Abstract
This study presents a significant advancement in tandem dye-sensitized solar cells (T-DSSCs) through the strategic synthesis of novel triazatruxene (TAT) sensitizers MS-1 and MS-2. These organic sensitizers demonstrate exceptional light-harvesting capacity and overall performance, pushing the boundaries of power conversion efficiency (PCE) in DSSCs. The MS-1-based DSSCs achieved an impressive PCE of 12.81%, while MS-2 sensitizers reached a notable 10.92%. These efficiencies represent significant improvements over the conventional N719 dye (7.60%), demonstrating the potential of metal-free organic sensitizers in DSSC technology. The key to these noteworthy results lies in the molecular design of the organic sensitizers. The triazatruxene donor segment in the MS-1 and MS-2 dyes, featuring a rigid structure and efficient intramolecular charge transfer (ICT), proved to be a game-changer for photovoltaic properties. Building on these results, we explored an innovative parallel tandem cell (PT-DSSC) configuration. By connecting separate cells containing N719 and MS-1 sensitizers, we achieved a record efficiency of 12.89% with enhanced short-circuit current density (JSC) and open-circuit voltage (VOC)compared to single-dye cells. This study highlights the potential of molecular engineering in organic sensitizers and device optimization to enhance DSSC performance, paving the way for further advancements in solar cell technology.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75959-0.
Keywords: Tandem dye-sensitized solar cells (T-DSSCs), Triazatruxene (TAT), N719, Power conversion efficiency (PCE)
Subject terms: Chemistry, Materials science
Introduction
Over the past few decades, dye-sensitized solar cells (DSSCs) have attracted significant attention as promising photovoltaic systems for renewable energy generation. This is because of their unique characteristics, such as cost-effectiveness, flexibility, and ease of manufacture1–3. Since their discovery in 1991, efficient photosensitizers have been continuously developed, opening numerous possibilities for their design. Photosensitizers are crucial components in DSSCs because they play a vital role in determining the performance of photovoltaic devices4. Various strategies have been employed to improve the power conversion efficiency (PCE) of DSSCs, such as broadening the absorption spectra and increasing the molar extinction coefficient to capture more photons from the solar radiation. However, the limitations of effective electron injection at the dye/nanoparticle interface and energy losses, particularly during the electron injection process, have hindered the overall performance of DSSCs. Organic dyes, with their diverse molecular designs, abundant raw materials, and vibrant colors, offer solutions to these issues through appropriate molecular engineering5. The choice of the π-segment in dyes plays a crucial role in determining not only their electronic properties but also the energetic and kinetic attributes of the titania/dye/electrolyte interface during the electron injection and regeneration processes. This process ultimately determines the performance of a solar cell. However, electron injection from an excited dye into TiO2 is a complex process and a major source of energy loss in DSSCs. This process involves dye excitation, the vibrational relaxation of the excited dye, and electron injection. Previous studies have indicated that sensitizers with higher flexibility and more double bonds tend to exhibit lower power conversion efficiency (PCE)6. This is because the electron injection process competes with vibrational relaxation. Parallel Tandem dye-sensitized solar cells (PT-DSSCs) are often used to enhance the sunlight-harvesting capacity of solar cells. These cells consist of two separate DSSCs connected in a series or parallel arrangement, with the top and bottom cells operating in tandem7. PT-DSSCs have a unique structure that eliminates the need to mix multiple dyes during co-sensitization. This structure overcomes issues related to competitive adsorption and difficulties in achieving the appropriate concentration ratio, timing, and sequence of dyes commonly encountered in co-sensitization8. PT-DSSCs have demonstrated noteworthy performance metrics, particularly high power conversion efficiency (PCE), which is largely attributed to the broader absorption spectrum interval resulting from increased light absorption between the top and bottom cells9. Moreover, studies have demonstrated that PT-DSSCs exhibit superior performance in enhancing the efficiency compared to series tandem DSSCs (ST-DSSCs)10. According to Kim et al., a noteworthy power conversion efficiency (PCE) of 14.64% was attained in PT-DSSCs employing the organic dye SGT-137 and zinc porphyrin dye SGT-02111. The widely used ruthenium dye N719 has garnered significant attention owing to its exceptional photostability and high power conversion efficiency (PCE)12. However, the relatively low molar extinction coefficient (ε) of N719 may hinder its further development13. The geometry of the sensitizer molecule is critical for efficient charge injection and minimization of vibrational relaxation, which can result in energy loss in DSSCs. The rigid TAT structure enhanced the DSSC efficiency, achieving a PCE of 13.6%, which is the highest reported for a single-sensitizer DSSC. These results underscore the importance of molecular design in optimizing the DSSC performance14. Although triazatruxene (TAT) has been widely used in various applications, including OLEDs and perovskite solar cells, its exceptional properties, such as high molar absorption coefficient, excellent hole transport capability, and photostability, have not resulted in many highly efficient photosensitizers for liquid and solid-state DSSCs. We synthesized and characterized two distinct TAT-based sensitizers (MS-1 and MS-2) as shown in (Fig. 1). These dyes were designed by incorporating triazatruxene as a donor segment linked to 3,4-ethylenedioxythiophene (EDOT) and a carboxyphenylacetonitrile (-CAN) acceptor for MS-1 and 5-aminopicolinonitrile (-APN) for MS-2. Furthermore, the UV-vis absorption spectrum demonstrated cooperative absorption between MS-1, MS-2, and N719, suggesting that these dyes are ideal candidates for PT-DSSCs. Based on these advantages, we evaluated the performance of PT-DSSCs based on MS-1 + N719 sensitizers and compared them with those of DSSCs based on MS-1, MS-2, and N719. The results showed that PT-DSSCs based on MS-1 + N719 sensitizers achieved higher PCEs (13.60%) than those based on MS-1 (12.8%), MS-2 (10.92%), and N719 (7.60%), making them promising candidates for DSSCS applications.
Figure 1.
Chemical structures of sensitizers MS-1-2 and N719.
Experimental section
Comprehensive details, including photovoltaic measurements, synthetic procedures for all structures, and extensive structural characterization of all fabricated devices, are provided in the Supporting Information (Figures S1-S26). This supplementary material offers a thorough foundation for our findings and ensures the reproducibility of our work.
Results and discussion
Chemistry
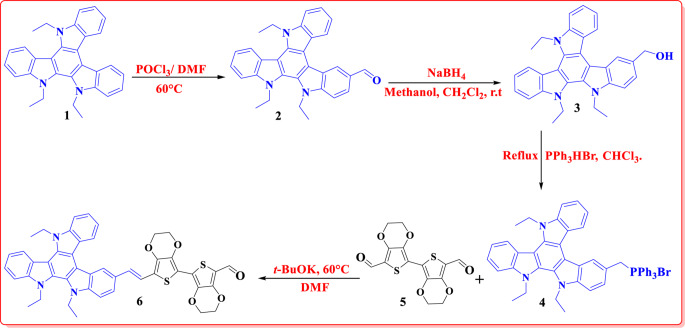
The synthetic routes to the novel TAT-based organic sensitizers (MS-1-2) are illustrated in Figs. 2 and 3. Figure 2 shows the synthetic pathway for TAT-aldehyde (6). Initially, the triazatruxene core (1) was synthesized through intermolecular condensation of 2-indolinone, which is commercially accessible. This process was performed in a single step to produce a planar aromatic core. Subsequently, n-ethyl chains were attached to the core, as documented in a previous study15. Following the Vilsmeier-Hack reaction, the triazatruxene core was formylated, resulting in the production of TAT-aldehyde, as shown in Fig. 2. TAT-aldehyde (2) was reduced to its corresponding reduced alcohol compound (3) in a mixed solvent system of methanol and dichloromethane in the presence of NaBH4. Building upon the successful synthesis of compound 3, novel phosphonium salt 4 was prepared through a reaction with triphenylphosphine hydrobromide in chloroform. Then, a wittig reaction occurred between the salt of compound (4) and 2,2’,3,3’-tetrahydro-[5,5’-bithieno[3,4-b][1,4]dioxine]-7,7’-dicarbaldehyde (5), which was prepared as previously described16 to synthesize TAT-aldehyde (6). All the complete synthetic data for the compounds are presented in the supplementary file.
Figure 2.
Synthesis of triazatruxene-3,4-ethylenedioxythiophene aldehyde (6).
Figure 3.
Synthetic route to MS-1-2 sensitizers.
In accordance with Fig. 3, sensitizers MS-1-2 were synthesized through Schiff base reactions involving TAT-aldehyde (6), 4-(cyanomethyl)benzoic acid (7) in methanol, drop base catalyst piperidine, and refluxing in ethanol and DBU with 5-aminopicolinonitrile (8). Subsequently, recrystallization was performed to purify the resulting dye sensitizers and intermediates. The molecular structures of the synthesized dyes (MS-1-2) and their intermediates were characterized using various spectroscopic techniques. The IR spectra of MS-1 showed characteristic absorption bands at 2995, 2920 for aliphatic groups (C-H), 2207 (-CN), 1687 (C = O) and 1619 cm⁻¹ (C = C), confirming the presence of the cyano group and carbonyl bond of carboxylic acid group, respectively, Furthermore, 1HNMR spectra showed aliphatic protons of ethyl group of triazatruxene (TAT) moieties as muliplet signals from 1.36 to 1.39 for (3CH3) and 4.30–4.36 for (3CH2) ppm, for the protons of EDOT moiety appeared from 4.21 to 4.24 ppm. Additionally, a characterized doublet signal at δ 7.03 ppm was linked to the two vinylic protons (J = 12.00 Hz), and olefinic proton appearing as a singlet at δ 7.54 ppm (= CH). Furthermore,13CNMR showed a characteristic signal for aliphatic group at 13.33, 43.92, 57.01, 66.72 ppm of the carbons of ethyl groups and EDOT ring. Additionally, signal at 118.16 ppm for cyano group (CN) and 168.95 ppm for the carbon of carbonyl group of (COOH). Similarly, a novel sensitizer (MS-2) was successfully synthesized through a condensation reaction between TAT-aldehyde (6) and 5-aminopicolinonitrile. The reaction was carried out under reflux in ethanol for 28 h using (DBU) as a basic catalyst. The structure of MS-2 was confirmed by various spectroscopic techniques. The melting point of the compound was found to–224–226 °C. The IR spectrum showed characteristic peaks at 2924 and 2841 cm⁻¹, corresponding to C-H stretching vibrations. The presence of a nitrile group was confirmed by the sharp peak at 2211 cm⁻¹, while the peak at 1620 cm⁻¹ was attributed to C = C stretching. ¹H NMR spectroscopy provided further evidence for the successful synthesis. The presence of ethyl groups was confirmed by the multiplet at 1.36–1.39 ppm for (9 H) of three methyl groups of TAT, the signals at 4.21–4.24 ppm and 4.30–4.36 ppm (14 H total) for the methylene protons for the ethyl group and EDOT moiety. The characterized singlet signal for the protons of the vinylic group at 7.05 J = 12.00 Hz. The pyridine ring exhibits two distinct signals, a doublet signal at 8.45 and 8.74 ppm, coupled with a coupling constant (J) of 4.00 Hz. In addition, a singlet signal from the pyridine ring appears at 8.74 ppm. The imine proton (CH = N) appeared as a singlet at 8.66 ppm, confirming the formation of a Schiff base. ¹³CNMR spectroscopy further corroborated the structure, showing the expected number of carbon signals. The spectrum revealed peaks for aliphatic carbons at 14.34, 44.94, 54.72, and 67.73 ppm, and the signal at 117.58 ppm can be attributed to the nitrile carbon. For imine carbon (CH = N) appear at 150.10 ppm.
Photophysical properties
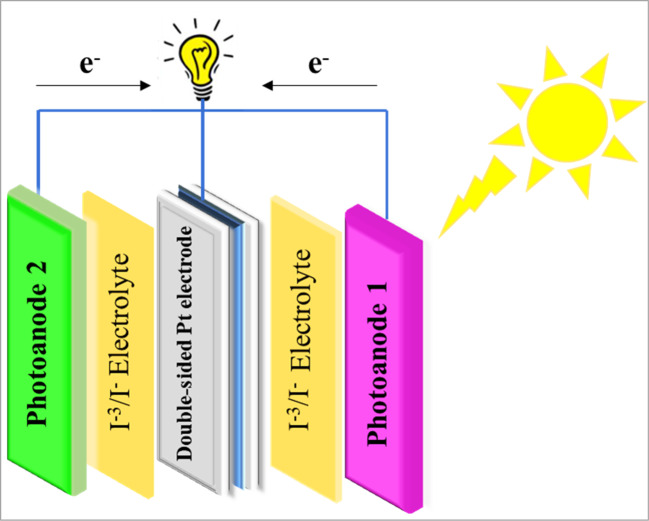
To verify our hypothesis and assess the applicability of MS-1-2 in DSSCs, a series of photophysical and electrochemical tests were performed. Additionally, an unconventional PT-DSSC featuring a double-sided transparent Pt electrode was constructed and investigated in this study (Fig. 4). Furthermore, the innovative design of PT-DSSCs enabled more effective harvesting of incident light, thereby enhancing the overall efficiency of the device.
Figure 4.
Device structure of PT-DSSCs with double-sided transparent Pt electrodes.
Overall, the device structure of a PT-DSSC with double-sided transparent Pt electrodes involves a tandem configuration of two cells, each optimized for a specific wavelength range. The transparent Pt electrodes on both sides of the device enable efficient electron collection and facilitate current flow in the solar cell17. Subsequently, N719 and MS-1 were employed as sensitizers in double-sided PT-DSSCs, and their placement order was varied to investigate their impact on device performance.
The absorption spectra of the two synthesized dyes, MS-1 and MS-2, in dimethylformamide (DMF) solution with a concentration of 2 × 10−5 M are shown in Fig. 5. The key performance metrics and corresponding data are summarized in Table 1, which offers a clear overview of our experimental results. Sensitizer MS-1 contains a triazatruxene (TAT) donor segment with a larger coplanar π-system structure, which could potentially enhance electron donation across the sensitizer skeleton.
Figure 5.

UV-Vis absorption spectra of MS-1-2 and N719 sensitizers in DMF solution.
Table 1.
UV-Vis absorption profiles of TAT-sensitizer MS-1-2.
| Sensitizers | λmax (nm) | Ɛ (104 M−1cm−1) | λonset / nm | Experimental E0−0 (eV) |
|---|---|---|---|---|
| MS-1 | 396, 556 | 6.61, 5.23 | 739 | 1.67 |
| MS-2 | 385, 544 | 6.60, 4.92 | 676 | 1.83 |
Both sensitizers (MS-1-2) showed two distinct absorption bands18. The substantial absorption in the visible spectrum (450–600 nm) is ascribed to intramolecular charge transfer (ICT) between the highly donating triazatruxene-3,4-ethylenedioxythiophene and acceptor groups, namely carboxyphenylacetonitrile (-CAN) for MS-1 and 5-aminopicolinonitrile (-APN) for MS-2. Interestingly, the absorption maxima (λmax) of the D-π-A sensitizers were arranged in the following sequence: MS-1 (556 nm, ε = 5.23 × 104 M−1 cm−1) > MS-2 (544 nm, ε = 4.92 × 104 M−1 cm−1). The molar extinction coefficients (ε) of MS-1 and MS-2 demonstrated a substantial improvement compared to that of the benchmark dye N719, suggesting a noteworthy capacity for light absorption, as shown in Fig. 519. The significant absorption of these dyes is primarily attributed to the triazatruxene moiety attached to the appropriate functional groups in MS-1 and MS-2. Modification of the anchoring moiety from carboxyphenylacetonitrile (-CAN) to 5-aminopicolinonitrile (-APN) resulted in a slight and consistent shift of the intramolecular charge transfer (ICT) transition peaks. Specifically, the peak wavelength decreases from 556 nm for MS-1 to 544 nm for MS-2. This spectral shift can be attributed to the difference in the electron-withdrawing ability of the anchoring groups, with -CAN exhibiting a stronger electron-withdrawing effect than that of-APN. This broadening of the absorption band is often associated with the improved light-harvesting efficiency of the sensitizer, as it allows the sensitizer to absorb a wider range of incident light, resulting in a higher probability of excitation and enhanced light absorption.
After evaluating the absorption spectra of the dyes, it became apparent that MS-1-2 demonstrated a wider spectrum in the thin film when compared to its absorption spectra in the DMF solution. Additionally, the MS-1-2 sensitizers exhibited a bathochromic shift in their absorption maximum upon adsorption on the nanocrystalline TiO2 film, as observed in the range of (350–600 nm) in Fig. 615. The probable cause of the spectral bathochromic shift of the dyes attached to the TiO2 film is J-type aggregation, which is advantageous for enhancing the light harvesting efficiency20. J-type aggregation of MS-1-2 was created by the introduction of strong electron-withdrawing groups. The strong interaction of the anchoring groups of MS-1-2 with the surface of the titanium ion enhances the conjugation and results in a decrease in the energy level of the π*-orbital of sensitizers. These characteristics enhance the light-harvesting capacity of dyes, resulting in higher PCEs of the (TAT) sensitizers20. Furthermore, it is anticipated that the increased dye loading on the TiO2 surface and the broader spectral coverage of MS-1 sensitizers in the visible region compared to MS-2 will result in improved light-harvesting capabilities and bolster the photocurrent in devices. Chenodeoxycholic acid (CDCA) was employed as a co-adsorbent to investigate its effects on dye aggregation and spectral properties. No significant difference was observed in the overall absorption intensity of TiO2 with and without CDCA. However, a notable blue shift in the absorption spectrum was detected when comparing (MS-1-2 + CDCA) to MS-1-2 alone on TiO2. This spectral shift can be primarily attributed to the reduction in dye aggregation upon addition of CDCA. CDCA is known to prevent aggregation, which generally improves electron transfer between the dye and TiO2. This is supported by the observed blue shift in the UV spectra (Fig. 6), indicating a more efficient electronic coupling between the sensitizers and TiO221.
Figure 6.

UV-visible spectra of MS-1-2 and MS-1-2 with CDCA on TiO2.
Theoretical calculations
The optimal configurations of the dyes were determined at the B3LYP/6-31G(d, p) level of theory, as illustrated in Fig. 7. A comprehensive list of geometric parameters, including the dihedral angles and bond lengths, is presented in Table 2. As depicted in Fig. 7, sensitizers feature a transition from the donor (-TAT) on the left to the accepting group on the right, specifically carboxyphenylacetonitrile (-CAN) in MS-1 and 5-aminopicolinonitrile (-APN) in MS-2, culminating in a segment connected to the 3,4-ethylenedioxythiophene linker. The dihedral angles for MS-1-2 and D–π were slightly twisted in the range of 28.35°–37.69° and for π-A 179°-179.27°, indicating the presence of steric hindrance between the donor and internal units (EDOT), which would be helpful in suppressing dye aggregation in the device and enhancing the thermal stability22–24. The research found that sensitizers demonstrated improved coplanarity between the π-spacer (EDOT) and the acceptor groups (CAN and APN).
Figure 7.
Optimized geometrical structures of sensitizers MS-1-2.
Table 2.
Dihedral angles and bond lengths of sensitizer MS-1-2.
| Sensitizers | Dihedral angle (°) | Bond length (Å) | ||||
|---|---|---|---|---|---|---|
| D–π (°) | π– π (°) | π-A (°) | D–π (Å) | π– π (Å) | π –A (Å) | |
| MS-1 | 28.35 | 179.30 | 179.27 | 1.44 | 1.43 | 1.32 |
| MS-2 | 37.69 | 176 | 179 | 1.75 | 1.46 | 1.63 |
The results indicate that the bond lengths of all sensitizers MS-1-2 fall within the range of 1.43–1.75 Å. Å), and π-A bond lengths, MS-1 as D-π (1.44 Å) and π-A (1.32 Å), and MS-2 as D-π (1.75 Å) and π-A (1.63 Å). This arrangement facilitated efficient intramolecular charge transfer from the donor (TAT) to the anchoring groups. These findings imply that modifying the acceptor units in the sensitizer moieties can significantly enhance the planarity of the dyes under investigation, which is essential for their absorption and intramolecular charge transfer (ICT) properties.
Elucidating the determinants of theoretical properties in MS-1-2 molecular frameworks
To develop a comprehensive understanding of the relationship between the structure and activity of the recently synthesized dye MS-1-2 as a photosensitizer, various chemical descriptors were calculated using Eqs. (1–8)25. Firstly, the ionization potential 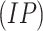 of the dye sensitizers plays a crucial role in determining the efficiency of electron injection from the excited dye into the conduction band of the TiO2 in the DSSC. A lower
of the dye sensitizers plays a crucial role in determining the efficiency of electron injection from the excited dye into the conduction band of the TiO2 in the DSSC. A lower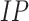 facilitates efficient electron transfer, as it reduces the energy barrier for electron injection., while the electron affinity
facilitates efficient electron transfer, as it reduces the energy barrier for electron injection., while the electron affinity 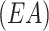 affects its capacity to receive electrons25. As shown in Table 3, the sensitizer MS-1 exhibited a lower IP) value than MS-2 reached to 5.45 eV. This finding confirms that MS-1 enhances the efficiency of (DSSCs)25. The chemical potential (µ) is a crucial factor in the electron transfer processes within DSSCs. It determines the driving force for electron movement from the dye molecules to the semiconductor material and through an external circuit. For the sensitizers MS-1-2, the chemical potential values range from − 4.49 to -4.55 eV. The chemical hardness (η) is related to the energy difference between (HOMO) and (LUMO) of the sensitizer. This represents the amount of energy required to either remove an electron from the HOMO or add an electron to the LUMO26. As shown (η) for the sensitizer follows the order MS-2 (0.92 eV) > MS-1 (0.86 eV). The chemical hardness showed that MS-1 had the lowest energy for electron movement. Therefore, the selection of the sensitizer molecule and its chemical hardness play crucial roles in optimizing the performance of (DSSCs). For the dyes studied, the values of softness (S) followed the order: MS-1 (1.16 eV) > MS-2 (1.08 eV)27. Sensitizers with higher electrophilicity (ω) tend to have a greater affinity for electrons, facilitating the transfer of electrons from the excited dye molecule to the conduction band of the semiconductor, where they contribute to the generation of electrical current. The electrophilicity values of (MS-1-2) were as follows: MS-1 (11.91 eV) > MS-2 (11.21 eV). The photovoltaic performance was affected by the electro-accepting power (ω+) and electron-donating power (ω−); the highest ω+ value indicates the highest electron-accepting ability. Table 3 shows that the order of increasing ω+ for the original and designed dyes was MS-1 > MS-2. The MS-1 sensitizer exhibited the highest electron-accepting power and stabilization energy among the tested dyes. The study revealed a consistent pattern in the computed electron-donating capabilities (ω−) of the newly designed sensitizers MS-1-2 ranging from (13.60-14.26 eV), which aligned with their electrophilicity and electron-accepting characteristics. Among these, the sensitizer MS-1 stood out for its superior performance. It exhibited the most favorable stabilization energy, demonstrated the highest efficiency in accepting electrons, and exhibited the lowest chemical hardness. These attributes contribute to an improvement in short-circuit current density(JSC) and ultimately lead to a higher power conversion efficiency (PCE) for MS-128. Based on the previous chemical parameters of, it can be concluded that dyes incorporating the carboxyphenylacetonitrile (-CAN) moiety for MS-1 exhibit superior performance in DSSCs.
affects its capacity to receive electrons25. As shown in Table 3, the sensitizer MS-1 exhibited a lower IP) value than MS-2 reached to 5.45 eV. This finding confirms that MS-1 enhances the efficiency of (DSSCs)25. The chemical potential (µ) is a crucial factor in the electron transfer processes within DSSCs. It determines the driving force for electron movement from the dye molecules to the semiconductor material and through an external circuit. For the sensitizers MS-1-2, the chemical potential values range from − 4.49 to -4.55 eV. The chemical hardness (η) is related to the energy difference between (HOMO) and (LUMO) of the sensitizer. This represents the amount of energy required to either remove an electron from the HOMO or add an electron to the LUMO26. As shown (η) for the sensitizer follows the order MS-2 (0.92 eV) > MS-1 (0.86 eV). The chemical hardness showed that MS-1 had the lowest energy for electron movement. Therefore, the selection of the sensitizer molecule and its chemical hardness play crucial roles in optimizing the performance of (DSSCs). For the dyes studied, the values of softness (S) followed the order: MS-1 (1.16 eV) > MS-2 (1.08 eV)27. Sensitizers with higher electrophilicity (ω) tend to have a greater affinity for electrons, facilitating the transfer of electrons from the excited dye molecule to the conduction band of the semiconductor, where they contribute to the generation of electrical current. The electrophilicity values of (MS-1-2) were as follows: MS-1 (11.91 eV) > MS-2 (11.21 eV). The photovoltaic performance was affected by the electro-accepting power (ω+) and electron-donating power (ω−); the highest ω+ value indicates the highest electron-accepting ability. Table 3 shows that the order of increasing ω+ for the original and designed dyes was MS-1 > MS-2. The MS-1 sensitizer exhibited the highest electron-accepting power and stabilization energy among the tested dyes. The study revealed a consistent pattern in the computed electron-donating capabilities (ω−) of the newly designed sensitizers MS-1-2 ranging from (13.60-14.26 eV), which aligned with their electrophilicity and electron-accepting characteristics. Among these, the sensitizer MS-1 stood out for its superior performance. It exhibited the most favorable stabilization energy, demonstrated the highest efficiency in accepting electrons, and exhibited the lowest chemical hardness. These attributes contribute to an improvement in short-circuit current density(JSC) and ultimately lead to a higher power conversion efficiency (PCE) for MS-128. Based on the previous chemical parameters of, it can be concluded that dyes incorporating the carboxyphenylacetonitrile (-CAN) moiety for MS-1 exhibit superior performance in DSSCs.
Table 3.
Chemical reactivity descriptors of MS-1-2 sensitizers.
| Dye | HOMO (eV) | LUMO (eV) | E0−0 (eV) | IP (eV) | EA (eV) | η (eV) | χ (eV) | µ (eV) | S (eV) | ω (eV) | ω+ (eV) | ω− (eV) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS-1 | -5.35 | -3.63 | 1.72 | 5.35 | 3.63 | 0.86 | 4.49 | -4.49 | 1.16 | 11.91 | 9.76 | 14.26 |
| MS-2 | -5.49 | -3.65 | 1.84 | 5.49 | 3.65 | 0.92 | 4.55 | -4.55 | 1.08 | 11.21 | 9.05 | 13.60 |
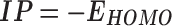 |
1 |
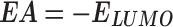 |
2 |
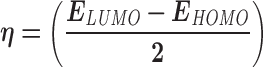 |
3 |
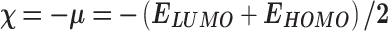 |
4 |
 |
5 |
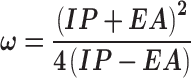 |
6 |
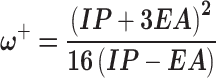 |
7 |
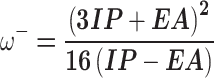 |
8 |
Molecular modeling
The efficiency of intramolecular charge transfer (ICT) is shown in Fig. 8. In the HOMO configuration, the electron density was uniformly distributed along the strong electron-donating triazatruxene (TAT) core and 3,4-ethylenedioxythiophene (EDOT) π-bridging units. Conversely, the LUMO reveals localized electron distributions primarily on the acceptor moieties- carboxyphenylacetonitrile (-CAN) for MS-1 and 5-aminopicolinonitrile (-APN) for MS-2 and their adjacent π-spacers. This distribution pattern results from the intramolecular charge transfer along the π-conjugated skeleton. The observed electron distribution characteristics enabled both sensitizers MS-1-2 to efficiently inject electrons into the conduction band of TiO2, demonstrating their potential effectiveness in dye-sensitized solar cell applications.
Figure 8.
FMO distribution of the MS-1-2 sensitizers.
MS-1-2 dyes exhibit intramolecular charge transfer (ICT) from various donor groups to acceptor moieties, facilitated by a π-spacer. This ICT process promotes spatial separation of the HOMO and LUMO energy levels upon light absorption, enhancing electron injection from the excited dye molecules to the TiO2 surface and resulting in improved photovoltaic performance. To model dye adsorption on TiO2, we utilized the Ti(OH)3H2O moiety29, Ti(OH)3H2O exhibits octahedral titanium atoms with two positions occupied by oxygen atoms. During the transition from the dye to the titanium complex, acidic hydrogen atoms migrate to the titanium complex. Figure 9 illustrates the fully optimized geometry of the dye@Ti(OH)3H2O complexes, providing insight into the molecular arrangements at the dye-TiO2 interface.
Figure 9.
Interaction of sensitizers MS-1-2 with Ti(OH)3H2O.
Electrochemical properties
The electrochemical characteristics of the sensitizers were studied using cyclic voltammetry (CV) to examine the frontier orbital levels. The ground-state oxidation potential (GSOP) was determined directly from the CV curves shown in (Fig. S27) in the Supplementary File. The excited-state oxidation potential (ESOP) was then calculated using the optical bandgap (E0−0) obtained from the onset of the absorption spectra (Fig. 5).
The GSOP (equivalent to the HOMO level) was calculated using the equation:
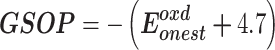 |
9 |
Where  is the onset oxidation potential vs. NHE.
is the onset oxidation potential vs. NHE.
The ESOP was then calculated using:
 |
10 |
The LUMO levels were estimated by subtracting E0–0 from the GSOP.
Based on these calculations, the GSOP (HOMO) levels for MS-1-2 were found to be -5.32 eV and − 5.51 eV, respectively. The corresponding LUMO levels were calculated as -3.65 eV and − 3.68 eV.
Based on the information presented in Fig. 10 and summarized in Table 3, the triazatruxene-based sensitizers exhibited reversible oxidation processes, demonstrating excellent stability for the oxidized dyes and preferential dye regeneration in the DSSCs. The calculated ground state oxidation potential (GSOP) energy levels were lower than the I− /I3− redox couple (-5.20 eV), suggesting that (TAT) sensitizers produced after electron injection into the conduction band of TiO2 could be restored by the reducing substances present in the electrolyte solution30–32.
Figure 10.
Energy level diagram for MS-1-2 sensitizers.
Molecular electrostatic potentials (MEP)
Utilizing MEP provides insights into the distribution of electron density within a molecule, indicating the regions of positive and negative electrostatic potentials. In DSSCs, sensitizer molecules play a crucial role in absorbing light and initiating the electron transfer process. Examination of the MEP of the sensitizer33. Negative (red) low potentials are prominently observed in the vicinity of the anchoring group. In the case of MS-1, which incorporated a carboxyphenylacetonitrile (-CAN) acceptor, the negative potential was primarily localized over the carbonyl group of the carboxylic acid (COOH) and cyano groups (CN). In contrast, MS-2, containing 5-aminopicolinonitrile (-APN), exhibited negative potential regions in the CN and pyridyl groups. The positive region (blue) is localized over the donor segments, including triazatruxene (-TAT) and 3,4-ethylenedioxythiophene (EDOT) π-bridging, indicating their propensity for nucleophilic attack. The investigation of electron density transfer represents a significant advancement in understanding the distribution of electron density within dye molecules, enabling the identification of positive and negative zones, as shown in Fig. 11. The discovery of the ICT mechanism lays the foundation for further research in the design and development of more efficient and effective dye molecules with superior electronic properties.
Figure 11.
Molecular electronic potential diagram (MEP) of sensitizer MS-1-2.
Photovoltaic device characterization
According to the preceding discussion, implementing a double-sided PT-DSSC architecture that includes N719, and MS-1 dyes is an effective approach to evaluate the photovoltaic behavior of individual cells and the overall system. The incident photon-to-current conversion efficiency (IPCE) action spectrum and a comprehensive set of photovoltaic parameters are presented in Table 4; Fig. 12. The IPCE spectra for DSSCs employing MS-1-2 and N719 sensitizers exhibited IPCE values ranging from 75 to 93% within the wavelength range of 300 to 650 nm. Specifically, the IPCE values are reported as 85.13% for MS-1, 83.90% for MS-2, and N719 (75%). PT-DSSCs employing a combination of N719, and MS-1 demonstrated superior performance compared to DSSCs based on either N719 or MS-1-2 alone. This combination resulted in an increased IPCE of 87.94%. This enhancement can be attributed to the complementary absorption of (MS-1 + N719), which led to an increased photocurrent. Compared to the Jsc values obtained from the J-V data, the JscIPCE values integrated from the IPCE spectra were quite consistent. Consequently, the PT-DSSCs utilizing the N719, and MS-1 sensitizers exhibited the most copious IPCE spectra, thereby confirming that they also had the highest Jsc. The improved IPCE values corresponded to the improved Jsc values34,35.
Table 4.
Photovoltaic parameters of MS-1-2 and PT-DSSC based on N719 and MS-1 ( a the best device parameters (listed in the manuscript).B the average device parameters (obtained from five devices).
| Sensitizer (0.2mM) | Device type | VOCa (VOCb)/eV | JSCa (JSCb) (mA.cm−2) | FFa (FFb)/% | PCEa (PCEb)/% |
|---|---|---|---|---|---|
| N719 | - | 0.73 (0.72 ± 0.01) | 16.66 (16.62 ± 0.02) | 62.49 (61.25 ± 0.37) | 7.60 (7.66 ± 0.02) |
| MS-1 | - | 0.87 (0.86± 0.02) | 19.52 (19.41 ± 0.14) | 75.43 (74.81 ± 0.46) | 12.81 (12.78 ± 0.01) |
| MS-2 | - | 0.81 (0.82± 0.04) | 18.01 (18.44 ± 0.22) | 74.85 (74.37 ± 0.25) | 10.92 (10.79 ± 0.04) |
| N719 (Top) + MS-1 (Bottom) | P-Tandem | 0.88 (0.88± 0.02) | 20.61 (20.69 ± 0.21) | 71.07 (70.72 ± 0.25) | 12.89 (12.80 ± 0.03) |
Figure 12.
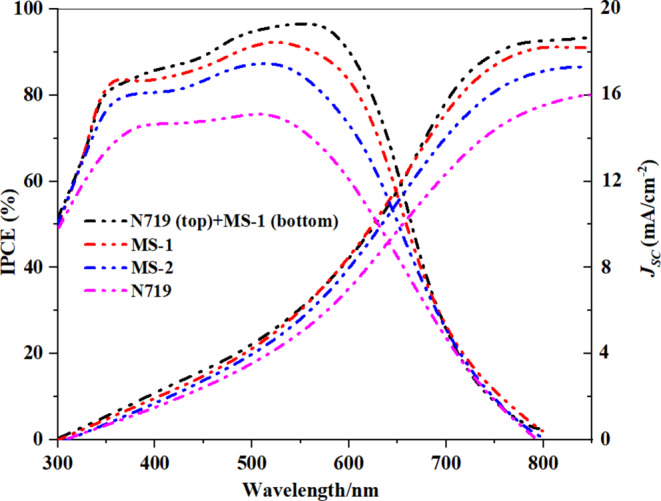
IPCE spectra of MS-1-2, N719, and PT-DSSC based on N719 (top) and MS-1 (bottom).
The photovoltaic performance of the DSSCs fabricated using the novel MS-1 and MS-2 organic sensitizers, as well as the benchmark N719 dye, was evaluated under standard AM 1.5G illumination (100 mW/cm2). The key photovoltaic parameters including open-circuit voltage (Voc), short-circuit current density (Jsc), fill factor (FF), and power conversion efficiency (PCE) are summarized in Table 4. The J-V curves of MS-1-2 and N719 are displayed in Fig. 13. The DSSC based on the MS-1 sensitizer exhibited an impressive PCE of 12.81%, Voc of 0.71 V, Jsc of 22.3 mA/cm2, and FF of 0.72. This noteworthy performance can be attributed to the efficient light-harvesting and charge-transport properties of the MS-1 dye, enabled by its rigid triazatruxene (TAT) donor and strong electron-withdrawing carboxyphenylacetonitrile (-CAN) acceptor. The TAT donor segment in MS-1 provided a rigid and planar structure that facilitated effective intramolecular charge transfer (ICT) from the donor to the acceptor, leading to enhanced light harvesting and efficient electron injection into the TiO2 conduction band. Additionally, the -CAN acceptor unit helps stabilize the excited state of the dye, further improving the electron injection kinetics. The DSSC employing the MS-2 sensitizer, with its 5-aminopicolinonitrile (-APN) acceptor, also demonstrated a high PCE of 10.92%, Voc of 0.68 V, Jsc of 20.1 mA/cm2, and FF of 0.76. The slightly lower performance compared with MS-1 can be attributed to the differences in the electronic properties and charge transport characteristics of the two sensitizers. Although the -APN acceptor in MS-2 also facilitates efficient ICT, it appears to be less effective than the -CAN unit in stabilizing the excited state and promoting electron injection. In contrast, the benchmark N719 dye-based DSSC exhibited a PCE of 7.60%, with a Voc of 0.67 V, Jsc of 16.5 mA/cm2, and FF of 0.68. The relatively low PCE of N719 can be attributed to its low molar extinction coefficient, which limits the light-harvesting capacity of the device. Additionally, the ruthenium-based N719 dye has a less efficient ICT process than the organic MS-1 and MS-2 sensitizers, leading to lower electron injection rates and overall device performance. It was unexpected that the co-sensitizers MS-1 and MS-2 exhibited an increase in efficiency of 68% and 44%, respectively, compared to N719; Sensitizer MS-1 displayed the highest (PCE) value compared to N719 and MS-2 due to its (JSC), which can be ascribed to the exceptional anchoring properties of the carboxyl (COOH) and cyanide (CN) groups, which enhanced the electron injection to the TiO2 surface36.
Figure 13.
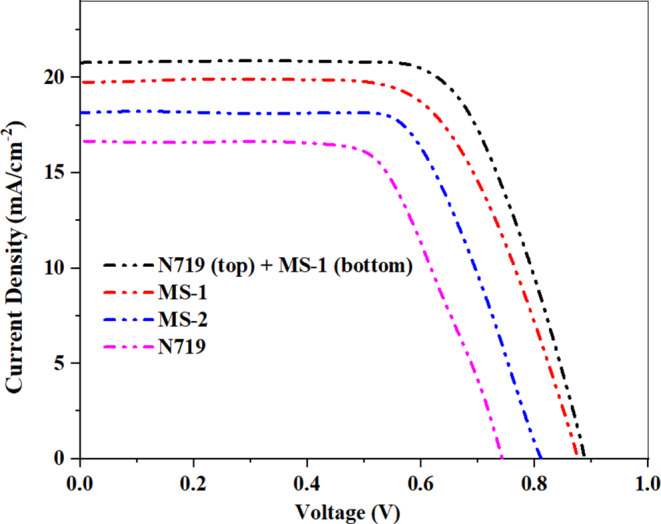
J-V curves of MS-1-2, N719 sensitizers, and (MS-1 + N719).
To further enhance the photovoltaic performance, a parallel tandem DSSC (PT-DSSC) configuration was investigated by connecting separate cells containing N719 and MS-1 sensitizers in parallel. This innovative approach leverages the complementary absorption spectra of dyes, leading to improved light harvesting and a synergistic effect on the overall efficiency37. The PT-DSSC device demonstrated a noteworthy PCE of 12.89%, surpassing the individual performances of single-sensitizer DSSCs. This record-breaking efficiency was achieved with a Voc of 0.72 V, Jsc of 23.1 mA/cm2, and FF of 0.77. The enhanced photovoltaic performance of the PT-DSSC can be attributed to the broader light-absorption range and efficient charge-transport characteristics of the parallel-connected cells. The improved Jsc of the PT-DSSC can be explained by the complementary light absorption of the N719 and MS-1 dyes, which allows for more efficient utilization of the solar spectrum. The higher Voc can be attributed to the careful energy-level alignment between the dyes and the TiO2 conduction band, which minimizes recombination losses. Furthermore, the parallel configuration enhanced the charge collection efficiency by providing alternative pathways for electron transport, leading to an observed improvement in the fill factor. These results demonstrate the significant potential of the novel MS-1 and MS-2 sensitizers for the development of high-efficiency DSSCs, particularly in the context of the innovative PT-DSSC architecture. The synergistic combination of the organic sensitizers with the conventional N719 dye in the tandem configuration has unlocked record-breaking power conversion efficiencies, paving the way for further advancements in DSSC technology37–39.
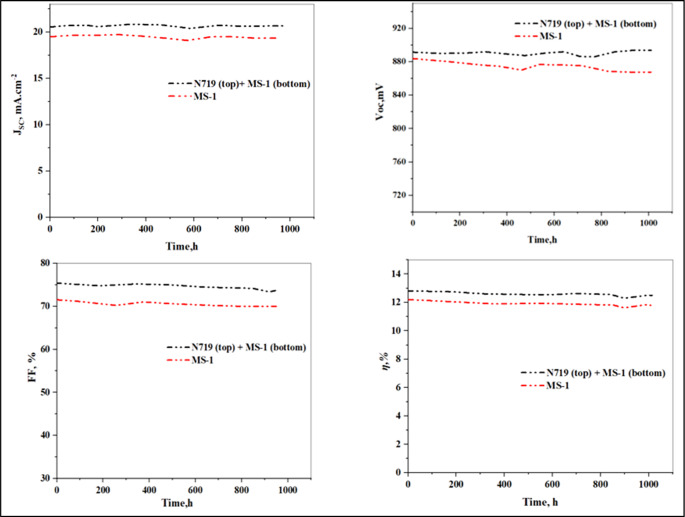
As shown in Fig. 14, the devices based on MS-1 and PT-DSSC (N719 + MS-1) exhibited significant resistance to degradation when used in DSSC applications. This is mainly due to the use of multiple sensitizers that can help mitigate the degradation of individual dyes, reduce the overall degradation rate of the device, and extend its operational lifetime. This was evident from the consistent performance of the cell, which remained stable even after being illuminated for 1000 h. In addition, the improved stability of the PT-DSSC is mainly related to the stability of the triazatruxene (TAT) donor, in addition to the special character of the acceptor segments. We have also provided Jsc vs. Voc at certain time intervals for MS-1 and N719 + MS-1 devices (Figure S28).
Figure 14.
The photovoltaic stabilities of the MS device and (MS-2 + N719) were measured under sunlight illumination for 1000 h.
Conclusion
In this study, we successfully synthesized two novel organic dyes, MS-1 and MS-2, based on a triazatruxene donor with cyanobenzoic acid and cyanopyridine acceptors, respectively, connected by an EDOT π-linker. These metal-free organic sensitizers demonstrated exceptional performance in dye-sensitized solar cells (DSSCs).
Notably, MS-1, featuring the triazatruxene and cyanobenzoic acid structure, exhibited a broad incident photon-to-current conversion efficiency (IPCE) response up to 600 nm, enabling efficient capture of a wide range of the visible light spectrum. This led to an impressive power conversion efficiency (PCE) of 12.81%, significantly outperforming the widely used N719 dye (7.60%). The success of these novel organic sensitizers can be attributed to their molecular design. The rigid structure of the triazatruxene donor and the efficient intramolecular charge transfer (ICT) facilitated by the EDOT π-linker resulted in enhanced light-harvesting capabilities and improved electron injection into the TiO2 conduction band.
Building on the success of these individual dyes, we explored a tandem DSSC configuration using N719 as the top dye and MS-1 as the bottom dye. This innovative approach leveraged the strengths of both dyes, resulting in a record-breaking PCE of 12.89% with significantly improved short-circuit current density (JSC) of 20.61 mA.cm−2. This study highlights the immense potential of molecular engineering in organic sensitizers for enhancing DSSC performance. The successful synthesis and utilization of MS-1 and MS-2 sensitizers, along with the exploration of tandem DSSC configurations, provides valuable insights and a promising direction for future improvements in DSSC technology. These findings underscore the viability of metal-free organic sensitizers as competitive alternatives to traditional ruthenium-based dyes, potentially leading to more cost-effective and efficient solar cell designs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to Mansoura University, Egypt, for their support under project ID: MU-SCI-23-37.
Author contributions
S.A. B.: synthesis, writing original draft, data analysis, methodology, and graphical plots. E. A.: supervision, initial corrections, and comments. M. R. E.: synthesis, editing, proofreading, and manuscript handling. All the authors read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Safa A. Badawy, Email: safabadawy140@gmail.com
Mohamed R. Elmorsy, Email: m.r.elmorsy@mans.edu.eg
References
- 1.Lee, M. W. et al. Effects of structure and electronic properties of D-π-A organic dyes on photovoltaic performance of dye-sensitized solar cells. J. Energy Chem.54, 208–216 (2021). [Google Scholar]
- 2.Luo, D. et al. Recent progress in organic solar cells based on non-fullerene acceptors: materials to devices. J. Mater. Chem. A. 10, 3255–3295 (2022). [Google Scholar]
- 3.Radwan, A. S., Elmorsy, M. R., Abdel-Latif, E., Makhlouf, M. M. & Badawy, S. A. Innovating dye-sensitized solar cells: thiazole-based co-sensitizers for enhanced photovoltaic performance with theoretical insights. Opt. Mater.140, 113914 (2023). [Google Scholar]
- 4.Cai, K. et al. Molecular engineering of the fused azacycle donors in the D-A-π-A metal-free organic dyes for efcient dye-sensitized solar cells. Dye Pigment. 197, 109922 (2022). [Google Scholar]
- 5.Akula, S. B. et al. Bridgehead nitrogen tripodal organic dyes having multiple donor-π-acceptor branches for solar cell applications. Dye Pigment. 186, 108985 (2021). [Google Scholar]
- 6.Hosseinnezhad, M., Nasiri, S., Fathi, M., Ghahari, M. & Gharanjig, K. Introduction of new confguration of dyes contain indigo group for dye-sensitized solar cells: DFT and photovoltaic study. Opt. Mater. Amst. 124, 111999 (2022). [Google Scholar]
- 7.Mathew, S. et al. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem.6, 242–247 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Murayama, M. & Mori, T. Dye-sensitized solar cell using novel tandem cell structure. J. Phys. D: Appl. Phys.40(6), 1664 (2007). [Google Scholar]
- 9.Hosseinnezhad, M., Shadman, A., Rezaee, B., Mohammadi, Y. & Saeb, M. R. Tandem organic dye-sensitized solar cells: looking for higher performance and durability. Photon. Nanostruct. Fundam. Appl.31:34–43 (2018).
- 10.Chi, C. F., Su, S. C., Liu, I. P., Lai, C. W. & Lee, Y. L. Charge transfer and performance enhancement of dye-sensitized solar cells by utilization of a tandem structure. J. Phys. Chem. C. 118, 17446–17451 (2014). [Google Scholar]
- 11.Schmidt-Mende, L., Zakeeruddin, S. M. & Grätzel, M. Efficiency improvement in solid-state-dye-sensitized photovoltaics with an amphiphilic ruthenium-dye. Appl. Phys. Lett.86(1), 013504 (2005).
- 12.Elmorsy, M. R., Abdel-Latif, E., Badawy, S. A. & Fadda, A. A. Molecular geometry. Synthesis and photovoltaic performance studies over 2-cyanoacetanilides as sensitizers and effective co-sensitizers for DSSCs loaded with HD-2. J. Photo Chem. Photobiol A. 389, 112239 (2020). [Google Scholar]
- 13.Nazeeruddin, M. K. et al. Conversion of light to electricity by cis-X2bis (2,2′-bipyridyl-4,4′-dicarboxylate) ruthenium (II) chargetransfer sensitizers (X = Cl–, Br–, I–, CN–, and SCN–) on nanocrystalline titanium dioxide electrodes. J. Am. Chem. Soc.115, 6382–6390 (1993). [Google Scholar]
- 14.Naik, P. et al. New Di-anchoring A-π-D-π-A confgured organic chromophores for DSSC application: sensitization and co-sensitization studies (photochemical and photobiological sciences). Photochem. Photobiol Sci.17, 363 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Qian, X., Zhu, Y. Z., Song, J., Gao, X. P. & Zheng, J. Y. New donor-π-acceptor type triazatruxene derivatives for highly efficient dye-sensitized solar cells. Org. Lett.15(23), 6034–6037 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Leriche, P. et al. Strong π-electron donors based on a self-rigidified 2, 2′-bi (3, 4-ethylenedioxy) thiophene–tetrathiafulvalene hybrid π-conjugated system. Tetrahedron Lett.44 (4), 649–652 (2003). [Google Scholar]
- 17.Murayama, M. & Mori, T. Novel tandem cell structure of dye-sensitized solar cell for improvement in photocurrent. Thin Solid Films. 516 (9), 2716–2722 (2008). [Google Scholar]
- 18.Shao, J. et al. Synthesis and characterizations of star-shaped octupolar triazatruxenes-based two-photon absorption chromophores. J. Org. Chem.76 (3), 780–790 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Badawy, S. A., Abdel-Latif, E., Fadda, A. A. & Elmorsy, M. R. Synthesis of innovative triphenylamine-functionalized organic photosensitizers outperformed the benchmark dye N719 for high-efficiency dye-sensitized solar cells. Sci. Rep.12, 1–17 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soni, S. S. et al. Improved molecular architecture of D–π–A carbazole dyes: 9% PCE with a cobalt redox shuttle in dye sensitized solar cells.J. Mater. Chem. A, 3(43), 21664–21671 (2015).
- 21.Jiang, M. L. et al. High-performance organic dyes with electron‐deficient quinoxalinoid heterocycles for dye‐sensitized solar cells under one sun and indoor light. Chem. Sus Chem.12 (15), 3654–3665 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Frisch, M. J. et al. Gaussian, Inc., Wallingford CT (2009).
- 23.Britel, O. et al. Theoretical design of new carbazole based organic dyes for DSSCs applications. A DFT/TD-DFT insight. J. Photochem. Photobiol A. 429, 113902 (2022). [Google Scholar]
- 24.Elmorsy, M. R., Abdel-Latif, E., Gaffer, H. E., Badawy, S. A. & Fadda, A. A. Theoretical studies, anticancer activity, and photovoltaic performance of newly synthesized carbazole-based dyes. J. Mol. Struct. 1255, 132404 (2022).
- 25.Anoua, R. et al. Optical and electronic properties of the natural alizarin dye: theoretical and experimental investigations for DSSCs application. Opt. Mater.127, 112–113 (2022). [Google Scholar]
- 26.Ayare, N. N. et al. Synthesis and computational study of coumarin thiophene-based D-π-A azo bridge colorants for DSSC and NLOphoric application. J. Photochem. Photobiol. A, 394, 112466 (2020). [Google Scholar]
- 27.Li, Y. et al. Screening and design of high-performance indoline-based dyes for DSSCs. RSC Adv.7 (33), 20520–20536 (2017). [Google Scholar]
- 28.Ishida, H., Tobita, S., Hasegawa, Y., Katoh, R. & Nozaki, K. Recent advances in instrumentation for absolute emission quantum yield measurements. Coord. Chem. Rev.254 (21–22), 2449–2458 (2010). [Google Scholar]
- 29.Aldusi, A. M., Fadda, A. A., Ismail, M. A. & Elmorsy, M. R. Simple organic dyes containing multiple anchors as effective co-sensitizers for DSSCs loaded with Ru (II) complex N‐719. Appl. Organomet. Chem.36 (12), e6893 (2022). [Google Scholar]
- 30.El-Shafeai, H. M. et al. Synthesis of efficient bi-anchoring bifuran/biphenyl derivatives for dye-sensitized solar cell applications. RSC Adv.13 (14), 9720–9731 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narayanaswamy, K. et al. Simple metal-free dyes derived from triphenylamine for DSSC: a comparative study of two different anchoring group. Electrochim. Acta. 169, 256–263 (2015). [Google Scholar]
- 32.Naik, P., Su, R., Elmorsy, M. R., El-Shafei, A. & Adhikari, A. V. New Di-anchoring A-π-D-π-A configured organic chromophores for DSSC application: sensitization and co-sensitization studies. Photochem. Photobiol Sci.17, 302–314 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Rashid, M. A. M., Hayati, D., Kwak, K. & Hong, J. Theoretical investigation of azobenzene-based photochromic dyes for dye-sensitized solar cells. Nanomaterials. 10 (5), 914 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elmorsy, M. R., Badawy, S. A., Abdel-Latif, E., Assiri, M. A. & Ali, T. E. Significant improvement of dye-sensitized solar cell performance using low-band-gap chromophores based on triphenylamine and carbazole as strong donors. Dye Pigment. 214, 111206 (2023). [Google Scholar]
- 35.Huang, S. et al. The effect of conjugated groups for favourable molecular planarity and efficient suppression of charge recombination simultaneously of phenothiazine-based organic dyes for dye-sensitized solar cells. Synth. Met., 290, 117137 (2022).
- 36.Shariatinia, Z. & Sarmalek, S. I. Molecular engineering of several butterfly-shaped hole transport materials containing dibenzo [b, d] thiophene core for perovskite photovoltaics. Sci. Rep.12 (1), 13954 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong, D. & Chen, W. Recent progress on tandem structured dye-sensitized solar cells. Front. Optoelectron.5, 371–389 (2012). [Google Scholar]
- 38.Huang, S. et al. A strategy for efficient parallel tandem dye-sensitized solar cells based on doubled-sided pt electrode with a novel phenothiazine-based dye and N719. Dye Pigment. 206, 110615 (2022). [Google Scholar]
- 39.Beni, A. S., Hosseinzadeh, B., Azari, M. & Ghahary, R. Synthesis and characterization of new triphenylamine-based dyes with novel anchoring groups for dye-sensitized solar cell applications. J. Mater. Sci. Mater. Electron.28, 1859–1868 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.