Abstract
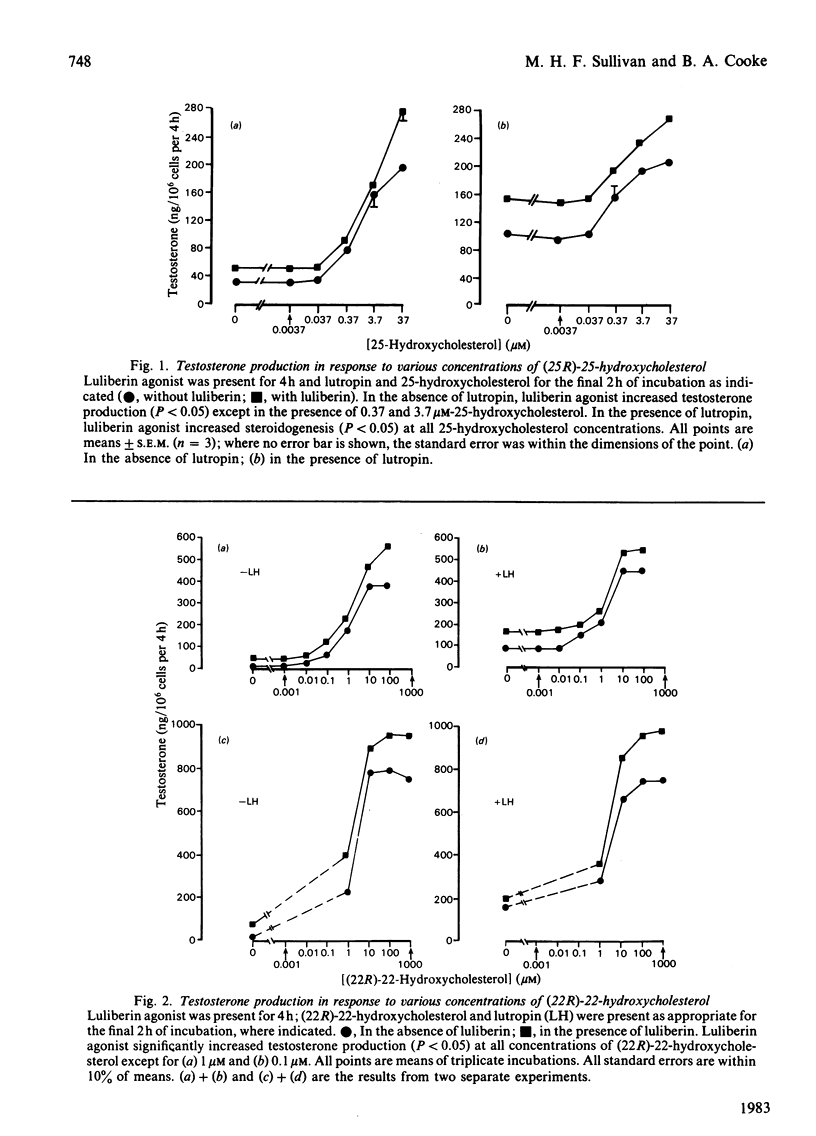
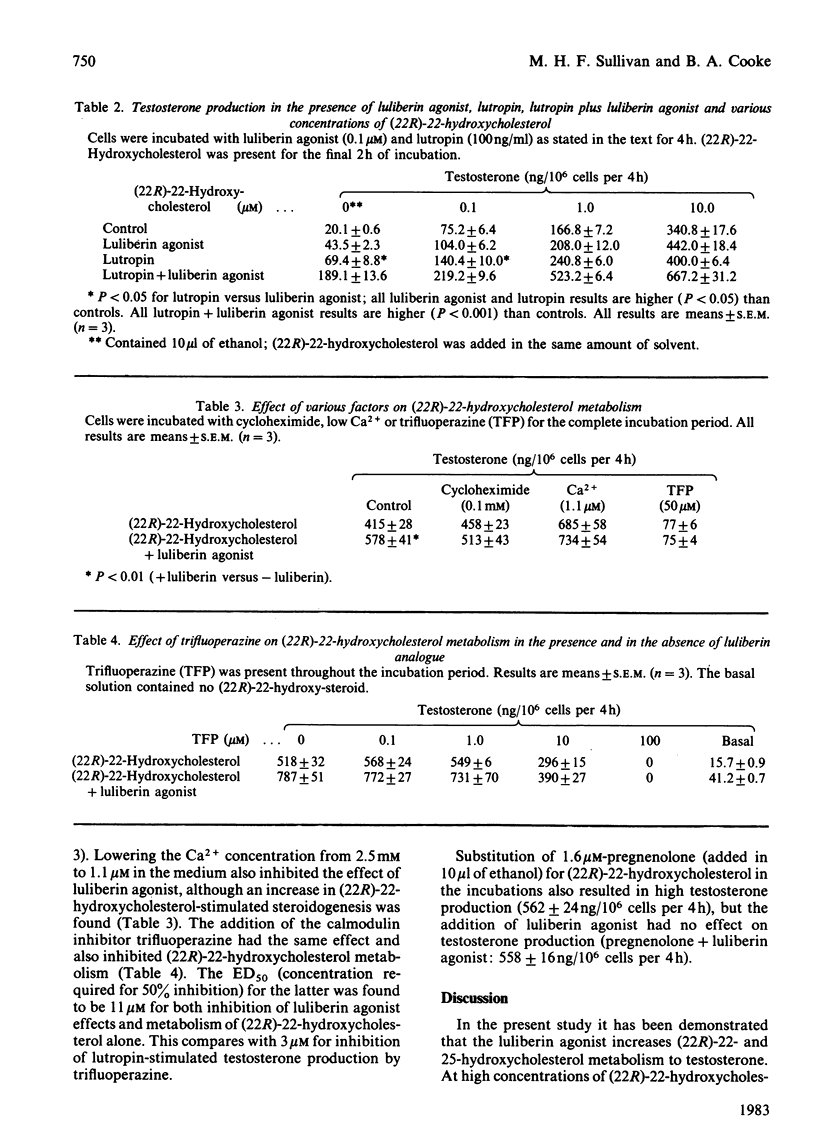
The action of a luliberin (luteinizing-hormone-releasing hormone) agonist (ICI 118630) and lutropin (luteinizing hormone) on the activity of the cytochrome P-450 cholesterol side-chain cleavage enzyme in rat Leydig cells has been investigated. This has been carried out by studying the metabolism of exogenous (22R)-22- and 25-hydroxycholesterol to testosterone. It was found that both hydroxycholesterols increased testosterone production to higher levels than achieved by lutropin alone. Addition of luliberin agonist but not lutropin was found to increase further the metabolism of the hydroxycholesterol to testosterone; this occurred in the presence of saturating and subsaturating levels of the hydroxycholesterols. This effect of luliberin agonist was potentiated in the presence of lutropin. The protein synthesis inhibitor, cycloheximide, inhibited the luliberin agonist-induced stimulation of the hydroxycholesterol metabolism. At low calcium levels (1.1 microM), testosterone production was increased by addition of (22R)-22-hydroxycholesterol but the luliberin agonist effect was negated. The calmodulin inhibitor trifluoperazine inhibited (22R)-22-hydroxycholesterol-stimulated steroidogenesis and negated the luliberin agonist effect. These results indicate that luliberin agonist specifically increases the synthesis of the cytochrome P-450 cholesterol side-chain cleavage enzyme in rat testis Leydig cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldred L. F., Cooke B. A. The deleterious effect of mechanical dissociation of rat testes on the functional activity and purification of Leydig cells using Percoll gradients. Int J Androl. 1982 Apr;5(2):191–195. doi: 10.1111/j.1365-2605.1982.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Bakker C. P., van der Plank-van Winsen M. P., van der Molen H. J. Effects of cholesterol, hydroxycholesterols and calcium on pregnenolone production rates in mitochondrial fractions from rat testes. Biochim Biophys Acta. 1979 Apr 18;584(1):94–103. doi: 10.1016/0304-4165(79)90239-3. [DOI] [PubMed] [Google Scholar]
- Cooke B. A., Dix C. J., Magee-Brown R., Janszen F. H., van der Molen H. J. Hormonal control of Leydig cell function. Adv Cyclic Nucleotide Res. 1981;14:593–609. [PubMed] [Google Scholar]
- Cooke B. A., Janszen F. H., Clotscher W. F., van der Molen H. J. Effect of protein-synthesis inhibitors on testosterone production in rat testis interstitial tissue and Leydig-cell preparations. Biochem J. 1975 Sep;150(3):413–418. doi: 10.1042/bj1500413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Hunter M. G., Sullivan M. H., Dix C. J., Aldred L. F., Cooke B. A. Stimulation and inhibition by LHRH analogues of cultured rat Leydig cell function and lack of effect on mouse Leydig cells. Mol Cell Endocrinol. 1982 Jun;27(1):31–44. doi: 10.1016/0303-7207(82)90060-0. [DOI] [PubMed] [Google Scholar]
- Purvis J. L., Canick J. A., Rosenbaum J. H., Hologgitas J., Latif S. A. Control of cytochrome P-450 in rat testis mitochondria by human chorionic gonadotrophin. Arch Biochem Biophys. 1973 Nov;159(1):31–38. [PubMed] [Google Scholar]
- Sharpe R. M., Cooper I. Stimulatory effect of LHRH and its agonists on Leydig cell steroidogenesis in vitro. Mol Cell Endocrinol. 1982 Apr;26(1-2):141–150. doi: 10.1016/0303-7207(82)90012-0. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Fraser H. M. HCG stimulation of testicular LHRH-like activity. Nature. 1980 Oct 16;287(5783):642–643. doi: 10.1038/287642a0. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Toaff M. E., Schleyer H., Strauss J. F., 3rd Metabolism of 25-hydroxycholesterol by rat luteal mitochondria and dispersed cells. Endocrinology. 1982 Dec;111(6):1785–1790. doi: 10.1210/endo-111-6-1785. [DOI] [PubMed] [Google Scholar]
- Verjans H. L., Cooke B. A., de Jong F. H., de Jong C. M., van der Molen H. J. Evaluation of a radioimmunoassay for testosterone estimation. J Steroid Biochem. 1973 Nov;4(6):665–676. doi: 10.1016/0022-4731(73)90042-3. [DOI] [PubMed] [Google Scholar]


