Abstract
Chronic histiocytic intervillositis of unknown origin (CHI) is a rare placental disorder associated with adverse pregnancy outcomes, frequent recurrence, and a lack of effective preventive strategies. Recent insights indicate a potential link between CHI-associated inflammatory lesions and the inflammasome pathway, suggesting innovative therapeutic avenues. Here we show a potential role of the inflammasome pathway in CHI through comprehensive transcriptomic analysis of grade 2 or 3 histopathologic CHI samples, paired with placental controls. Additionally, we present case studies of three individuals with recurrent CHI, who have undergone treatment with anakinra and colchicine throughout pregnancy, resulting in improved perinatal outcomes. Notably, all cases are characterized by the birth of healthy, full-term infants, with reduced or absent intervillositis recurrence. Placental assessment unveils heightened activation of the NLRP3-PYCARD inflammasome pathway and IL-1β processing in CHI samples, with downregulation observed in treated pregnancy samples, devoid of intervillositis. Collectively, these findings suggest a potential therapeutic role for targeting the inflammasome pathway in preventing recurrent CHI in pregnant individuals.
Subject terms: Immunosuppression, Autoinflammatory syndrome, Translational research, Urogenital reproductive disorders
Chronic histiocytic intervillositis of unknown origin is a rare but serious and hard-to-prevent condition, leading to repeated pregnancy losses. Here authors show that high-grade cases are associated with inflammasome activation in placental samples, and they report the birth of healthy full-term infants following sustained inflammasome inhibition in three high-risk pregnancies.
Introduction
Chronic histiocytic intervillositis (CHI) stands as a rare inflammatory placental anomaly that profoundly impacts maternal and fetal health. This condition is characterized by an extensive influx of white blood cells, primarily macrophages, into the intervillous space – the specialized region of the placenta where maternal and fetal blood interact1. This infiltration initiates local inflammation and trophoblast necrosis, accompanied by the deposition of fibrin, a clotting factor1,2. This cascading process disrupts the placental exchanges crucial for nourishing the developing fetus and culminates in a spectrum of adverse perinatal outcomes, including miscarriages, severe intra-uterine growth restriction, stillbirth, and premature birth3–7.
Despite its rarity, CHI casts a formidable shadow due to its devastating consequences, affecting a range of pregnancies and gestational stages. Its prevalence, estimated at 0.17%, exhibits variability between different stages of pregnancy, with a higher incidence in the first trimester compared to later stages8. This discrepancy underscores the complexity of CHI’s pathogenesis, possibly involving a convergence of distinct mechanisms.
While therapeutic interventions encompassing various medications have offered some amelioration of CHI impact, the underlying immune-related mechanisms driving the disease remain elusive9–12. Emerging insights into the maternal-fetal interface suggest that inflammasome activation – a cellular machinery involved in immune responses – may orchestrate pathological conditions like fetal growth restriction, pre-eclampsia, and placental infections13–16. Notably, two therapeutic agents, anakinra and colchicine, targeting key components of the inflammasome pathway, have demonstrated efficacy in non-pregnancy-related disorders17,18.
This review aims to explore the intricate interplay between CHI and inflammasome activation. We present evidence from transcriptomic analyses suggesting the presence of an inflammasome-related signature in CHI, specifically involving the NLRP3-PYCARD pathway. Inspired by this molecular connection, we investigate the feasibility of an innovative therapeutic strategy involving inflammasome blockade. To this end, we present a case series of three women afflicted by severe recurrent CHI in successive pregnancies, who underwent treatment with anakinra and colchicine. By examining the intersection of CHI’s pathophysiology with inflammasome biology, we aspire to illuminate a promising avenue for potential intervention, offering newfound hope for the improved management of this rare and perplexing placental disorder.
Results
Global assessment of transcriptome profiles in CHI and controls
To investigate the genes and pathways that are disrupted in placentas from CHI, the gene expression profiles of placental tissues from 18 CHI and 6 controls were assessed by RNA‐Seq. The samples were obtained from a retrospective analysis of 122 cases with CHI sourced from the fetal and placental pathology archives.
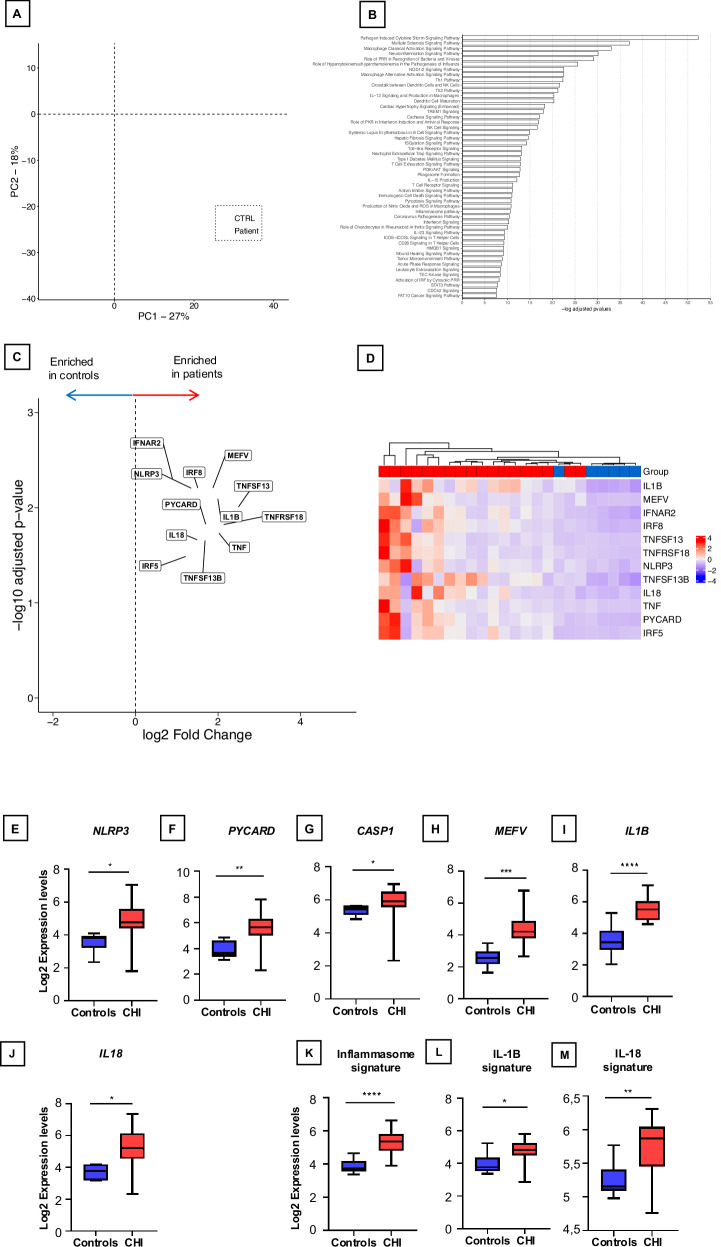
Unsupervised principal components analysis (PCA) was instrumental in discerning CHI patients from control samples. Specifically, on dimensions 1 and 2 (among the 10 highlighted dimensions), the transcriptome profiles for CHI and controls were readily distinguishable, suggesting significant differences between both groups (Fig. 1A). Clinical characteristics of the patients are described in Supplementary Table 1. When exclusively considering a significance threshold of P-value (<0.05), a total of 145 mRNA genes exhibited differential expression (DE), with 133 genes upregulated in individuals with CHI compared to controls. We employed the Ingenuity Pathway Analysis (IPA) package to identify the most significant canonical pathways and biological networks involved in CHI. Only DE genes with a significance threshold of P-value (<0.05) and a fold change > 0.5 were included in this analysis. As predicted by the activation z‐score, the 50 top canonical pathways activated in CHI included macrophage signaling, innate immunity and inflammasome pathways (Fig. 1B). In addition to the identification of upregulated canonical pathways, IPA analysis identified significant networks associated with the DE genes in CHI. These networks were scored based on the number of genes participating in any particular network. This analysis revealed the presence of various transcriptional regulators closely linked to cytokines, including interferon type I (IFN type I), tumor necrosis factor (TNF), interleukin-6 (IL-6), but also interleukin-1B (IL-1B), and interleukin-18 (IL-18) (Supplementary Fig. 1). These findings suggest that the inflammasome pathway may play a pivotal role in the pathogenesis of CHI.
Fig. 1. Transcriptomic Profiling of the Inflammasome Pathway in CHI Placentas.
A Principal component analysis (PCA plot) of the placental transcriptomes. The PCA plot reveals a significant separation between the controls (CTRL) and CHI placentas (Patient). B Top canonical pathways upregulated in CHI. In red, canonical pathways involved in innate immunity, macrophage activation and inflammasome pathway. C Volcano plots identifying differentially expressed genes between CHI and controls. Genes involved in the inflammasome pathway are represented. D. Heatmap representation of inflammasome-related gene expression, organized by hierarchical clustering, depicting differences between healthy controls (n = 6) and CHI patients (n = 18). E–J Box-whisker plots of transcriptomic analysis reveals the log2 expression levels of key components involved in the inflammasome pathway: NLRP3, PYCARD, CASPASE-1, MEFV, IL-1β, and IL-18, respectively, in comparison between healthy controls and patients afflicted with CHI. K–M Box-whisker plots of transcriptional signature of the inflammasome pathway, IL-1β, and IL-18, respectively, across these groups. The horizontal line in each box is the median. The lower and upper ends of each box are limits of the first and third quartiles, and height of each box is the interquartile range. Statistical analysis was conducted using GraphPad Prism 9. P values between each group were determined by a Mann-Whitney U test; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. All tests were two sided.
Inflammasome Pathway is dysregulated in CHI
To provide a more comprehensive analysis of the engagement of the inflammasome pathway in CHI, we focused on the expression of several pivotal genes associated with this pathway (Fig. 1C). A heatmap representation of supervised hierarchical clustering involving inflammasome genes underscored the differences between healthy controls and patients with CHI (Fig. 1D).
Specifically, NLRP3 demonstrated a fold change of 2.57 (p < 0.01), PYCARD exhibited a fold change of 3.25 (p = 0.02), CASPASE displayed a fold change of 1.59 (p < 0.05), and MEFV (Pyrin) demonstrated a fold change of 3.24 (p < 0.01). Importantly, genes encoding pro-inflammatory cytokines generated by the inflammasome, including IL-1β with a fold change of 3.97 (p < 0.01) and IL-18 with a fold change of 2.83 (p = 0.02), were significantly overexpressed in CHI samples (Fig. 1E–J). To further characterize the impact of inflammasome pathway dysregulation in CHI, we calculated transcriptional signatures for the inflammasome, IL-1β, and IL-18. Notably, each of these signatures demonstrated upregulation in CHI samples compared to control samples (Fig. 1K–M and Supplementary Table 2).
These findings collectively emphasize the significant dysregulation of the inflammasome pathway in CHI, providing compelling evidence of its involvement in the pathophysiology of this rare placental disorder and highlighting the potential for inflammasome blockade strategies.
Case Studies:
To establish a direct link between the identified molecular signatures and therapeutic outcomes, we implemented a proof-of-concept strategy. This involved administering an inflammasome pathway blockade strategy to three consecutive pregnant patients (not included in the initial transcriptomic analysis) with a history of recurrent chronic intervillositis.
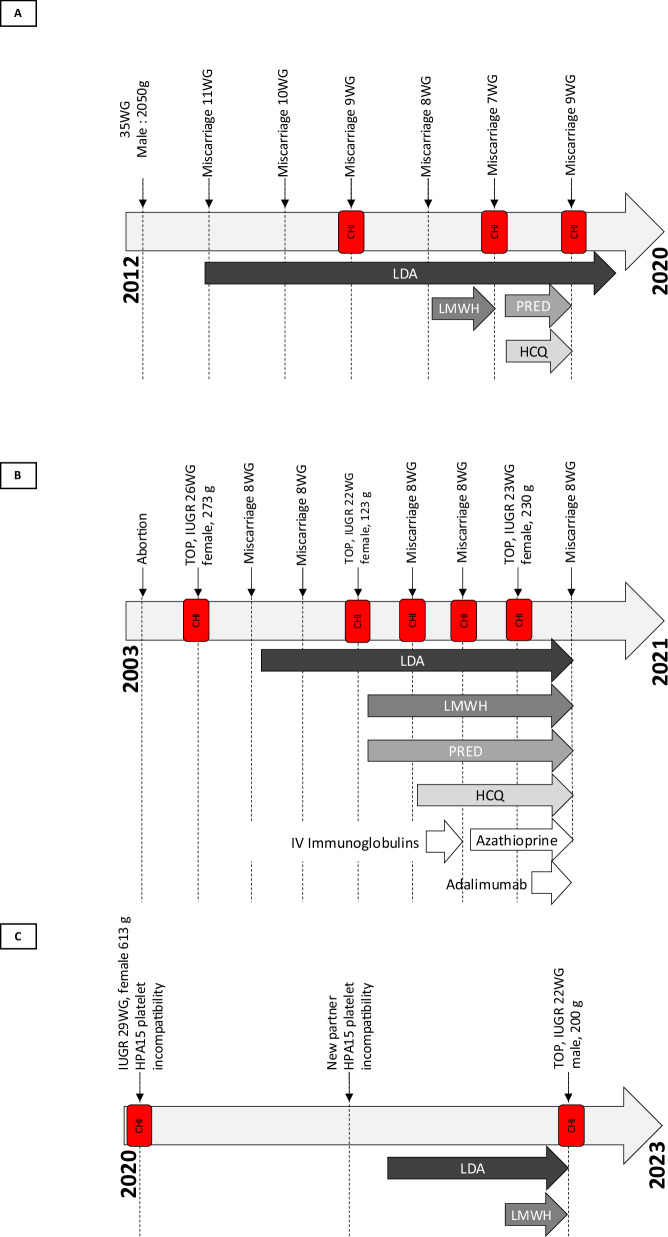
Patient 1, in the age range of 30 to 35 years, Gravida 7 Para 1, presented with a history of recurrent miscarriages associated with CHI. Her obstetric history included a prior pregnancy nine years ago with a diagnosis of intrauterine growth restriction (IUGR) at 34 weeks of gestation (WG). She gave birth vaginally to a live-born boy weighing 2050 g (13th percentile19 at 35 + 1 WG). Placental analysis revealed maternal vascular lesions but no evidence of CHI. Subsequently, she had six consecutive miscarriages occurring between 7 and 11 WG (Fig. 2A). Pathological examination of the conceptus, performed three times (third, fifth, and sixth miscarriage), consistently showed typical grade 3 CHI lesions (Figure 3A1). The patient maintained a normal body weight and abstained from addictive substances such as tobacco and alcohol.
Fig. 2. Obstetrical treatment chronology across patients’ obstetric histories.
A–C depict the comprehensive obstetrical treatment timelines for each patient. HCQ (Hydroxychloroquine), IUGR (Intrauterine Growth Restriction), IV (Intravenous), CHI (Chronic histiocytic intervillositis), LDA (Low-Dose Aspirin), LMWH (Low Molecular Weight Heparin), PRED (Prednisone), TOP (Termination of Pregnancy), and WG (Week of Gestation).
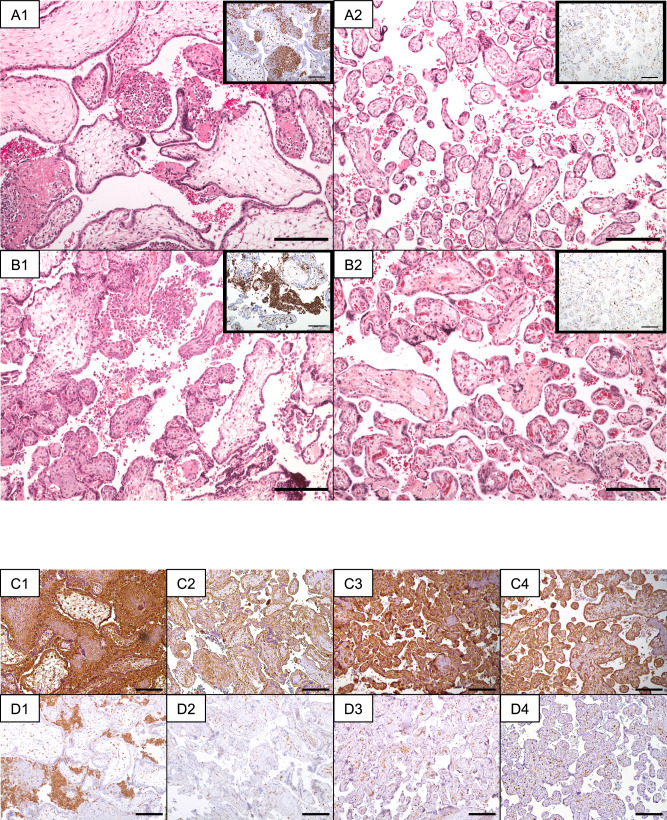
Fig. 3. Placental histological analysis and immunohistochemistry staining in patients diagnosed with CHI and treated with Inflammasome Blockade strategy.
Stainings were repeated on multiple different sections of the placental samples, and the most representative data are presented (scale-bar: 200μ). Panels A1 and B1 present microscopic placental features from Patient 1 (A1) and Patient 2 (B1), both diagnosed with CHI. Hematoxylin-eosin-saffron staining of placental tissue reveals the presence of macrophages (magnification: x100). Insets provide a closer view, highlighting histiocytic infiltration through CD68 immunostaining. Panels A2 and B2 display microscopic placental features from the consecutive pregnancies of Patient 1 (A2) and Patient 2 (B2), following treatment with anakinra and colchicine. Hematoxylin-eosin-saffron staining of placental tissue reveals limited lesions associated with vascular malperfusion, with an absence of CHI-related lesions (magnification: x100). Insets provide an amplified view, emphasizing the very limited histiocytic infiltration through CD68 immunostaining. Panel C (1–4) showcases placental immunohistochemistry staining using anti-NLRP3 antibodies for Patient 1, diagnosed with CHI (C1), and after undergoing treatment with an inflammasome blockade strategy (C3). Panels C2 and C4 serve as term-matched controls for C1 and C3, respectively (magnification: x100). Panel D (1–4) illustrates placental immunohistochemistry staining with anti-PYCARD antibodies for Patient 1, diagnosed with CHI (D1), and following treatment with an inflammasome blockade strategy (D3). Panels D2 and D4 function as term-matched controls for D1 and D3, respectively (magnification: x100).
Extensive investigations failed to identify any causative factors for these recurrent miscarriages, encompassing anatomical, immunological, metabolic, genetic, and hormonal assessments. Antiphospholipid antibody screening was negative, as were parental karyotyping results.
Despite previous pregnancies involving various therapeutic combinations, including low-dose aspirin (LDA), low molecular weight heparin, prednisone, and hydroxychloroquine to prevent recurrent CHI, they were all unsuccessful. Consequently, the therapeutic approach was adapted. Colchicine and hydroxychloroquine were initiated prior to conception, with the addition of LDA and anakinra at 8 weeks of gestation. The subsequent pregnancy, under this inflammasome-targeted therapy, proceeded without complications. Regular ultrasound monitoring throughout the pregnancy revealed normal findings with no signs of small gestational age or congenital malformations. Due to her medical history, labor was induced at 37 WG and 3 days, culminating in the vaginal birth of a 2490 g live-born girl (14th percentile19) at 37 WG and 4 days. Placental examination confirmed normal placental weight and few vascular lesions, without any CHI lesions (Figure 3A2).
Patient 2, in the age range of 40 to 45 years, Gravida 10 Para 0, presented at the beginning of a new pregnancy with severe recurrent CHI. Her obstetric history encompassed nine pregnancies without achieving a viable child (Fig. 2B). This included one surgical abortion, and three pregnancies with early and severe IUGR, resulting in termination at 26, 22, and 23 WG (with respective fetal weights of 273 g, 123 g, and 230 g), and five miscarriages occurring around 8 WG. Placental and conceptus examinations performed during the terminations of pregnancy and miscarriages consistently revealed grade 3 CHI lesions (Figure 3B1). Extensive investigations failed to reveal any other underlying causes for the recurrent miscarriages or IUGR. She had a normal body weight, but was an active smoker. Following the first episode of CHI, subsequent pregnancies experienced recurrences despite varying empiric treatment regimens, including LDA, low molecular weight heparin, prednisone, hydroxychloroquine, polyvalent immunoglobulins, azathioprine, and adalimumab. In a subsequent pregnancy, a treatment regimen comprising colchicine, anakinra, hydroxychloroquine, and LDA was initiated at 8 weeks of gestation. The pregnancy proceeded without complications, with regular ultrasound monitoring every 4 weeks indicating normal fetal growth and the absence of small gestational age. To mitigate the risk associated with CHI and recurrent pregnancy loss and due to proctologic history, an elective cesarean section was performed at 37 WG, resulting in the birth of a 2600 g live-born boy (21st percentile19). Placental examination confirmed normal placental weight, low-grade villitis of unknown etiology, and no CHI lesions (Fig. 3B2).
Patient 3, in the age range of 25 to 30 years, Gravida 2 Para 1, had a history of recurrent severe IUGR associated with CHI (Fig. 2C). Her medical history included juvenile polyarthritis and migraine. Her first pregnancy was complicated by IUGR at 22 WG, culminating in a corporeal cesarean section delivery of a 613 g live-born girl at 29 + 4 WG. Placental analysis revealed placental malperfusion lesions and CHI grade 3. Additional investigations identified obstetrical anti-phospholipid syndrome (positive lupus anticoagulant confirmed at 12 weeks) but yielded negative results for genetic and CMV testing. Unfortunately, the newborn passed away at 7 days of life due to severe thrombocytopenia and multiple cerebral hematomas. Subsequent maternal-fetal platelet incompatibility testing confirmed an HPA15 platelet incompatibility.
During her second pregnancy with a different partner, the patient received LDA and low molecular weight heparin due to obstetrical anti-phospholipid syndrome. HPA15 platelet incompatibility was also present with this partner. Nevertheless, severe IUGR was diagnosed at 18 WG, and recurrent CHI was suspected. Genetic and CMV testing yielded negative results. Ultrasound monitoring every 2 weeks indicated inadequate fetal growth, leading to termination of pregnancy at 22 + 5 WG. The patient delivered a stillborn boy weighing 200 g, with placental analysis confirming grade 3 CHI.
For her subsequent pregnancy with a new partner, the therapeutic approach was adapted. Colchicine and hydroxychloroquine were initiated before conception, with the addition of LDA, low molecular weight heparin, and anakinra at 8 weeks of gestation. The pregnancy proceeded without complications, with fetal platelet genotyping excluding the risk of HPA15 platelet alloimmunization. Ultrasound monitoring every 4 weeks indicated normal fetal growth with no signs of small gestational age or congenital malformations. Due to the patient’s history and recurrent pregnancy loss, an iterative cesarean section was performed at 37 WG and 3 days. She gave birth to a live-born girl weighing 2660 g (27th percentile19). The placental analysis confirmed the presence of CHI grade 2, a milder presentation than observed in the two previous pregnancies.
No severe maternal or neonatal side effects were reported for the women or their newborns. The treatment administered did not exert discernible effects on maternal immune parameters across the observed time points (before, during, and after pregnancy). Details regarding maternal characteristics of the three women are provided in Supplementary Table 3 and 4.
Immunohistochemical profiling of the inflammasome pathway
We conducted immunohistochemical staining to assess the expression of the NLRP3-PYCARD inflammasome in the placental tissues of patients 1 and 2. In both instances, our immunohistochemical analysis revealed a substantial presence of histiocytes within the intervillous space, exhibiting extensive staining for NLRP3 and PYCARD (Fig. 3C1-4). Remarkably, treatment with anakinra and colchicine elicited a significant downregulation in the expression of these critical genes (Fig. 3D1-4). These findings underscore the potent impact of the therapeutic intervention on mitigating the inflammasome pathway activation, potentially contributing to the favorable outcomes observed in these cases.
Discussion
In this study, we present compelling evidence from three patients that the implementation of inflammasome-targeted therapies during pregnancy holds the potential to prevent severe recurrent CHI and culminate in successful full-term births. Our comprehensive tissue analysis further suggests that aberrant inflammasome regulation may play a pivotal role in the pathogenesis of CHI.
Currently, the management of recurrent CHI lacks standardized or optimal treatment approaches, leading to inconsistency in the literature and compelling some clinicians to rely on reinforced obstetrical surveillance as a primary strategy5–7,9,20. Regrettably, the high risk of CHI recurrence underscores the urgent need for novel therapeutic strategies. Transcriptomic data has unveiled a distinctive inflammasome gene expression signature in CHI patient samples, underscoring the potential value of inflammasome-targeted therapies as a preventive measure, complemented by the assessment of inflammasome-related genes and associated proteins.
At the maternal-fetal interface, innate immunity hinges on the constitutive release of IL-1β and IL-18, facilitated by NLRP3-PYCARD inflammasome activation in response to placental infections. For instance, during Listeria monocytogenes infection, inflammasome activity serves as a protective mechanism against the adverse effects of microbial invasion, whereas in placental malaria, IL-1 signaling contributes to adverse pregnancy outcomes16. In the context of pre-eclampsia, NLRP3-PYCARD activation triggers placental dysfunction13–15. These observations collectively underscore the significance of inflammasome signaling in placental pathogenesis, implying that in specific cases, inhibiting IL-1 signaling may improve pregnancy outcomes.
In the three cases elucidated in this study, the administration of inflammasome blockade through an IL-1 receptor antagonist and colchicine proved effective in preventing CHI recurrence or severity. Notably, placental examination revealed in two cases an absence of intervillositis lesions, accompanied by a robust downregulation of the inflammasome pathway. It’s worth highlighting that this study stands as the pioneering effort to implement immunotherapy specifically in the context of a pregnancy-related disorder.
Crucially, the safety profile of this intervention was found to be tolerable, with no reported maternal or neonatal side effects, aside from mild reactions at the anakinra injection site. This aligns with existing safety data from 69 pregnancies involving women with inflammatory diseases, who were treated with daily doses of 50 to 200 mg of anakinra, revealing no discernible increase in adverse perinatal outcomes despite these women presenting inflammatory diseases associated with an elevated risk of pregnancy complications21. For colchicine, a systematic review and meta-analysis involving 550 pregnancies of women, primarily with familial Mediterranean fever (FMF), found no significant increase in the incidence of fetal malformations or miscarriages when the drug was taken during pregnancy at doses of 1–2 mg per day22. Low-dose aspirin (LDA) has been extensively studied in high-risk pregnancies and shown to improve outcomes. A Cochrane review comprising 77 trials involving 40,249 women and their babies provided high-quality evidence that antiplatelet agents, mostly LDA up to 150 mg/day, reduced the incidence of pre-eclampsia and its complications, with a favorable safety profile23. Regarding hydroxychloroquine, the systematic literature review supporting the 2022 British Society for Rheumatology guidance consistently found no statistically significant difference in outcomes, including congenital malformations, across various studies24.
While this study holds great promise, a major limitation stems from the small sample size, comprising only three patients. Nonetheless, their recurrent CHI profiles, resistance to multiple combined therapies, and our comprehensive tissue analysis collectively offer compelling evidence for the potential of this innovative therapeutic strategy, warranting further exploration.
In conclusion, our findings underscore the potential of inflammasome pathway blockade as a preventive measure in recurrent CHI, leading to successful live births in the cases presented.
Methods
Study Design
Study Design involved 4 steps
Global Assessment of Transcriptome Profiles among CHI and control samples not treated with an inflammasome blockade strategy,
Analysis of Inflammasome Pathway Genes in order to identify molecular signatures and dysregulations within the inflammasome pathway associated with recurrent chronic intervillositis,
Proof of Concept with Patient Treatment to establish a direct link between the identified molecular signatures and therapeutic outcomes. This involved administering an inflammasome pathway blockade to three consecutive pregnant patients with a history of recurrent chronic intervillositis,
Immunohistochemical Profiling to assess the expression of the NLRP3-PYCARD inflammasome in the placental tissues of the treated patients.
Transcriptomic profile
The transcriptomic study was performed on samples obtained from a retrospective analysis of 122 cases with CHI sourced from the fetal and placental pathology archives before the SARS-CoV-2 pandemic, to avoid cases of CHI due to SARS-Cov2-infection. Eighteen samples were matched in a 3:1 ratio with placental controls based on gestational age, maternal age, and fetal sex. Details are provided in Section 1.1 of the Supplementary Appendix.
Three individuals with a history of severe recurrent CHI, characterized by at least two pregnancy losses attributed to grade 3 CHI, were consecutively selected as participants for this study.
For each participant, placental tissues from the pregnancies were collected post-delivery, yielding both frozen and paraffin-embedded samples. Paraffin blocks containing placental tissues from prior pregnancies with a CHI diagnosis were also sourced from the fetal and placental pathology archives.
Study medication
The four following drugs were administrated as soon as the intra-uterine pregnancy was confirmed according to this protocol approved from the local ethics committee: Anakinra 100 mg once daily subcutaneously, Hydroxychloroquine (HCQ) 400 mg orally per day, Colchicine 1 mg orally per day, Low dose of aspirin (LDA) (100 mg) orally per day (in the evening).
Anakinra, a pharmaceutical agent sanctioned by the European Medicines Agency for the management of conditions such as rheumatoid arthritis and autoinflammatory disorders, was administered in conjunction with colchicine, with the specific aim of modulating the NLRP3-PYCARD inflammasome pathway. Low-dose aspirin was administered as a prophylactic measure against pregnancy-related complications, including preeclampsia and preterm delivery, as documented in previous studies25. Additionally, hydroxychloroquine was administered due to its recognized potential for beneficial immunomodulatory effects in the context of placental inflammation9,26. The detailed treatment protocol is outlined in Section 1.2 of the Supplementary Appendix.
Immunohistochemical staining
Serial 3 µm-thick-sections of placental tissues embedded in paraffin were subjected to immunostaining using anti CD68, anti-PYCARD and anti-NLRP3 antibodies. Immunoreactive signals were visualized using the Dako EnVision FLEX peroxidase detection system. Hematoxylin counterstaining was performed, and the sections were mounted. Further specifics are outlined in Section 1.3 of the Supplementary Appendix.
Gene expression analysis
Total RNA extraction was carried out using the NanoString nCounter Flex System and the nCounter Immune Profiling Panel, designed to probe 730 immune-related genes along with 40 reference controls. Differentially expressed genes compared to the baseline control were identified as significant using nSolver 4.0 Analysis Software. Gene expression and signatures were calculated according to predefined gene lists (Supplementary Table 2)27,28. Normalized gene expression data were used for unsupervised analyses and visualized using z-score transformation and heatmap visualization. Further details are provided in Section 1.4 and 1.5 of the Supplementary Appendix.
Ingenuity Pathway Analysis (IPA) was used to identify gene pathways and regulatory networks to which DE genes belong to.
Statistical analysis and reproductibility
Statistical analyses were performed using GraphPad Prism 9. P values between each group were determined by a Mann-Whitney U test; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. All tests were two-sided. Principal component analysis and visualization using ade4 were performed for unsupervised analyses29. Data analyses were executed in R environment (v.4.1.3) and RStudio (v.2022.02.0), and figure creation utilized Prism 9 (GraphPad Software).
For the transcriptomic analysis, the run was performed only once due to resource constraints; gene expression data were normalized using control probes and housekeeping genes selected using the geNorm method of the Advanced Analysis package (nSolver Analysis Software 4.0). For the immunohistochemical stainings, the analysis was repeated on multiple different sections of the placental samples, and the most representative data are presented.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
Immunohistochemistry staining was performed at the Histopathology Platform, TBM-Core, a service unit of the University of Bordeaux. We thank Emily Cornish for her kind advice and critical reading of the manuscript and JetPub Scientific Communications for their help in editing the manuscript.
Author contributions
A.M., F.S. L.S., P.B. and E.L. designed research; A.M., F.S., T.F., N.D-S., L.S., P.B. and E.L. performed research; A.M., F.S., T.F., E.W., C.L., M.C., N.D-S., L.S., P.B. and E.L. analyzed data; A.M. and F.S. wrote the first draft of the paper; E.W., C.L., I.D., D.D., C.B., M-E.T., C.R., E.F., P.D., J-F.V., L.S. and P.B. edited the paper; E.L. wrote the paper.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The transcriptomic data generated in this study have been deposited in the GEO (Gene Expression Omnibus database) under accession code GSE272505.
Competing interests
The authors declare no competing interests.
Ethics
The study adhered to French law MR-004 and obtained approval from the local ethics committee (CNIL number: CER-BDX-2022-34). Each participant provided written informed consent. None of the study participants received compensation.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Aurélien Mattuizzi, Fanny Sauvestre.
These authors jointly supervised this work: Loïc Sentilhes, Patrick Blanco.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-53591-w.
References
- 1.Labarrere, C. & Mullen, E. Fibrinoid and trophoblastic necrosis with massive chronic intervillositis: an extreme variant of villitis of unknown etiology. Am. J. Reprod. Immunol. Microbiol15, 85–91 (1987). [DOI] [PubMed] [Google Scholar]
- 2.Jacques, S. M. & Qureshi, F. Chronic intervillositis of the placenta. Arch. Pathol. Lab Med117, 1032–1035 (1993). [PubMed] [Google Scholar]
- 3.Parant, O., Capdet, J., Kessler, S., Aziza, J. & Berrebi, A. Chronic intervillositis of unknown etiology (CIUE): Relation between placental lesions and perinatal outcome. Eur. J. Obstet. Gynecol. Reprod. Biol.143, 9–13 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Marchaudon, V. et al. Chronic histiocytic intervillositis of unknown etiology: Clinical features in a consecutive series of 69 cases. Placenta32, 140–145 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Mekinian, A. et al. Chronic histiocytic intervillositis: Outcome, associated diseases and treatment in a multicenter prospective study. Autoimmunity48, 40–45 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Mattuizzi, A. et al. Adverse perinatal outcomes of chronic intervillositis of unknown etiology: an observational retrospective study of 122 cases. Sci. Rep.10, 12611 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homatter, C. et al. Is chronic histiocytic intervillositis a severe placental disease? A case-control study. Placenta91, 31–36 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Simula, N. K. et al. Chronic Intervillositis of Unknown Etiology (CIUE): Prevalence, patterns and reproductive outcomes at a tertiary referral institution. Placenta100, 60–65 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Brady, C. A. et al. Immunomodulatory therapy reduces the severity of placental lesions in chronic histiocytic Intervillositis. Front Med8, 753220 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brady, C. A. et al. Chronic histiocytic intervillositis: A breakdown in immune tolerance comparable to allograft rejection? Am. J. Reprod. Immunol.85, e13373 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benachi, A. et al. Chronic histiocytic intervillositis: manifestation of placental alloantibody-mediated rejection. Am. J. Obstet. Gynecol.225, 662.e1–662.e11 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Nedberg, N. H. et al. Platelet alloimmunization is associated with low grade chronic histiocytic intervillositis - A new link to a rare placental lesion? Placenta112, 89–96 (2021). [DOI] [PubMed] [Google Scholar]
- 13.C.Weel, I. et al. Increased expression of NLRP3 inflammasome in placentas from pregnant women with severe preeclampsia. J. Reprod. Immunol.123, 40–47 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Kohli, S. et al. Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood128, 2153–2164 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Stødle, G. S. et al. Placental inflammation in pre-eclampsia by Nod-like receptor protein (NLRP)3 inflammasome activation in trophoblasts. Clin. Exp. Immunol.193, 84–94 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Megli, C., Morosky, S., Rajasundaram, D. & Coyne, C. B. Inflammasome signaling in human placental trophoblasts regulates immune defense against Listeria monocytogenes infection. J. Exp. Med.218, e20200649 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silvis, M. J. M. et al. Colchicine reduces extracellular vesicle NLRP3 inflammasome protein levels in chronic coronary disease: A LoDoCo2 biomarker substudy. Atherosclerosis334, 93–100 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Leung, Y. Y., Yao Hui, L. L. & Kraus, V. B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheumat.45, 341–350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenton, T. R. & Kim, J. H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr.13, 59 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contro, E., deSouza, R. & Bhide, A. Chronic intervillositis of the placenta: A systematic review. Placenta31, 1106–1110 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Brien, M.-E. et al. A systematic review of the safety of blocking the IL-1 system in human pregnancy. JCM11, 225 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Indraratna, P. L. et al. Use of colchicine in pregnancy: a systematic review and meta-analysis. Rheumatology57, 382–387 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Duley L., Henderson-Smart D. J., Meher S. & King J. F. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev 2007;CD004659 [DOI] [PubMed]
- 24.Russell, M. D. et al. British Society for Rheumatology guideline on prescribing drugs in pregnancy and breastfeeding: immunomodulatory anti-rheumatic drugs and corticosteroids. Rheumatology62, e48–e88 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Askie L. M., Duley L., Henderson-Smart D. J. & Stewart L. A. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet.369, 1791–1798 (2007). [DOI] [PubMed]
- 26.Bouariu, A. et al. The potential benefit of hydroxychloroquine in chronic placental inflammation of unknown etiology associated with adverse pregnancy outcomes. Healthcare10, 168 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danaher, P. et al. Gene expression markers of Tumor Infiltrating Leukocytes. J. Immunother. Cancer5, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urrutia, A. et al. Standardized whole-blood transcriptional profiling enables the deconvolution of complex induced immune responses. Cell Rep.16, 2777–2791 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Dray S. & Dufour A.-B. The ade4 Package: Implementing the duality diagram for ecologists. J Stat Soft 2007;22. Available from: http://www.jstatsoft.org/v22/i04/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The transcriptomic data generated in this study have been deposited in the GEO (Gene Expression Omnibus database) under accession code GSE272505.