Abstract
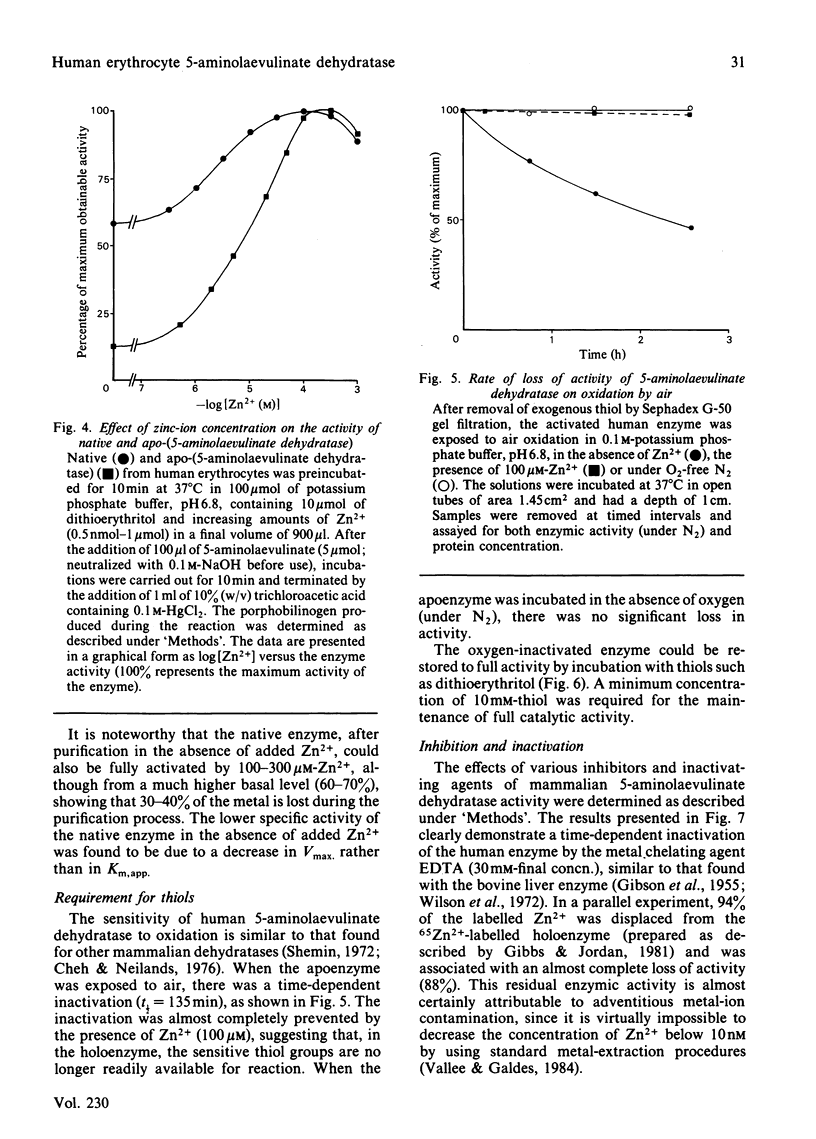
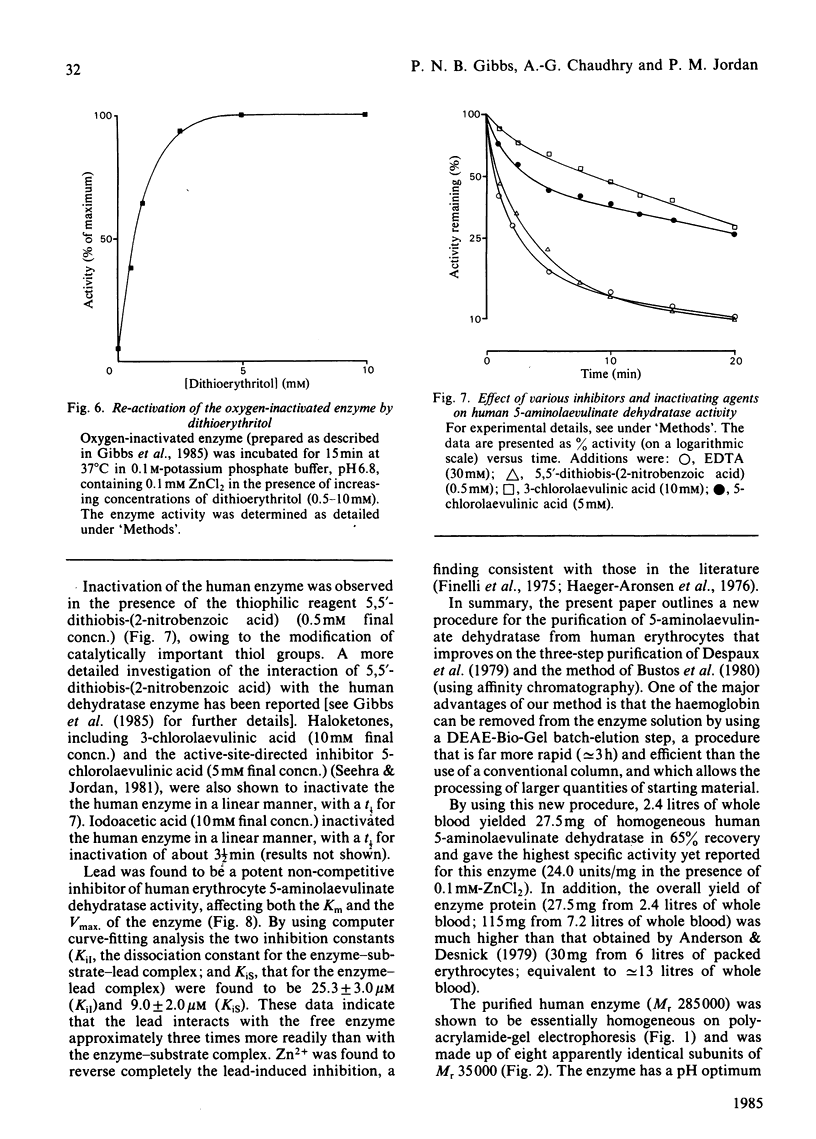
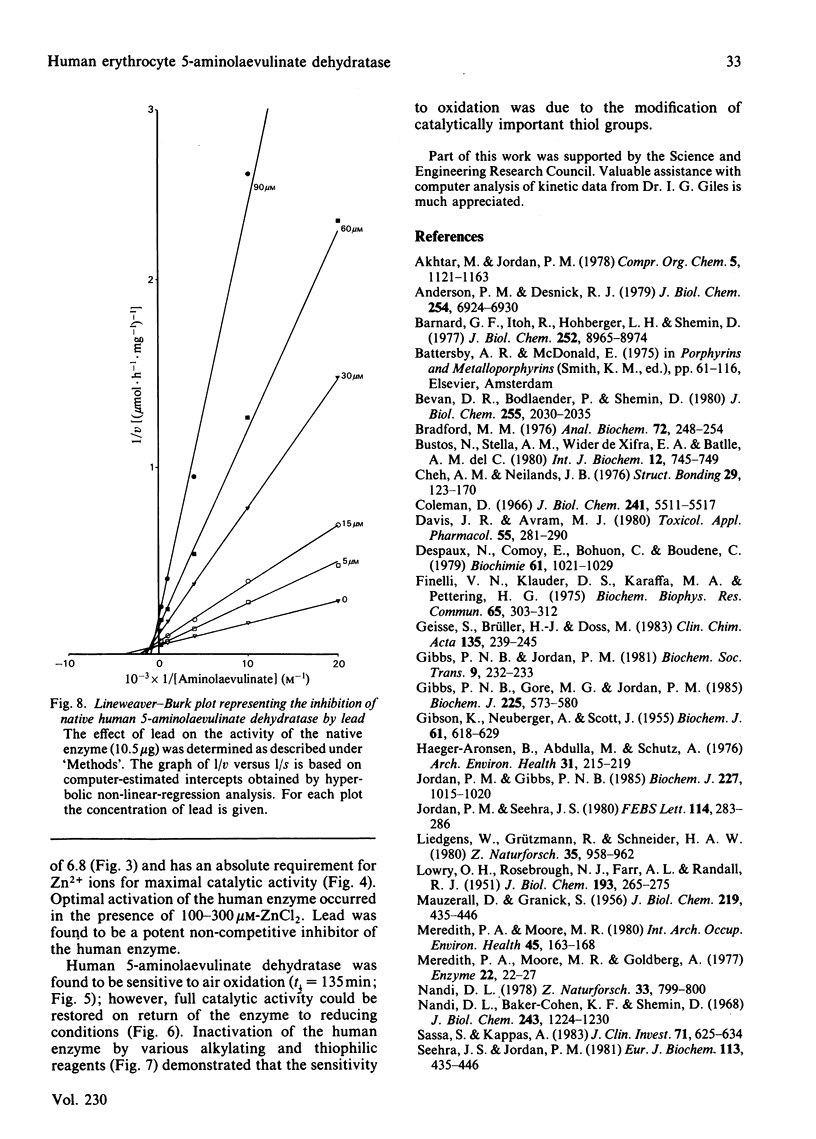
A new procedure for the isolation of homogeneous human 5-aminolaevulinate dehydratase (porphobilinogen synthase, EC 4.2.1.24) is described in which the enzyme is purified 35000-fold and in 65-74% yield. The specific activity of the purified enzyme, 24 units/mg, is the highest yet reported. An efficient stage for the removal of haemoglobin is incorporated in the method, which has general application to the purification of other erythrocyte enzymes. The erythrocyte dehydratase (Mr 285 000) is made up of eight apparently identical subunits of Mr 35 000. The enzyme is sensitive to oxygen, and its activity is maintained by the presence of thiols such as dithioerythritol. Zn2+ is obligatory for enzyme activity, the apoenzyme being essentially inactive (approximately equal to 12% of control) when assayed in buffers devoid of Zn2+. Addition of Zn2+ to the apoenzyme restores activity as long as the sensitive thiol groups are fully reduced; optimal stimulation occurs between 100 and 300 microM-Zn2+. The human enzyme is inhibited by Pb2+ in a non-competitive fashion [KiI (dissociation constant for E X S X Pb2+ complex) = 25.3 +/- 3.0 microM; KiS (dissociation constant for E X Pb2+ complex) = 9.0 +/- 2.0 microM]. Modification of thiol groups, inactivation by oxidation, alkylation or reaction with thiophilic reagents demonstrates the importance of sensitive thiol groups for full enzymic activity.
Full text
PDF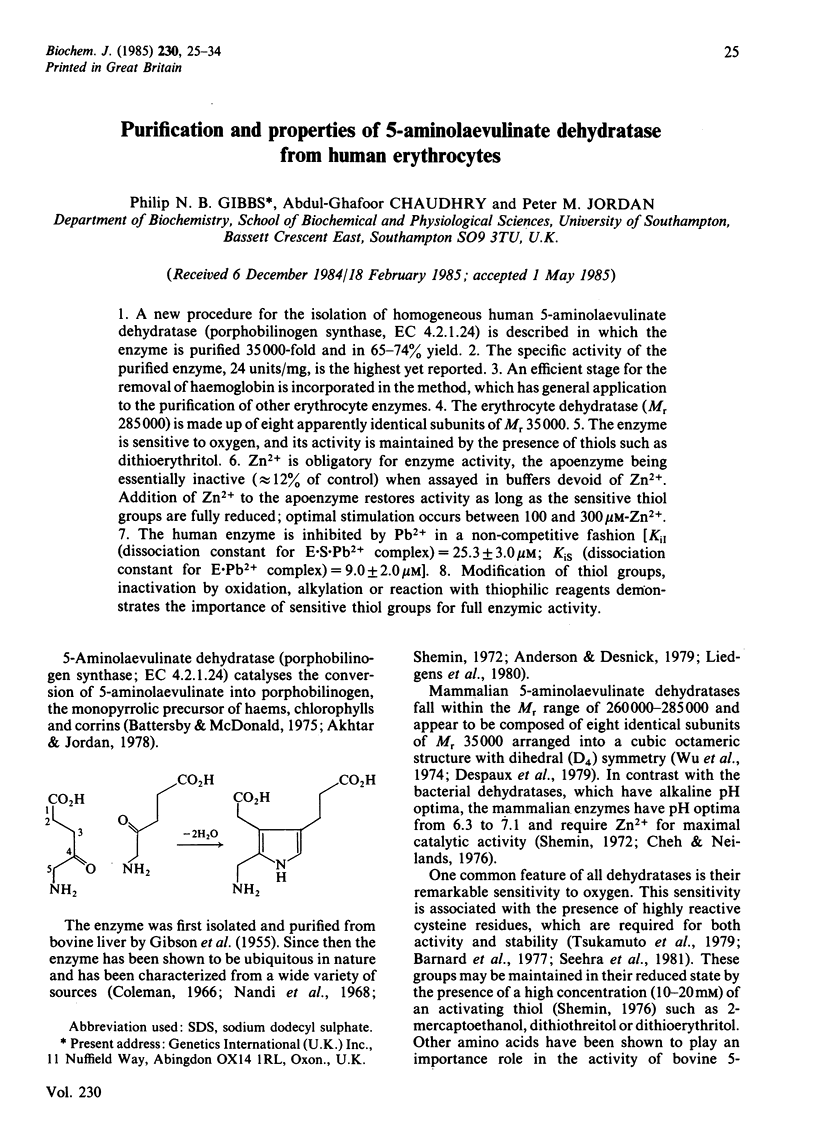



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Purification and properties of delta-aminolevulinate dehydrase from human erythrocytes. J Biol Chem. 1979 Aug 10;254(15):6924–6930. [PubMed] [Google Scholar]
- Barnard G. F., Itoh R., Hohberger L. H., Shemin D. Mechanism of porphobilinogen synthase. Possible role of essential thiol groups. J Biol Chem. 1977 Dec 25;252(24):8965–8974. [PubMed] [Google Scholar]
- Bevan D. R., Bodlaender P., Shemin D. Mechanism of porphobilinogen synthase. Requirement of Zn2+ for enzyme activity. J Biol Chem. 1980 Mar 10;255(5):2030–2035. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bustos N., Stella A. M., Wider de Xifra E. A., Batlle A. M. Studies or erythrocyte aminolaevulinate dehydratase. I. Its purification and possible therapeutic applications. Int J Biochem. 1980;12(5-6):745–749. doi: 10.1016/0020-711x(80)90156-1. [DOI] [PubMed] [Google Scholar]
- Coleman D. L. Purification and properties of delta-aminolevulinate dehydratase from tissues of two strains of mice. J Biol Chem. 1966 Dec 10;241(23):5511–5517. [PubMed] [Google Scholar]
- Davis J. R., Avram M. J. Correlation of the physicochemical properties of metal ions with their activation and inhibition of human erythrocytic delta-aminolevulinic acid dehydratase (ALAD) in vitro. Toxicol Appl Pharmacol. 1980 Sep 15;55(2):281–290. doi: 10.1016/0041-008x(80)90090-3. [DOI] [PubMed] [Google Scholar]
- Despaux N., Comoy E., Bohuon C., Boudène C. Purification and properties of human erythrocyte delta-amino-levulinic acid dehydratase (EC 4-2-1-24). Biochimie. 1979;61(9):1021–1028. doi: 10.1016/s0300-9084(80)80256-2. [DOI] [PubMed] [Google Scholar]
- Finelli V. N., Klauder D. S., Karaffa M. A., Petering H. G. Interaction of zinc and lead on delta-aminolevulinate dehydratase. Biochem Biophys Res Commun. 1975 Jul 8;65(1):303–312. doi: 10.1016/s0006-291x(75)80093-3. [DOI] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., SCOTT J. J. The purification and properties of delta-aminolaevulic acid dehydrase. Biochem J. 1955 Dec;61(4):618–629. doi: 10.1042/bj0610618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisse S., Brüller H. J., Doss M. Porphobilinogen synthase (delta-aminolevulinic acid dehydratase) activity in human erythrocytes: reactivation by zinc and dithiothreitol depending on influence of storage. Clin Chim Acta. 1983 Dec 15;135(2):239–245. doi: 10.1016/0009-8981(83)90141-9. [DOI] [PubMed] [Google Scholar]
- Gibbs P. N., Gore M. G., Jordan P. M. Investigation of the effect of metal ions on the reactivity of thiol groups in human 5-aminolaevulinate dehydratase. Biochem J. 1985 Feb 1;225(3):573–580. doi: 10.1042/bj2250573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeger-Aronsen B., Schütz A. Antagonistic effect in vivo of zinc on inhibition of delta-aminolevulinic acid dehydratase by lead. Arch Environ Health. 1976 Jul-Aug;31(4):215–220. doi: 10.1080/00039896.1976.10667222. [DOI] [PubMed] [Google Scholar]
- Jordan P. M., Gibbs P. N. Mechanism of action of 5-aminolaevulinate dehydratase from human erythrocytes. Biochem J. 1985 May 1;227(3):1015–1020. doi: 10.1042/bj2271015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Seehra J. S. 13C NMR as a probe for the study of enzyme-catalysed reactions: mechanism of action of 5-aminolevulinic acid dehydratase. FEBS Lett. 1980 Jun 2;114(2):283–286. doi: 10.1016/0014-5793(80)81134-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liedgens W., Grützmann R., Schneider H. A. Highly efficient purificaton of the labile plant enzyme 5-aminolevulinate dehydratase (EC 4.2.1.24) by means of monoclonal antibodies. Z Naturforsch C. 1980 Nov-Dec;35(11-12):958–962. doi: 10.1515/znc-1980-11-1215. [DOI] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Meredith P. A., Moore M. R., Goldberg A. Effects of aluminium, lead and zinc on delta-aminolaevulinic acid dehydratase. Enzyme. 1977;22(1):22–27. doi: 10.1159/000458503. [DOI] [PubMed] [Google Scholar]
- Meredith P. A., Moore M. R. The in vivo effects of zinc on erythrocyte delta-aminolaevulinic acid dehydratase in man. Int Arch Occup Environ Health. 1980 Feb;45(2):163–168. doi: 10.1007/BF01274135. [DOI] [PubMed] [Google Scholar]
- Nandi D. L., Baker-Cohen K. F., Shemin D. Delta-aminolevulinic acid dehydratase of Rhodopseudomonas spheroides. J Biol Chem. 1968 Mar 25;243(6):1224–1230. [PubMed] [Google Scholar]
- Nandi D. L. Lysine as the substrate binding site of porphobilinogen synthase of Rhodopseudomonas spheroides. Z Naturforsch C. 1978 Sep-Oct;33(9-10):799–802. doi: 10.1515/znc-1978-9-1036. [DOI] [PubMed] [Google Scholar]
- Sassa S., Kappas A. Hereditary tyrosinemia and the heme biosynthetic pathway. Profound inhibition of delta-aminolevulinic acid dehydratase activity by succinylacetone. J Clin Invest. 1983 Mar;71(3):625–634. doi: 10.1172/JCI110809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehra J. S., Gore M. G., Chaudhry A. G., Jordan P. M. 5-Aminolevulinic acid dehydratase. The role of sulphydryl groups in 5-aminolevulinic acid dehydratase from bovine liver. Eur J Biochem. 1981 Feb;114(2):263–269. doi: 10.1111/j.1432-1033.1981.tb05145.x. [DOI] [PubMed] [Google Scholar]
- Seehra J. S., Jordan P. M. 5-Aminolevulinic acid dehydratase: alkylation of an essential thiol in the bovine-liver enzyme by active-site-directed reagents. Eur J Biochem. 1981 Jan;113(3):435–446. doi: 10.1111/j.1432-1033.1981.tb05083.x. [DOI] [PubMed] [Google Scholar]
- Shemin D. 5-Aminolaevulinic acid dehydratase: structure, function, and mechanism. Philos Trans R Soc Lond B Biol Sci. 1976 Feb 5;273(924):109–115. doi: 10.1098/rstb.1976.0004. [DOI] [PubMed] [Google Scholar]
- Trevisan A., Gori G. P., Zangirolami A., Benevento C., Rosa A., Chiesura P. Site of action of metals on the aminolevulinic acid dehydratase of human erythrocytes. Enzyme. 1980;25(1):33–36. doi: 10.1159/000459212. [DOI] [PubMed] [Google Scholar]
- Tsukamoto I., Yoshinaga T., Sano S. Evidence for histidine as another functional group of delta-aminolevulinic acid dehydratase from beef liver. Biochem Biophys Res Commun. 1975 Nov 3;67(1):294–300. doi: 10.1016/0006-291x(75)90315-0. [DOI] [PubMed] [Google Scholar]
- Tsukamoto I., Yoshinaga T., Sano S. The role of zinc with special reference to the essential thiol groups in delta-aminolevulinic acid dehydratase of bovine liver. Biochim Biophys Acta. 1979 Sep 12;570(1):167–178. doi: 10.1016/0005-2744(79)90211-0. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Galdes A. The metallobiochemistry of zinc enzymes. Adv Enzymol Relat Areas Mol Biol. 1984;56:283–430. doi: 10.1002/9780470123027.ch5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilson E. L., Burger P. E., Dowdle E. B. Beef-liver 5-aminolevulinic acid dehydratase. Purification and properties. Eur J Biochem. 1972 Sep 25;29(3):563–571. doi: 10.1111/j.1432-1033.1972.tb02022.x. [DOI] [PubMed] [Google Scholar]
- Wu W. H., Shemin D., Richards K. E., Williams R. C. The quaternary structure of delta-aminolevulinic acid dehydratase from bovine liver. Proc Natl Acad Sci U S A. 1974 May;71(5):1767–1770. doi: 10.1073/pnas.71.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]



