Abstract
BACKGROUND
Pediatric intracranial aneurysms present unique diagnostic and therapeutic challenges due to their rarity and their distinct anatomical and physiological considerations compared with those of adult intracranial aneurysms. The authors present the case of a symptomatic pediatric patient who required emergency microsurgical treatment after a thrombosed dissecting aneurysm was identified in the right M1 segment of the middle cerebral artery.
OBSERVATIONS
The lesion completely occluded its parent vessel, although distal blood flow was reconstituted through leptomeningeal collaterals. However, aneurysm wall contrast enhancement and signs of early perfusion changes were noticed, which prompted emergency treatment consisting of microsurgical aneurysm trapping, decompression, and extracranial/intracranial revascularization to be successfully performed through a minipterional craniotomy. After 1 year, the bypass occluded, although the patient remained asymptomatic. A slight enlargement of the ipsilateral anterior cerebral artery suggested the possibility of a benign hemodynamic rearrangement.
LESSONS
Emergency treatment may be necessary when signs of lesion instability or hemodynamic compromise are present; however, a comprehensive multidisciplinary evaluation is required. Treatment of complex vascular lesions using a minipterional approach is feasible even in pediatric patients, and delayed bypass occlusion may be a benign phenomenon reflecting gradual blood flow reorganization.
Keywords: dissecting aneurysm, middle cerebral artery, minipterional approach, pediatric intracranial aneurysm, cerebral revascularization, STA-MCA bypass, superficial temoral artery–middle cerebral artery bypass
ABBREVIATIONS: ACA = anterior cerebral artery, CBF = cerebral blood flow, CBV = cerebral blood volume, CTA = computed tomography angiography, DWI = diffusion-weighted imaging, EC-IC = extracranial-intracranial, IA = intracranial aneurysm, ICA = internal carotid artery, IGVA = indocyanine green video-angiography, MCA = middle cerebral artery, MRI = magnetic resonance imaging, mRS = modified Rankin Scale, MTT = mean transit time, NCCT = noncontrast computed tomography, SAH = subarachnoid hemorrhage, STA = superficial temporal artery, TTP = time to peak.
Less than 0.8% of all intracranial aneurysms (IAs) are diagnosed in the pediatric population;1 however, when ruptured, they account for up to 15% of hemorrhagic strokes and 57% of spontaneous subarachnoid hemorrhages (SAHs) in patients younger than 20 years of age.2 Several differences have been described in terms of pathophysiology, natural history, and treatment options compared to those in adult IAs. Congenital, traumatic, and hemodynamic risk factors seem to have a greater role in their development, and population-based studies have shown a predisposition toward male patients and posterior circulation locations. Additionally, almost half of these lesions are considered large or giant, and a higher proportion of nonsaccular morphologies, including dissecting aneurysms, is usually diagnosed.3–5 Dissecting IAs are complex and evolving lesions defined by an abrupt disruption of the endothelium, intima, and internal elastic lamina, with subsequent penetration of circulating blood into the media. The clinical presentation depends on the fate of the subadventitial hematoma and can range from entirely asymptomatic cases to ischemic symptoms, hemorrhage, or mass effect.6 There is limited evidence on how to treat these lesions; however, for cases with recent bleeding, ischemic symptoms, or radiological signs indicating lesion instability, treatment is often considered necessary, sometimes urgently.7–10
The relatively straightforward access to aneurysms located on the middle cerebral artery (MCA), along with their frequently complex morphological features, such as wide necks and perforator involvement, is usually best managed through traditional neurosurgical techniques.11 However, dissecting and partially thrombosed IAs often lack a compliant neck, which makes them unsuitable for direct clipping;12 thus, advanced microsurgical techniques such as aneurysm trapping and decompression with revascularization can become necessary, especially if endovascular options are technically unfeasible.7, 12 This article examines the case of a symptomatic pediatric patient with a complex dissecting MCA aneurysm successfully treated with microsurgical trapping, decompression, and extracranial/intracranial revascularization performed through a minipterional approach.
Illustrative Case
A 7-year-old male patient with an unremarkable medical history presented with a 2-year history of intermittent right periorbital headaches, without neurological signs. The frequency and intensity of his symptoms had worsened in the days before the consultation. After clinical examination, the patient was found to be in good general condition, and meningeal and neuro-ophthalmological findings were discarded. Additionally, routine laboratory and coagulation tests were normal. Noncontrast computed tomography (NCCT) revealed a slightly hyperdense, ovoid-shaped, 12-mm lesion located in the right sylvian fissure, without evident signs of SAH or perilesional edema. Computed tomography angiography (CTA) showed an M1 occlusion with distal branch reconstitution by pial collaterals from the ipsilateral anterior cerebral artery (ACA) and posterior cerebral artery. Digital subtraction angiography revealed a completely occluded distal M1 segment after a short lumen tapering. The lenticulostriate arteries and an early temporal branch originated proximally to the occlusion, andcomplete retrograde filling of the MCA’s distal branches was noted, arising from prominent leptomeningeal collaterals via the ipsilateral A4 and P4 segments. No signs of double lumen, intimal flaps, or synangiosis from the extracranial circulation were noted (Fig. 1). Magnetic resonance imaging (MRI) confirmed the presence of a thrombosed dissecting MCA aneurysm after a noticeable paramagnetic susceptibility artifact was associated with the lesion. Furthermore, focal, linear enhancement of the anterior aspect of the aneurysm wall after contrast administration suggested lesion instability (Fig. 2). No abnormalities were seen on diffusion-weighted imaging (DWI); however, a focal delay in the parameters of time to peak (TTP) and mean transit time (MTT) on dynamic susceptibility contrast perfusion-weighted imaging was noted at the distal MCA vascular territory, while cerebral blood flow (CBF) and cerebral blood volume (CBV) did not show signs of hypoperfusion (Fig. 3). Although no irreversible ischemic damage was noted, these findings suggested early hemodynamic compromise and compensatory vascular changes to ischemia in the affected territory.
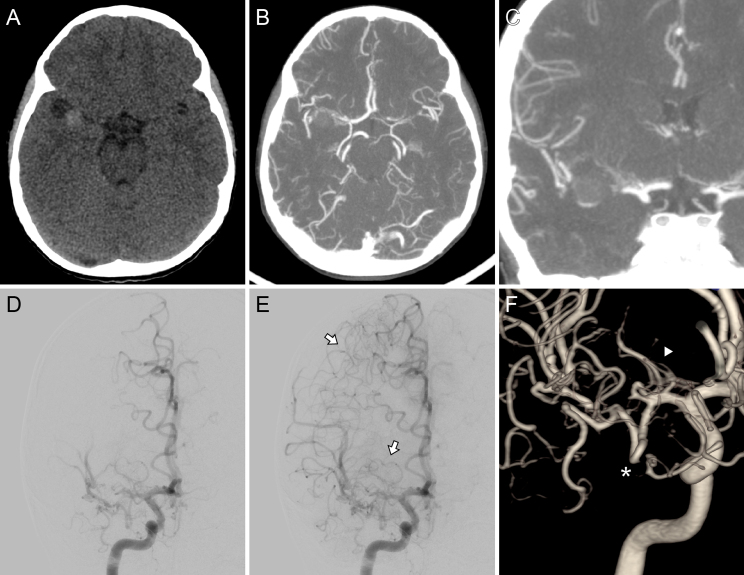
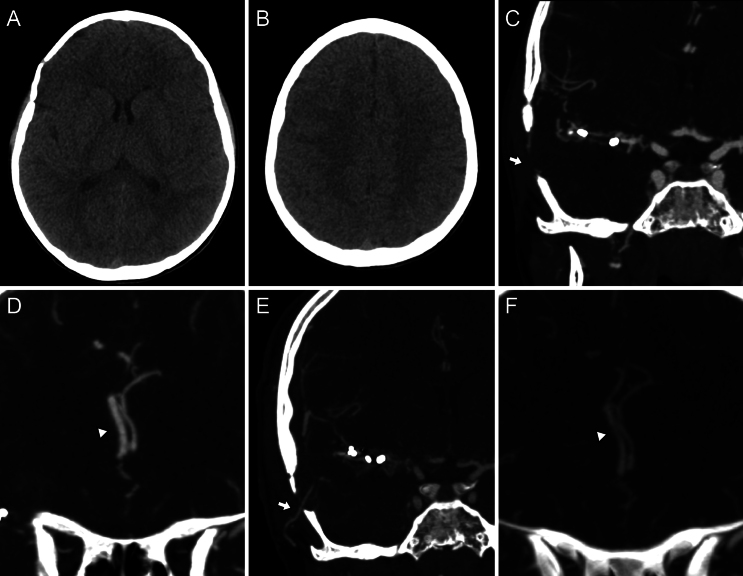
FIG. 1.
Preoperative imaging. Axial NCCT scan (A) showed a slightly hyperdense ovoid lesion in the right sylvian cistern. Axial (B) and coronal (C) CTA demonstrated an MCA occlusion at the level of the distal end of the M1 segment, although its distal branches were present. Digital subtraction angiography after right ICA injection, posteroanterior view, early (D) and late (E) arterial time, and three-dimensional (3D) rendering (F). This angiographic study revealed the lesion’s specific location and a lumen tapering before it, suggestive of an occlusive dissecting aneurysm. Distal flow reconstitution was noted through leptomeningeal collaterals originating from the ACA and from an anastomotic network involving deep perforators (white arrows, E). Additionally, the lenticulostriate arteries (white arrowhead, F) and an early temporal branch (white asterisk) originated proximal to the lesion.
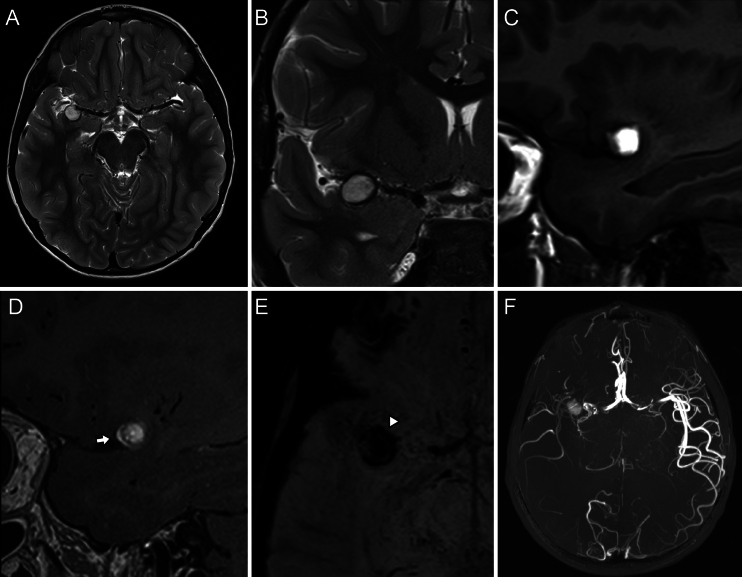
FIG. 2.
Preoperative MRI. The aneurysm dilation exhibited a hyperintense signal on T2-weighted (A and B) and T1-weighted (C) imaging. Sagittal two-dimensional T1 black-blood MRI (D) showed focal linear enhancement after contrast injection at the level of the aneurysm anterior wall (white arrow), which was interpreted as a sign of aneurysm instability. A paramagnetic susceptibility artifact at the level of the lesion was identified (white arrowhead, E) on susceptibility-weighted imaging, which suggested thrombosis. Maximum intensity projection of the time-of-flight angiogram revealed diminished blood flow at the frontal and parietal distal territories (F).
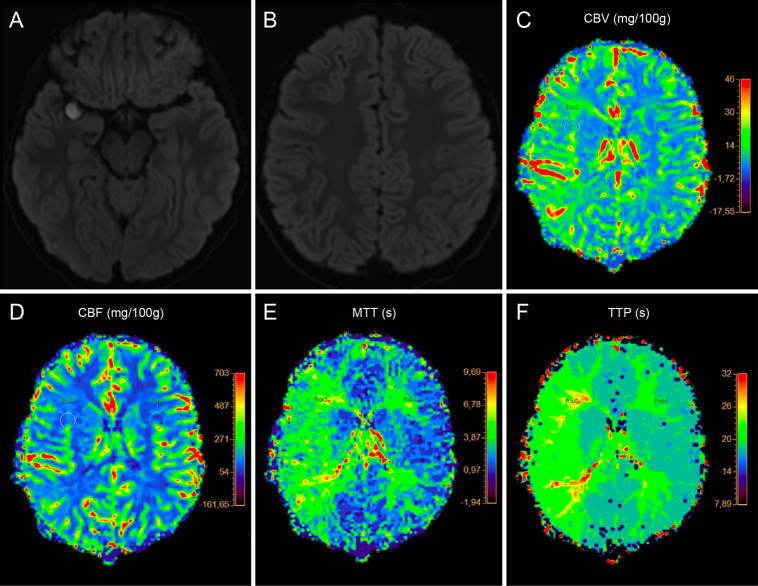
FIG. 3.
Preoperative imaging. No signs of restricted diffusion were noted on DWI (A and B), and perfusion asymmetry affecting the MCA territories was evident on perfusion-weighted imaging. CBV (C) and CBF (D) did not show any signs of hypoperfusion, although delays in the parameters MTT (E) and TTP (F) indicated early hemodynamic changes in the right MCA territory, possibly reflecting a still-compensated autoregulatory mechanism.
This case was discussed by a multidisciplinary team involving vascular neurosurgeons, interventional neuroradiologists, and neurologists. Since the MCA seemed entirely occluded and radiological signs of early perfusion compromise in a sizable eloquent territory were noted, treatment was deemed necessary to avoid potentially devastating ischemic complications. Furthermore, the patient’s progressive symptoms and the presence of vessel wall enhancement, indicating potential lesion instability, argued against conservative therapy. Any attempt to perform a reconstructive endovascular procedure was abandoned after no visible lumen suitable for safe catheterization was identified, and proximal coiling was also ruled out to avoid the risk of a striatocapsular infarct due to iatrogenic lenticulostriate artery occlusion. As a result, open surgery was decided; however, the partially thrombosed and dissected features made the lesion unclippable. Upon confirming that the ipsilateral superficial temporal artery (STA) had a suitable diameter for an STA-MCA bypass through Doppler ultrasonography, it was determined that microsurgical revascularization followed by aneurysm trapping, decompression, and resection was the most appropriate strategy.
The patient’s head was fixed and slightly rotated 15° to the left while supine. A right-sided interfascial dissection, followed by a 3 × 4–cm minipterional craniotomy, was performed as previously described,13, 14 allowing for the identification and dissection of the donor vessel, as well as an uneventful splitting of the sylvian fissure. After careful microsurgical dissection (Mitaka MM80 operating microscope), a large fusiform aneurysm located between the M1 and M2 segments of the right MCA was encountered. Intraoperative indocyanine green video-angiography (IGVA) and micro-Doppler ultrasonography confirmed the absence of blood flow inside the lesion. A successful STA-M2 terminolateral bypass was performed, and then the aneurysm was trapped using a straight 11-mm Yasargil clip (Aesculap) and a straight 9-mm Peter Lazic L-Aneurysm Clip (Peter Lazic GmbH), followed by careful coagulation and resection (Fig. 4A–C). The proximally located lateral lenticulostriate arteries and the early temporal branch were identified and spared, and distal MCA blood flow, as well as flow through the newly created bypass, was confirmed by IGVA at the end of the procedure.
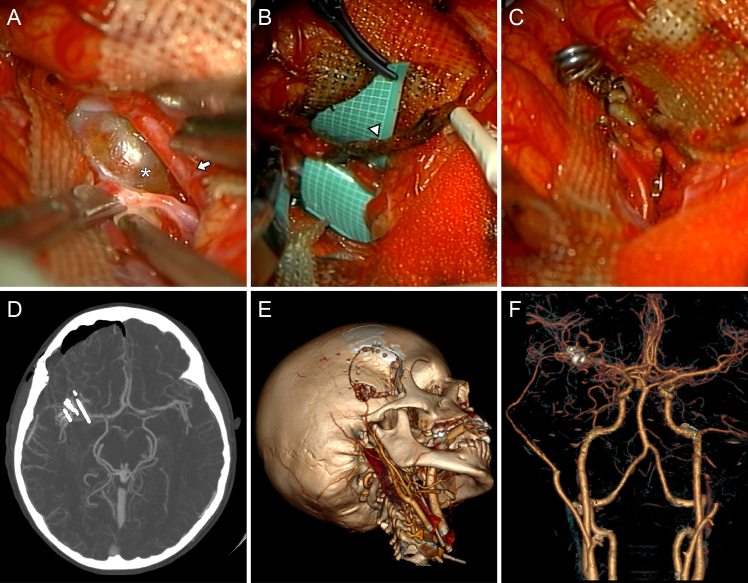
FIG. 4.
Intraoperative views after sylvian fissure splitting. According to IGVA and intraoperative micro-Doppler ultrasonography, the dissecting aneurysm (white asterisk, A) exhibited no flow, confirming its thrombosed status. Distal to the lesion, a large M2 branch presented normal blood flow and, considering its diameter and accessibility, was selected as the recipient vessel for the bypass (white arrow). An uneventful end-to-side STA (white arrowhead, B) to M2 bypass was performed, and the aneurysm was trapped, decompressed, and resected (C). Postoperative axial CTA, maximum intensity projection (D), and 3D renderings (E and F). After surgery, the bypass was patent, and no complications were noted.
After surgery, CTA confirmed the complete resection of the lesion and bypass patency without any apparent hemorrhagic or ischemic complications (Fig. 4D and E). A daily oral dose of 100 mg of aspirin (Bayer Vital GmbH) was started immediately after surgery, and no neurological deficit was identified during recovery. The patient was successfully discharged 9 days later with a modified Rankin Scale (mRS) score of 0 and was prescribed aspirin treatment for life. Later histological examination confirmed the diagnosis. Microscopic signs of chronic inflammatory changes at the aneurysm wall and a recanalized luminal thrombus were also identified. After 1 year, follow-up CTA revealed no signs of aneurysm remnants and the unexpected occlusion of the bypass. However, the distal MCA branches showed an adequate caliber, and a slight enlargement of the ipsilateral ACA was evident (Fig. 5). At the time of writing, the patient remained in good clinical condition (mRS score 0) without experiencing any further episodes of headaches or transitory neurological deficits.
FIG. 5.
Long-term follow-up imaging. After 1 year, the patient remained asymptomatic and in good neurological condition, without cosmetic sequela from the craniotomy. Axial NCCT (A and B) showed no signs of ischemic or hemorrhagic complications; however, CTA revealed the occlusion of the bypass (white arrow, C), while a relative enlargement was observed in the right ACA (white arrowhead, D). The distal MCA branches exhibited a robust signal. For comparison, postoperative CTA demonstrated initial bypass patency (white arrow, E), and preoperative CTA showed a comparatively thinner ipsilateral ACA (white arrowhead, F).
Informed Consent
The necessary informed consent was obtained in this study.
Discussion
Observations
This report presents the unusual case of a child with a thrombosed dissecting aneurysm located in the anterior circulation that exhibited signs of potential instability and early hemodynamic compromise. To the best of our knowledge, this is the first pediatric case report in which a complex revascularization procedure was successfully performed through a minipterional craniotomy. Dissecting IAs are uncommon and complex lesions with relatively scarce representation in the literature, particularly concerning the pediatric population. Unlike in our case, these lesions are predominantly found within the vertebrobasilar circulation.7 Chuang et al. found that only 24 cases located on the MCA were published between 1990 and 2011.15 These lesions presented with bleeding or ischemia or were discovered incidentally in 63%, 29%, and 8% of cases, respectively. Moreover, these lesions carry a higher risk of rebleeding than saccular MCA-located IAs.16 Our patient did not present with acute hemorrhage; however, both the family and the patient recalled a clear increase in headache intensity and frequency in the days before the consultation. Although clinical presentation is not specific, some reports indicate that up to 80% of patients with intracranial arterial dissections can have prodromal headaches before SAH or cerebral ischemia.7, 17 The optimal treatment is currently unknown since available studies offer a limited level of evidence; however, it is widely agreed that ruptured cases require urgent treatment to avoid a high risk of rebleeding.16 In contrast, there is no clear consensus on the best approach for unruptured cases, and the decision mostly depends on the perceived risk of future rupture or stroke.18 Although some authors recommend medical treatment in the absence of hemorrhage,7 the recent progression of symptoms, the presence of aneurysm wall contrast enhancement (Fig. 2), and early perfusion changes affecting a large and eloquent cortical region (Fig. 3) were considered compelling reasons in favor of active treatment in the present case.
Ischemic Considerations and Lesion Instability
More than half of patients with dissecting MCA aneurysms who develop ischemic symptoms have poor outcomes, with progressive brain infarction as the leading cause of clinical deterioration.15 A previous review demonstrated that 71% of patients presenting with an MCA dissecting IA and ischemia died under conservative treatment.19 The well-developed leptomeningeal collateral network seen in our patient may have prevented acute neurological deterioration despite having a completely occluded MCA (Fig. 1). The general consensus is that children have a robust network of leptomeningeal collaterals and that developing brains can better endure ischemic insults. One example of this is the lower incidence of delayed neurological deficits seen in children in spite of presenting with a higher frequency of angiographic vasospasm after aneurysmal SAH.20 Furthermore, aging has been proven to cause insufficiency of collateral circulation in rodents.21 However, despite the protection provided by the patient’s grade of collateralization and the absence of infarcted tissue on DWI, early perfusion changes were already taking place, probably reflecting an initial autoregulatory hemodynamic response to ischemia. MTT and TTP changes with normal CBV and CBF parameters, as seen in our case (Fig. 3), have been linked to hemodynamic compromise and tissue at risk in patients with transient ischemic attacks and ischemic strokes.22, 23 In addition to the risk of ischemia, the lesion exhibited aneurysm vessel wall contrast enhancement (Fig. 2), which has been associated with instability due to inflammatory changes in both saccular24, 25 and nonsaccular26 morphologies. This radiological sign can predict morphological deterioration, meaning progressive dilation or stenosis, in unruptured intracranial arterial dissections.8 Interestingly, postoperative histological examination of the collected sample confirmed the presence of chronic inflammatory changes in the aneurysm wall.
Treatment Considerations
A comprehensive multidisciplinary evaluation of the patient’s symptoms, radiological findings, and angioarchitecture is essential for establishing the need for treatment and determining the optimal approach, particularly since no systematic studies comparing endovascular or surgical interventions exist. Regardless, the goal of treatment is to reduce blood flow into the dissected segment to prevent rupture, either by reconstructive or by deconstructive techniques.7 At first, the possibility of undergoing endovascular treatment was considered, although it was ultimately dismissed; a reconstructive approach was deemed unsafe due to the risk of vessel perforation when navigating a guidewire through an unidentifiable lumen. Similarly, a deconstructive approach, such as proximal coiling, would have risked occluding the M1 perforators leading to striatocapsular infarction, resulting in severe clinical consequences. Furthermore, this approach cannot prevent retrograde aneurysm filling, which has been associated with aneurysm recanalization, enlargement, and rupture.27, 28 A traditional open neurosurgical approach was therefore decided on. Surgery has been a mainstay of MCA IA treatment for several decades due to its relatively straightforward access and complex morphological features,11 although not all thrombosed or dissecting IAs can be treated with direct clipping. While this technique is generally considered the safest option, it requires a compliant aneurysm neck; when absent or if the risk of infarction due to parent artery occlusion is unacceptably high, more advanced techniques, such as aneurysm trapping and decompression with or without revascularization, become necessary.7, 12
Surgical Revascularization
Extracranial-intracranial (EC-IC) cerebral revascularization has been used in various conditions, including moyamoya disease, intracranial atherosclerotic stenosis, and complex aneurysms unsuitable for endovascular treatment or simple clipping. When performed at high-volume neurovascular centers, it is considered a safe and effective treatment for IAs in both adults and children.29–31 As an adjuvant technique, it allows excluding the affected arterial segment, as well as preventing further bleeding and longitudinal dissection progression while maintaining blood flow supply into areas without adequate collateral circulation.32 In a recent meta-analysis involving 915 cases with anterior circulation aneurysms, the surgery-related mortality rate was 0.3%, and the incidence of long-term ischemic and hemorrhagic complications was 3% and 1%, respectively.30 Unfortunately, evidence for children is limited to case reports and small case series. The largest study to date, which included 29 IAs treated with various revascularization techniques, reported a 92.3% rate of complete aneurysm occlusion and a 96.3% rate of long-term favorable outcomes after an average follow-up period of 46 months. Four patients experienced a stroke during the perioperative period, but only one remained neurologically dependent.31 Similarly, a literature review concluded that adjuvant EC-IC bypass for pediatric aneurysm surgery resulted in a complete aneurysm occlusion rate of over 90%, with less than 10% of long-term complications.29
The long-term STA-MCA bypass patency rate for both pediatric and adult patients is usually over 90%.33–35 While bypass occlusion is typically regarded as a treatment-related complication, some cases have shown no neurological repercussions. In a single-surgeon case series of 430 consecutive procedures, 4 of 12 patients with occluded bypasses remained asymptomatic;36 however, the underlying factors determining this event’s clinical impact are not fully understood. A multicenter study on adult hemorrhagic moyamoya disease treated with end-to-side STA-MCA bypass concluded that good collateral baseline circulation and compensated preoperative cerebral perfusion were independent predictors of long-term bypass occlusion. At the same time, this was not associated with long-term angiographic or neurological outcomes, suggesting that it could represent a relatively benign phenomenon.37 The perfusion profile associated with delayed bypass occlusion included delayed TTP and MTT without decreased CBF and CBV, which coincidentally were also observed in our patient (Fig. 3). While perioperative bypass occlusion usually results from thrombotic complications leading to stroke, delayed occlusions could reflect an adaptative compensation. It is possible that our patient’s well-developed collaterals at baseline prevented ischemic damage during the acute phase, allowing for progressive vascular reorganization after surgical revascularization. A gradual blood flow takeover through leptomeningeal collaterals arising from the ipsilateral ACA could have resulted in its slight enlargement, while the bypass blood flow eventually diminished, leading to its closure (Fig. 5). Although there are no studies on this phenomenon following aneurysm surgery, we believe that the absence of neurological deficit in our patient ultimately serves as indirect evidence of adequate hemispheric perfusion reorganization.
Minipterional Approach
After careful preoperative planning, we determined that a minipterional craniotomy was feasible and could lead to equally effective outcomes while providing less soft tissue damage, faster postoperative recovery, and better cosmetic results.13 When complemented with interfascial dissection, this approach provides adequate surgical exposure with reduced soft tissue damage, as it avoids unnecessary manipulation of the frontal branch of the facial nerve and does not require the complete dissection of the temporal muscle. However, this benefit alone does not warrant using minimally invasive approaches if a sufficiently wide surgical corridor is not guaranteed. The minipterional craniotomy, unlike the classic pterional craniotomy, is not centered on the pterion; instead, it is centered in the main axis of the sylvian fissure, specifically around its anterior ascending ramus, with the pterion defining its posterior and distal limits. Anatomical studies suggest that more distal sylvian dissections do not provide additional exposure benefit, at least for lesions around the internal carotid artery (ICA) bifurcation, anterior communicating artery, or MCA.13 The reduced area of the exposed cortex provides sufficient brain retraction for a noninferior transsylvian access compared to a traditional pterional craniotomy while also reducing the risk of unintended cortical injury. Furthermore, using the superior temporal line as the craniotomy’s upper margin prevents access to the frontal sinus, which starts pneumatizing around the 2nd year of life.38 This avoids potential postoperative infections and cerebrospinal fluid leakage. It is important to note that for cases in which a greater degree of temporal lobe retraction is necessary or with deeper lesions requiring a more anterior trajectory limited by the lack of frontal bone extension, this approach might be insufficient or require modifications. However, a wider surgical corridor can be created if complemented with meningo-orbital band detachment and extradural anterior clinoidectomy, compensating for some of these potential drawbacks.14 Likewise, lesions requiring a more distal transsylvian dissection can be managed with a simple posterior craniotomy extension. This technique has been used for treating complex aneurysms, parasellar and posterior fossa tumors, petroclival meningiomas, and moyamoya disease,14, 39–41 although there are only anecdotal reports concerning children.42 Regardless, the use of minipterional variations has been shown to be effective, even for complex revascularization procedures,43, 44 which was ultimately demonstrated by our case as well. The skin flap was tailored to allow the dissection of the STA’s common trunk and a sufficient segment of its frontal branch to ensure tension-free mobility of the donor vessel, while the craniotomy provided sufficient surgical maneuverability for a wide sylvian fissure split, bypass, and aneurysm trapping/resection (Fig. 4A–C).
Limitations
The natural history of these challenging lesions in this specific population is unknown, and currently, no randomized clinical trials comparing different surgical and endovascular techniques exist. More cases with longer follow-up periods are required to assess the presented approach and elucidate the factors behind delayed bypass occlusion; however, given the rarity of these lesions, such studies are unlikely to emerge.
Lessons
Pediatric dissecting IAs require multidisciplinary management, and certain conditions, such as previous rupture, aneurysm instability, or risk of ischemic stroke, may require urgent care. Aneurysms without a compliant neck can be managed through advanced microsurgical techniques, and revascularization should be considered when the risk of infarction due to parent artery occlusion is unacceptably high. Delayed bypass occlusion could be a benign phenomenon reflecting gradual blood flow reorganization, and good preoperative collateralization and compensated perfusion changes could predict a clinically silent occurrence. To our knowledge, this constitutes the first report of a complex vascular revascularization procedure successfully performed using a minipterional craniotomy in a pediatric patient, demonstrating the versatility of this approach.
Disclosures
Dr. Henkes reported personal fees from WallabyPhenox for consulting and proctoring outside the submitted work.
Author Contributions
Conception and design: Albiña-Palmarola, Mura. Acquisition of data: Muñoz, Lopez. Analysis and interpretation of data: Díaz-Peregrino, Henkes. Drafting the article: Albiña-Palmarola, Díaz-Peregrino. Critically revising the article: Muñoz, Henkes, Mura. Reviewed submitted version of manuscript: Albiña-Palmarola, Mura. Approved the final version of the manuscript on behalf of all authors: Albiña-Palmarola. Administrative/technical/material support: Albiña-Palmarola, Mura.
Correspondence
Pablo Albiña-Palmarola: Klinikum Stuttgart, Germany. pablo.a.med@gmail.com.
References
- 1.Amacher LA, Drake CG. Cerebral artery aneurysms in infancy, childhood and adolescence. Childs Brain. 1975;1(1):72-80. [DOI] [PubMed] [Google Scholar]
- 2.Jordan LC, Johnston SC, Wu YW, Sidney S, Fullerton HJ. The importance of cerebral aneurysms in childhood hemorrhagic stroke: a population-based study. Stroke. 2009;40(2):400-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gemmete JJ, Toma AK, Davagnanam I, Robertson F, Brew S. Pediatric cerebral aneurysms. Neuroimaging Clin N Am. 2013;23(4):771-779. [DOI] [PubMed] [Google Scholar]
- 4.Jian BJ, Hetts SW, Lawton MT, Gupta N. Pediatric intracranial aneurysms. Neurosurg Clin N Am. 2010;21(3):491-501. [DOI] [PubMed] [Google Scholar]
- 5.Lasjaunias P, Wuppalapati S, Alvarez H, Rodesch G, Ozanne A. Intracranial aneurysms in children aged under 15 years: review of 59 consecutive children with 75 aneurysms. Childs Nerv Syst. 2005;21(6):437-450. [DOI] [PubMed] [Google Scholar]
- 6.Krings T, Choi IS. The many faces of intracranial arterial dissections. Interv Neuroradiol. 2010;16(2):151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debette S, Compter A, Labeyrie MA, et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. 2015;14(6):640-654. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto Y, Matsushige T, Shimonaga K, et al. Magnetic resonance vessel wall imaging predicts morphological deterioration in unruptured intracranial artery dissection. J Stroke Cerebrovasc Dis. 2020;29(9):105006. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima S, Tsukahara T, Minematsu K. A study of vertebrobasilar artery dissection with subarachnoid hemorrhage. Acta Neurochir Suppl. 2010;107:45-49. [DOI] [PubMed] [Google Scholar]
- 10.Ohkuma H, Suzuki S, Shimamura N, Nakano T. Dissecting aneurysms of the middle cerebral artery: neuroradiological and clinical features. Neuroradiology. 2003;45(3):143-148. [DOI] [PubMed] [Google Scholar]
- 11.Nussbaum ES, Madison MT, Goddard JK, Lassig JP, Kallmes KM, Nussbaum LA. Microsurgical treatment of unruptured middle cerebral artery aneurysms: a large, contemporary experience. J Neurosurg. 2018;130(5):1498-1504. [DOI] [PubMed] [Google Scholar]
- 12.Lawton MT, Quiñones-Hinojosa A, Chang EF, Yu T. Thrombotic intracranial aneurysms: classification scheme and management strategies in 68 patients. Neurosurgery. 2005;56(3):441-454. [DOI] [PubMed] [Google Scholar]
- 13.Figueiredo EG, Deshmukh P, Nakaji P, et al. The minipterional craniotomy: technical description and anatomic assessment. Neurosurgery. 2007;61(5suppl 2):256-264. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Perez R, Joswig H, Tsimpas A, et al. The extradural minipterional approach for the treatment of paraclinoid aneurysms: a cadaver stepwise dissection and clinical case series. Neurosurg Rev. 2020;43(1):361-370. [DOI] [PubMed] [Google Scholar]
- 15.Chuang MJ, Lu CH, Cheng MH. Management of middle cerebral artery dissecting aneurysm. Asian J Surg. 2012;35(1):42-48. [DOI] [PubMed] [Google Scholar]
- 16.Aoki N, Sakai T. Rebleeding from intracranial dissecting aneurysm in the vertebral artery. Stroke. 1990;21(11):1628-1631. [DOI] [PubMed] [Google Scholar]
- 17.Mizutani T. Natural course of intracranial arterial dissections. J Neurosurg. 2011;114(4):1037-1044. [DOI] [PubMed] [Google Scholar]
- 18.Kim BM, Kim SH, Kim DI, et al. Outcomes and prognostic factors of intracranial unruptured vertebrobasilar artery dissection. Neurology. 2011;76(20):1735-1741. [DOI] [PubMed] [Google Scholar]
- 19.Kurino M, Yoshioka S, Ushio Y. Spontaneous dissecting aneurysms of anterior and middle cerebral artery associated with brain infarction: a case report and review of the literature. Surg Neurol. 2002;57(6):428-438. [DOI] [PubMed] [Google Scholar]
- 20.Moftakhar P, Cooke DL, Fullerton HJ, et al. Extent of collateralization predicting symptomatic cerebral vasospasm among pediatric patients: correlations among angiography, transcranial Doppler ultrasonography, and clinical findings. J Neurosurg Pediatr. 2015;15(3):282-290. [DOI] [PubMed] [Google Scholar]
- 21.Faber JE, Zhang H, Lassance-Soares RM, et al. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler Thromb Vasc Biol. 2011;31(8):1748-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37(4):979-985. [DOI] [PubMed] [Google Scholar]
- 23.Zaro-Weber O, Moeller-Hartmann W, Heiss WD, Sobesky J. Maps of time to maximum and time to peak for mismatch definition in clinical stroke studies validated with positron emission tomography. Stroke. 2010;41(12):2817-2821. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto Y, Matsushige T, Kawano R, et al. Focal aneurysm wall enhancement in vessel wall imaging as a surrogate marker for predicting aneurysm instability. J Vasc Interv Neurol. 2023;3(6):e001029. [Google Scholar]
- 25.Edjlali M, Gentric JC, Régent-Rodriguez C, et al. Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms? Stroke. 2014;45(12):3704-3706. [DOI] [PubMed] [Google Scholar]
- 26.Peng F, Fu M, Xia J, et al. Quantification of aneurysm wall enhancement in intracranial fusiform aneurysms and related predictors based on high-resolution magnetic resonance imaging: a validation study. Ther Adv Neurol Disord. 2022;15:17562864221105342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KC, Kim DI. Fatal rupture of a giant carotid aneurysm following high-flow bypass and endovascular occlusion. Surg Cereb Stroke. 1993;21(1):69-73. [Google Scholar]
- 28.Park JC, Kwon BJ, Cho YD, Han MH. Growing thrombosed dissecting aneurysm of the vertebral artery after endovascular proximal artery occlusion: the role of the vasa vasorum. NeuroIntervention. 2009;4(1):33-37. [Google Scholar]
- 29.Strickland BA, Attenello F, Russin JJ. Extracranial to intracranial bypass for the treatment of cerebral aneurysms in the pediatric population. J Clin Neurosci. 2016;34:6-10. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Chen P, Duan G, Li R, Li Z, Guo G. Extracranial-intracranial bypass surgery for intracranial aneurysm of the anterior cerebral circulation: a systematic review and meta-analysis. Front Neurol. 2023;14:1174088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalani MY, Elhadi AM, Ramey W, et al. Revascularization and pediatric aneurysm surgery. J Neurosurg Pediatr. 2014;13(6):641-646. [DOI] [PubMed] [Google Scholar]
- 32.Raper DMS, Rutledge WC, Winkler EA, et al. Controversies and advances in adult intracranial bypass surgery in 2020. Oper Neurosurg (Hagerstown). 2020;20(1):1-7. [DOI] [PubMed] [Google Scholar]
- 33.EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med. 1985;313(19):1191-1200. [DOI] [PubMed] [Google Scholar]
- 34.Matano F, Murai Y, Tateyama K, et al. Long-term patency of superficial temporal artery to middle cerebral artery bypass for cerebral atherosclerotic disease: factors determining the bypass patent. Neurosurg Rev. 2016;39(4):655-661. [DOI] [PubMed] [Google Scholar]
- 35.Morshed RA, Abla AA, Murph D, et al. Clinical outcomes after revascularization for pediatric moyamoya disease and syndrome: a single-center series. J Clin Neurosci. 2020;79:137-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon S, Burkhardt JK, Lawton MT. Long-term patency in cerebral revascularization surgery: an analysis of a consecutive series of 430 bypasses. J Neurosurg. 2019;131(1):80-87. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Lin F, Yan DB, et al. Delayed anastomotic occlusion after direct revascularization in adult hemorrhagic moyamoya disease. Brain Sci. 2021;11(5):536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scuderi AJ, Harnsberger HR, Boyer RS. Pneumatization of the paranasal sinuses: normal features of importance to the accurate interpretation of CT scans and MR images. AJR Am J Roentgenol. 1993;160(5):1101-1104. [DOI] [PubMed] [Google Scholar]
- 39.Ahn JY, Kim ST, Yi KC, Lee WH, Paeng SH, Jeong YG. Superficial temporal artery-sparing mini-pterional approach for cerebral aneurysm surgery. J Korean Neurosurg Soc. 2017;60(1):8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martínez-Pérez R, Silveira-Bertazzo G, Rangel GG, et al. The historical perspective in approaches to the spheno-petro-clival meningiomas. Neurosurg Rev. 2021;44(1):51-60. [DOI] [PubMed] [Google Scholar]
- 41.Mura J, Perales I, Rabelo NN, et al. Extradural minipterional approach: evolving indications of the minipterional craniotomy. Surg Neurol Int. 2020;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Connor KP, Pelargos PE, Palejwala AH, Shi H, Villeneuve L, Glenn CA. Resection of pediatric trigeminal schwannoma using minimally invasive approach: case report, literature review, and operative video. World Neurosurg. 2019;127:518-524. [DOI] [PubMed] [Google Scholar]
- 43.Ravina K, Rennert RC, Kim PE, Strickland BA, Chun A, Russin JJ. Orphaned middle cerebral artery side-to-side in situ bypass as a favorable alternative approach for complex middle cerebral artery aneurysm treatment: a case series. World Neurosurg. 2019;130:e971-e987. [DOI] [PubMed] [Google Scholar]
- 44.Kusdiansah M, Benet A, Suzuki Y, Ota N, Noda K, Tanikawa R. Dome resection and end-to-end reanastomosis for a middle cerebral artery fusiform aneurysm of the M1 segment: 2-dimensional operative video. World Neurosurg. 2023;178:114. [DOI] [PubMed] [Google Scholar]