Pegg A. E. Binding of radioactive alpha-difluoromethylornithine to rat liver ornithine decarboxylase. Biochem Biophys Res Commun. 1981 Jun;100(4):1597–1603. doi: 10.1016/0006-291x(81)90701-4. [DOI] [PubMed] [Google Scholar]
- Prouty W. F. Ornithine decarboxylase inactivation in HeLa cells. J Cell Physiol. 1976 Sep;89(1):65–76. doi: 10.1002/jcp.1040890107. [DOI] [PubMed] [Google Scholar]
- Pösö H., Guha S. K., Jänne J. Stabilization of ornithine decarboxylase in rat liver. Biochim Biophys Acta. 1978 Jun 9;524(2):466–473. doi: 10.1016/0005-2744(78)90185-7. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Ornithine decarboxylase as a biological and pharmacological tool. Pharmacology. 1980;20(3):117–129. doi: 10.1159/000137355. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Posttranslational modification of ornithine decarboxylase by its product putrescine. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1167–1172. doi: 10.1016/0006-291x(81)90741-5. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol Pharmacol. 1969 May;5(3):253–262. [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Changes in mouse kidney ornithine decarboxylase activity are brought about by changes in the amount of enzyme protein as measured by radioimmunoassay. J Biol Chem. 1983 Feb 25;258(4):2496–2500. [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Effect of androgens on turnover of ornithine decarboxylase in mouse kidney. Studies using labeling of the enzyme by reaction with [14C] alpha-difluoromethylornithine. J Biol Chem. 1982 Jul 10;257(13):7549–7553. [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Measurement of the number of ornithine decarboxylase molecules in rat and mouse tissues under various physiological conditions by binding of radiolabelled alpha-difluoromethylornithine. Biochem J. 1982 Aug 15;206(2):311–318. doi: 10.1042/bj2060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Purification of ornithine decarboxylase from kidneys of androgen-treated mice. Biochemistry. 1982 Jul 6;21(14):3394–3399. doi: 10.1021/bi00257a023. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Tuomi K., Raina A., Mäntyjärvi R. 1,3-Diaminopropane rapidly inhibits protein synthesis and virus production in BKT-1 cells. FEBS Lett. 1980 Mar 10;111(2):329–332. doi: 10.1016/0014-5793(80)80820-9. [DOI] [PubMed] [Google Scholar]
- Wilkinson K. D., Urban M. K., Haas A. L. Ubiquitin is the ATP-dependent proteolysis factor I of rabbit reticulocytes. J Biol Chem. 1980 Aug 25;255(16):7529–7532. [PubMed] [Google Scholar]
- Woodside K. H. Effects of cycloheximide on protein degradation and gluconeogenesis in the perfused rat liver. Biochim Biophys Acta. 1976 Jan 14;421(1):70–79. doi: 10.1016/0304-4165(76)90170-7. [DOI] [PubMed] [Google Scholar]


