Abstract
BACKGROUND
Helicobacter pylori (H. pylori) eradication rates have declined with the rise of antibiotic-resistant strains in recent years. Although highly effective with a low prevalence of resistance, standard dose tetracycline is associated with frequent adverse events. The efficacy and safety of low-dose tetracycline as part of tetracycline and amoxicillin-containing bismuth quadruple therapy are not well described.
AIM
To compare the efficacy and safety of low-dose compared to standard dose tetracycline with combined amoxicillin-containing bismuth quadruple therapy in patients with H. pylori infection.
METHODS
Consecutive patients with H. pylori infection receiving tetracycline, amoxicillin, proton pump inhibitor, and bismuth for 14 days at Sir Run Run Shaw Hospital (1/2022-6/2023) were evaluated. The low-dose tetracycline group received tetracycline 500 mg twice daily (bid) while the standard dose group received 750 mg bid or 500 mg three times daily (tid). Primary endpoints were H. pylori eradication rate and treatment-related adverse events.
RESULTS
The mean age of the 218 patients was 48.7 ± 14.0 years, 120 (55%) were male, and 118 (54.1%) received treatment as primary therapy. Furthermore, 73 (33%) patients received low-dose tetracycline (500 mg bid) and 145 (67%) received standard dose tetracycline including 500 mg tid in 74 (33%) and 750 mg bid in 71 (33%). On intention-to-treat analysis, H. pylori eradication rates were 89% [95% confidence interval (CI): 82%-96%] in the 500 mg bid group, 82% (95%CI: 74%-91%) in the 500 mg tid group, and 79% (95%CI: 69%-89%) in the 750 mg bid group without a statistically significant difference (P = 0.25). The incidence of adverse events was lower in the low-dose compared to the standard dose group (12.3% vs 31.1% or 23.9%; P = 0.02).
CONCLUSION
Low-dose tetracycline combined with amoxicillin quadruple therapy for 14 days achieved a high eradication rate and fewer adverse events compared to the standard dose tetracycline regimen in patients with H. pylori infection.
Keywords: Helicobacter pylori, Tetracycline, Amoxicillin, Eradication, Adverse events, Bismuth quadruple therapy
Core Tip: Helicobacter pylori (H. pylori) eradication rates have declined with the rise of antibiotic-resistant strains in recent years. Although highly effective, the standard dose of tetracycline as part of the H. pylori treatment regimen is associated with a high incidence of adverse events. Our results demonstrated that low-dose tetracycline combined with amoxicillin as part of bismuth-containing quadruple therapy can achieve a high eradication rate and fewer adverse events compared to standard dose tetracycline in patients with H. pylori infection.
INTRODUCTION
Helicobacter pylori (H. pylori) is a spiral gram-negative, rod-shaped microaerophilic bacterium with a flagella that colonizes the gastric and duodenal mucosa, triggering a host inflammatory response and resulting in damage to the structure and function of the stomach[1]. H. pylori infection is a common chronic infectious disease, associated with a risk of developing peptic ulcer, gastric cancer, and gastric mucosa-associated lymphoid tissue lymphoma[1]. H. pylori has infected more than 50% of the global population[2]. Although 80% of infected individuals are asymptomatic, most are accompanied by different degrees of gastritis[2]. Furthermore, approximately 80%-90% of gastric cancer is attributed to H. pylori infection worldwide[3,4]. Given that H. pylori is regarded as a group I human carcinogen and H. pylori-associated gastritis is considered an infectious disease, eradication is highly recommended[5-7].
According to the 2022 Chinese Society of Gastroenterology guidelines on H. pylori therapy, bismuth quadruple therapy is strongly recommended as a first-line regimen in primary and rescue therapy, in addition to other society guidelines[6,8-10]. The most commonly used antibiotics include amoxicillin, metronidazole, clarithromycin, tetracycline, and levofloxacin. However, the incidence of treatment failure has risen in recent years given the increased prevalence of H. pylori infection resistant to standard antibiotics[11]. Poor adherence and host genetics (CYP2C19 polymorphisms and low gastric pH associated with gene multidrug resistance protein 1, interleukin-1β) also likely contribute to the reasons for failed therapy[12,13]. A meta-analysis of 351 studies showed that resistance rates were 30% for clarithromycin, 61% for metronidazole, 35% for levofloxacin, 4% for tetracycline, and 6% for amoxicillin as primary therapy for H. pylori in the Asia-Pacific region[14]. The Maastricht VI/Florence Consensus Report recommended tetracycline and metronidazole-containing bismuth quadruple therapy as first-line treatment[11]. However, the increase in the prevalence of H. pylori resistant to metronidazole may impact treatment success[15].
Tetracycline is a broad-spectrum antibiotic produced by actinomycetes that inhibits bacterial reproduction and viability by interfering with protein synthesis through binding reversibly to a pocket in the 30S subunit of bacterial ribosomes containing 16S rRNA[16]. Amoxicillin is a penicillin-type antibiotic that interferes with cell wall synthesis and can exert excellent antibacterial activity during the bacterial multiplication period[17]. Given the low rate (0.4%) of dual-resistance with the combination of tetracycline and amoxicillin[18], quadruple therapy containing the two antibiotics is highly effective[19-21]. A randomized trial conducted in China showed that tetracycline and amoxicillin-based quadruple therapy achieved high eradication rates of 87.2% and 91.9% on intention-to-treat (ITT) and per-protocol (PP) analyses[22]. However, recommended standard doses of tetracycline [500 mg three or four times daily (tid or qid)] may result in poor adherence and treatment failure given frequent dosing requirements and the high incidence of antibiotic-related adverse events.
In our previous study, low-dose tetracycline [500 mg twice a day (bid)] as a component of tetracycline and furawzolidone quadruple therapy for 14 days resulted in a high eradication rate of 92.4%, comparable to standard tetracycline dose regimens, with a lower incidence of adverse events[23]. The aim of the present study was to evaluate the efficacy and safety of low-dose compared to standard dose tetracycline combined with amoxicillin-containing bismuth quadruple therapy for 14 days in achieving H. pylori eradication.
MATERIALS AND METHODS
Study design and population
This was a retrospective study conducted in Sir Run Run Shaw Hospital (Hangzhou, China) between January 2022 and June 2023. The study was approved by the Institutional Review Board of Sir Run Run Shaw Hospital, Medical School, Zhejiang University, Hangzhou (SRRSH: 2024-0053). Demographic characteristics including medical co-morbidities (diabetes, hypertension, hyperlipidemia), endoscopic findings, adverse events, treatment history, regimens, and outcomes were collected by reviewing clinical records, and conducting telephone surveys. Consecutive patients > 18 years of age with H. pylori infection diagnosed by a positive 13C/14C-urea breath test (UBT) treated with tetracycline and amoxicillin-containing quadruple therapy for 14 days were evaluated. Patients with a history or active gastrointestinal malignancy, previous upper gastrointestinal surgery, severe comorbidities, pregnant or lactating women, or incomplete information were excluded. Repeat 13C/14C-UBT was performed one month after completion of therapy to evaluate eradication.
Study definitions
H. pylori infection was defined by positive 13C/14C-UBT. Eradication was defined as negative on follow-up UBT. Tetracycline and amoxicillin-containing bismuth quadruple therapy was defined by 14 days treatment with tetracycline, amoxicillin, proton pump inhibitor (PPI) and bismuth at different doses and frequencies. Tetracycline was prescribed as 500 mg bid in the low-dose tetracycline group, and 750 mg bid or 500 mg tid in the standard dose tetracycline group, taken 30 minutes after meals. Amoxicillin was given as 1000 mg twice daily in all groups, taken 30 minutes before meals. PPIs including omeprazole, rabeprazole, pantoprazole and vonoprazan were taken bid 30 minutes before meals. Colloidal bismuth pectin or bismuth potassium citrate was also taken bid 30 minutes before meals. Specific drug dosage information was as follows: Omeprazole 20 mg, rabeprazole 10 mg, pantoprazole 40 mg, vonoprazan 20 mg, colloidal bismuth pectin 0.2-0.4 g, and bismuth potassium citrate 0.6 g per dose. Poor adherence was defined as taking less than 80% of the total prescribed doses.
Study endpoints
The primary endpoint of the study was the eradication rate of H. pylori in each treatment group by ITT analysis. The secondary endpoints were the frequency of adverse events and adherence rate.
Statistical analysis
All data were analyzed by SPSS version 26.0 software (IBM Corp., United States). Categorical variables were described as numbers and percentages and were analyzed by Pearson χ2 or Fisher’s exact test, as appropriate. Continuous variables were described as mean ± SD, and were analyzed by one-way ANOVA. Multivariate logistic regression model was used to identify independent factors associated with eradication failure. All P values were two-sided and were considered statistically significant when < 0.05.
RESULTS
Demographic data
During the study period, 218 patients with H. pylori infection received 14 days of quadruple therapy containing tetracycline, amoxicillin, PPI, and bismuth. The mean age of the patients was 48.7 ± 14.0 years, 120 (55.0%) were male, and 118 (54.1%) received treatment as primary therapy for H. pylori infection. Among those who received prior therapy, 74 (33.9%) received one and 26 (11.9%) received two or more H. pylori therapies.
Seventy-three (33.5%) patients received low-dose and 145 (66.5%) received standard dose tetracycline including 74 (33.9%) in the 500 mg tid group and 71 (32.6%) in the 750 mg bid group. There were no differences between the three groups in terms of baseline demographic and clinical characteristics including age, gender, smoking history, alcohol drinking history, family history of gastric cancer, hypertension, diabetes, and hyperlipidemia (Table 1). However, the types of PPI and bismuth differed (P < 0.001) among the treatment groups. Prior to therapy, 157 (72.0%) patients received gastroscopy without differences in the diagnosis of chronic gastritis, peptic ulcer, or intestinal metaplasia among the three groups. Finally, a higher proportion of patients received low-dose tetracycline (82.2% vs 56.8% and 22.5%, P < 0.001) compared to those who received standard dose tetracycline for primary therapy (Table 1).
Table 1.
Demographic and clinical data of patients
|
Variables
|
500 mg bid (n = 73)
|
500 mg tid (n = 74)
|
750 mg bid (n = 71)
|
P value
|
| Age, mean ± SD | 49.2 ± 12.0 | 50.2 ± 13.9 | 46.6 ± 15.8 | 0.348 |
| Age range (years) | 20-72 | 23-72 | 22-77 | |
| Age, ≤ 50 years | 36 | 35 | 40 | 0.523 |
| Sex, male/female | 41/32 | 39/35 | 40/31 | 0.899 |
| Family history of gastric cancer | 1 | 2 | 1 | 1.000 |
| NSAIDS | 2 | 3 | 0 | 0.375 |
| Probiotics | 7 | 2 | 3 | 0.202 |
| Smoking | 15 | 16 | 15 | 1.000 |
| Alcohol drinking | 10 | 14 | 13 | 0.689 |
| Hypertension | 17 | 17 | 15 | 0.958 |
| Diabetes | 5 | 6 | 3 | 0.694 |
| Hyperlipidemia | 1 | 3 | 1 | 0.623 |
| Endoscopy diagnosis | 0.600 | |||
| Gastritis | 49 | 42 | 36 | |
| Ulcer | 14 | 7 | 9 | |
| Unknown | 10 | 25 | 26 | |
| Intestinal metaplasia | 0.325 | |||
| No | 40 | 27 | 22 | |
| Yes | 23 | 22 | 23 | |
| Unknown | 10 | 25 | 26 | |
| Treatment times | < 0.001 | |||
| 1 | 60 | 42 | 16 | |
| 2 | 8 | 27 | 39 | |
| ≥ 3 | 5 | 5 | 16 | |
| Rescue therapy1 | 13 | 32 | 55 | |
| PPI | < 0.001 | |||
| Omeprazole | 2 | 5 | 27 | |
| Rabeprazole | 66 | 63 | 37 | |
| Pantoprazole | 1 | 5 | 1 | |
| Vonoprazan | 4 | 1 | 6 | |
| Bismuth | < 0.001 | |||
| Bismuth potassium citrate | 4 | 27 | 37 | |
| Colloidal bismuth pectin | 69 | 47 | 34 |
Rescue therapy was defined as patients who had received eradication therapy once or more but failed.
P values were from two-sided comparisons of the differences between the three groups. 500 mg bid: Tetracycline twice daily; 500 mg tid: Tetracycline three times daily; 750 mg bid: Tetracycline twice daily; NSAIDS: Nonsteroidal anti-inflammatory drugs; PPI: Proton pump inhibitor.
Eradication rates
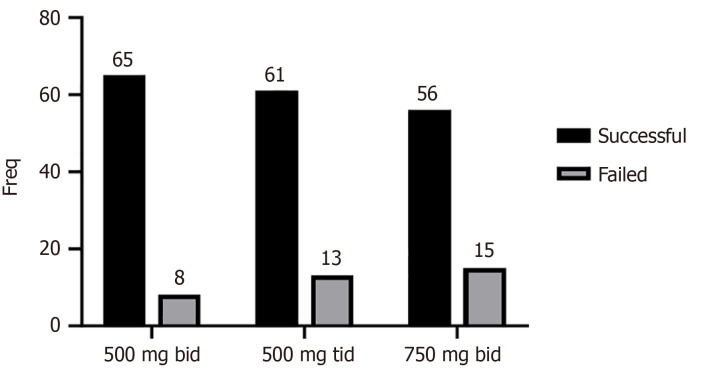
Overall, 182 (83.4%) patients achieved successful H. pylori eradication (Figure 1). With regard to ITT, H. pylori eradication rates were 89.0% in the 500 mg bid group, 82.4% in the 500 mg tid group, and 78.9% in the 750 mg bid group (P = 0.25) (Figure 1). Of the 118 patients receiving primary therapy, eradication rates were 90.0%, 88.1%, and 87.5% in the 500 mg bid group, 500 mg tid group and 750 mg bid group, respectively, without statistically significant differences (P = 0.85) (Table 2). Of the 100 patients receiving rescue therapy, eradication rates were 84.6%, 75.0%, and 76.4% in the 500 mg bid group, 500 mg tid group and 750 mg bid group, respectively, without statistically significant differences (P = 0.89) (Table 2).
Figure 1.
Eradication efficacy of the different groups. 500 mg bid: Tetracycline twice daily; 500 mg tid: Tetracycline three times daily; 750 mg bid: Tetracycline twice daily.
Table 2.
Treatment times of patients and the eradication rates
|
|
Eradication rate
|
P value
|
||
|
500 mg bid (n = 73)
|
500 mg tid (n = 74)
|
750 mg bid (n = 71)
|
||
| All | 65, 89.0% | 61, 82.4% | 56, 78.9% | 0.25 |
| Primary therapy | 54/60, 90.0% | 37/42, 88.1% | 14/16, 87.5% | 0.85 |
| 1 prior therapy | 8/8, 100% | 22/27, 81.5% | 27/39, 69.2% | |
| ≥ 2 prior therapies | 3/5, 60.0% | 2/5, 40.0% | 15/16, 93.8% | |
| Rescue therapy1 | 11/13, 84.6% | 24/32, 75.0% | 42/55 76.4% | 0.89 |
Rescue therapy was defined as patients who had received eradication therapy once or more but failed.
P values were from two-sided comparisons of the differences between the three groups. 500 mg bid: Tetracycline twice daily; 500 mg tid: Tetracycline three times daily; 750 mg bid: Tetracycline twice daily.
Failure to complete treatment
Seven (3%) patients failed to complete the prescribed treatment due to adverse events or non-adherence. In the low-dose tetracycline group, one of two patients who failed to complete therapy achieved eradication. In the standard dose tetracycline group, two of five patients who failed to complete therapy achieved eradication, both in the 500 mg tid group (Supplementary Table 1). After excluding 7 patients who failed to complete the prescribed therapy, the overall eradication rates in the 500 mg bid, 500 mg tid, and 750 mg bid group were 90.1%, 83.0%, and 81.1%, respectively, without statistically significant differences (P = 0.30). Of the 115 patients receiving treatment as primary therapy, the eradication rates were 89.8%, 87.8%, and 93.3%, respectively (P = 0.92). Among the 96 patients receiving the treatment as rescue therapy, the eradication rates were 91.6%, 76.6%, and 77.7%, respectively (P = 0.63) (Supplementary Table 1). Patients who received incomplete therapy were less likely to achieve eradication (42.9% vs 84.8%, P = 0.047) compared to those who completed therapy (Supplementary Table 2).
Adverse events
Overall, 36 patients (16.5%) suffered from at least one adverse event during the treatment period, with a cumulative number of 49 adverse events (Table 3). The incidence of adverse events was lower in the low-dose tetracycline (12.3% vs 31.1% or 23.9%, P = 0.02) compared to the standard dose groups. The most common adverse events were abdominal discomfort (14, 28.6%) followed by nausea (5, 10.2%).
Table 3.
Adverse events in the different treatment groups
|
Adverse effect
|
All
|
500 mg bid (n = 73)
|
500 mg tid (n = 74)
|
750 mg bid (n = 71)
|
P value
|
| Total, n/N (%)1 | 22.5 (49/218) | 12.3 (9/73) | 31.1 (23/74) | 23.9 (17/71) | 0.023 |
| Taste distortion | 1 | 0 | 1 | 0 | |
| Belching | 1 | 0 | 1 | 0 | |
| Loss of appetite | 3 | 0 | 1 | 2 | |
| Nausea | 5 | 1 | 3 | 1 | |
| Vomiting | 4 | 1 | 1 | 2 | |
| Abdominal pain | 1 | 0 | 1 | 0 | |
| Bloating | 3 | 0 | 3 | 0 | |
| Diarrhea | 3 | 1 | 0 | 2 | |
| Skin rash | 4 | 2 | 1 | 1 | |
| Fever | 3 | 0 | 1 | 2 | |
| Dizziness | 3 | 1 | 1 | 1 | |
| Melena | 2 | 0 | 1 | 1 | |
| Constipation | 2 | 0 | 1 | 1 | |
| Abdominal discomfort2 | 14 | 3 | 7 | 4 |
The symptoms of a single adverse event are counted as 1, and a patient may have multiple adverse events.
Abdominal discomfort, but not specified.
P values were from two-sided comparisons of the differences among the three treatment groups. 500 mg bid: Tetracycline twice daily; 500 mg tid: Tetracycline three times daily; 750 mg bid: Tetracycline twice daily. N: Number of total patients; n: Number of patients with adverse events.
Predictors of eradication failure
On univariate analysis, age, gender, alcohol drinking history, hypertension, diabetes, hyperlipidemia and other demographic characteristics were not associated with failed eradication. However, patients with a family history of gastric cancer (P = 0.02), smoking history (P = 0.007), and those receiving rescue therapy (P = 0.03) were associated with failed eradication (Supplementary Table 3). Multivariate analysis confirmed that patients with a family history of gastric cancer [odds ratio (OR) = 12.4, 95% confidence interval (CI): 1.1-140.6, P = 0.04], smoking history (OR = 2.8, 95%CI: 1.2-6.2, P = 0.01), and those receiving rescue therapy (OR = 2.3, 95%CI: 1.05-4.8, P = 0.04) were associated with failed eradication (Supplementary Table 4).
DISCUSSION
In this single center study evaluating the efficacy and safety of tetracycline combined with amoxicillin, bismuth and PPI quadruple therapy for H. pylori infection, the low-dose tetracycline (500 mg bid) regimen achieved a high eradication rate with few adverse events compared to the standard dose tetracycline regimen. The rise in antibiotic resistance is the primary factor leading to the increase in failed H. pylori therapy, posing a major public health concern. A high proportion of patients with prior failed therapy harbor antibiotic-resistant H. pylori infection. In a randomized trial of 312 patients with H. pylori infection who previously failed therapy performed in China, resistance rates of H. pylori to metronidazole of 87.8%, clarithromycin 90.2%, levofloxacin 85.4%, amoxicillin 8.3%, and tetracycline 1.0% were observed[24]. Similarly, H. pylori resistance rates to metronidazole, clarithromycin, levofloxacin, and amoxicillin tetracycline were 43.2%, 33.9%, 24%, 2.1% and 0%, respectively, in 1050 patients that included more than a quarter who experienced prior treatment failure[25]. Despite the high prevalence of H. pylori resistant to metronidazole, clarithromycin, and levofloxacin in both studies, rates of resistance to tetracycline and amoxicillin remained low, even among patients who failed prior therapy. Our study did not show a difference in eradication rates between patients receiving primary or rescue therapy.
Tetracycline, as a common component of bismuth-based quadruple therapy, is an antibiotic that maintains a high resistance barrier to H. pylori strains, because three adjacent point mutations (AGA-926 to 928-TTC) are required for the development of resistance. A single or double mutations at these sites result in only low to intermediate levels of resistance[25,26]. Similarly, amoxicillin resistance in H. pylori is rare as simultaneous mutations at multiple sites in penicillin-binding proteins associated genes are required to significantly increase the minimal inhibitory concentration[27].
Tetracycline as a component of various treatment regiments remains highly effective against H. pylori infection. In a multicenter, randomized trial of 339 patients with H. pylori infection naïve to treatment, combination therapy with amoxicillin (1 g bid), tetracycline (500 mg tid), esomeprazole, and colloidal bismuth pectin or bismuth potassium citrate for 14 days demonstrated high eradication rates of 90.5% and 92.3% on ITT analysis[28]. However, given common treatment-related adverse events and the inconvenience of frequent dosing, low-dose tetracycline (500 mg bid) has been evaluated as part of quadruple therapy in H. pylori eradication. A prospective, single-center study of 118 patients showed that patients receiving tetracycline 500 mg bid combined with omeprazole, metronidazole, and bismuth subcitrate for 14 days resulted in eradication rates of 95% in the ITT analysis and 98% in the PP analysis, with only 5% experiencing moderate or severe adverse events[29]. Another study evaluating the same regimen showed an eradication rates of 93% in ITT analysis and 97% in PP analysis among 68 patients with previous eradication failure, with only 2 patients suffering moderate adverse events[30]. A simplified low-dose 10 days quadruple therapy containing a formulation of tetracycline 500 mg bid, bismuth salicylate, metronidazole and rabeprazole, showed that the eradication rate could reach 95.1% by PP analysis and 92.9% by ITT analysis, with excellent adherence with minimal adverse events[31]. Although a number of studies have confirmed the high eradication rate of low-dose tetracycline, comparative studies evaluating different doses of tetracycline are lacking. In our previous study, low-dose tetracycline (500 mg bid) as part of tetracycline and furazolidone quadruple therapy for 14 days demonstrated a similar eradiation rate (92.4% vs 93.2% or 92.4%; P = 0.96) with a favorable safety profile (15.3% vs 32.3% and 29.4%; P = 0.002) compared to those who received standard dose tetracycline regimens[23].
In addition to antibiotic resistance, treatment-related adverse events and patient adherence are important factors affecting eradication rates, while age, smoking, CYP2C19 phenotype, diabetes, and use of probiotics are factors that may affect efficacy[32-34]. Multiple dosing and side effects are common causes of poor patient adherence[35]. Although the sample size was small, our study showed that patients with poor adherence were less likely to achieve eradication, consistent with previous studies[27,36-38]. Furthermore, our study demonstrated that smoking increased (OR = 2.8, 95%CI: 1.2-6.2) the odds of failed H. pylori therapy. In a meta-analysis evaluating 5538 patients receiving H. pylori therapy, patients who smoked were nearly twice more likely to have persistent H. pylori infection after treatment compared with non-smokers[32]. The proposed biologic mechanism includes increased gastric acid secretion from smoking leading to impaired mucus secretion and gastric blood flow, thereby reducing local antibiotic delivery[13].
Gut microbiota play an important role in human health, including regulating intestinal inflammation, maintaining human metabolic homeostasis, and regulating the immune system[39]. Dysregulation of the gut microbiota is associated with the development of inflammatory bowel disease, obesity, type 2 diabetes, and other systemic diseases[40]. Studies have shown that H. pylori infection can influence the structure, diversity, and biological function of gastric microbiota associated with the incidence of gastrointestinal diseases[41]. However, H. pylori therapy with antibiotics may reduce beneficial bacteria, leading to a decrease in microbial diversity, and even increased abundance of potentially pathogenic bacteria[42,43]. In addition, acid suppression with PPI as part of the treatment regimen may also affect gut microbial diversity[44]. However, in a meta-analysis of 1218 patients receiving H. pylori therapy, change in gut microbiota appeared transient, largely resolving after a year[45].
Supplemental probiotics in addition to standard antibiotic therapies have been evaluated as treatment for H. pylori. Multiple meta-analyses have demonstrated that probiotics (i.e., Lactobacillus, Saccharomyces boulardii, and Bacillus clausii) reduced treatment-related adverse events and enhanced eradication rates[45,46]. Probiotics may affect the survival of H. pylori in the stomach by the synthesis of antibacterial substances (e.g., organic fatty acids, ammonia, H2O2), change in local pH, direct competition with H. pylori for space and nutrients, influence on H. pylori colonization and adhesion, modification of the toxin or its receptors, and immunoregulation[47,48]. Although our study failed to demonstrate the association of probiotic therapy with eradication rate or adverse events, additional high-quality studies are needed.
Our study has limitations related to the retrospective study design. Although the baseline characteristics were generally similar, factors other than the tetracycline dose may have affected study endpoints. For example, differences in the type and dose of bismuth and PPI agents and a higher proportion of patients taking standard dose tetracycline as rescue therapy, may have introduced bias. Furthermore, important information regarding prior H. pylori therapy was not consistently available. Finally, evaluation of the effect of low-dose tetracycline regimen on gut microbiota was beyond the scope of the study and was not examined but may be invaluable in future studies.
CONCLUSION
In conclusion, low-dose tetracycline combined with amoxicillin-containing bismuth quadruple therapy achieved a high eradication rate with fewer treatment-related adverse effects compared to standard dose tetracycline quadruple therapy. Given its superior safety profile, low-dose tetracycline may provide an alternate treatment option as a component of the H. pylori treatment regimen. High quality randomized trials are needed to validate our findings.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Institutional Review Board of Sir Run Run Shaw Hospital, Medical School, Zhejiang University, Hangzhou (Approval No. SRRSH: 2024-0053).
Informed consent statement: Patient consent was waived due the impossibility of identifying patients and the retrospective design of the investigation.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade B
Novelty: Grade B, Grade B
Creativity or Innovation: Grade B, Grade C
Scientific Significance: Grade A, Grade B
P-Reviewer: Farwati R; Zou YT S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Yu HG
Contributor Information
Yi-Ru Zhao, Department of Gastroenterology, Sir Run Run Shaw Hospital, Medical School, Zhejiang University, Hangzhou 310016, Zhejiang Province, China; Department of Gastroenterology, The First Affiliated Hospital, Medical School, Zhejiang University, Hangzhou 310003, Zhejiang Province, China.
Xin-Jie Wang, Department of Gastroenterology, Sir Run Run Shaw Hospital, Medical School, Zhejiang University, Hangzhou 310016, Zhejiang Province, China.
Meng-Jia Zhu, Department of Gastroenterology, Sir Run Run Shaw Hospital, Medical School, Zhejiang University, Hangzhou 310016, Zhejiang Province, China.
Ang-Li Chen, Department of Gastroenterology, Sir Run Run Shaw Hospital, Medical School, Zhejiang University, Hangzhou 310016, Zhejiang Province, China.
Dian Zhang, Department of Gastroenterology, Sir Run Run Shaw Hospital, Medical School, Zhejiang University, Hangzhou 310016, Zhejiang Province, China.
Qin Du, Department of Gastroenterology, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310000, Zhejiang Province, China.
John J Kim, Keck School of Medicine, University of Southern California, Los Angeles, CA 90089-9021, United States.
Wei-Ling Hu, Department of Gastroenterology, Sir Run Run Shaw Hospital, Medical School, Zhejiang University, Hangzhou 310016, Zhejiang Province, China; Institute of Gastroenterology, Zhejiang University, Hangzhou 310016, Zhejiang Province, China. huweiling@zju.edu.cn.
Data sharing statement
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
References
- 1.Malfertheiner P, Camargo MC, El-Omar E, Liou JM, Peek R, Schulz C, Smith SI, Suerbaum S. Helicobacter pylori infection. Nat Rev Dis Primers. 2023;9:19. doi: 10.1038/s41572-023-00431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Liou JM, Malfertheiner P, Lee YC, Sheu BS, Sugano K, Cheng HC, Yeoh KG, Hsu PI, Goh KL, Mahachai V, Gotoda T, Chang WL, Chen MJ, Chiang TH, Chen CC, Wu CY, Leow AH, Wu JY, Wu DC, Hong TC, Lu H, Yamaoka Y, Megraud F, Chan FKL, Sung JJ, Lin JT, Graham DY, Wu MS, El-Omar EM Asian Pacific Alliance on Helicobacter and Microbiota (APAHAM) Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. 2020;69:2093–2112. doi: 10.1136/gutjnl-2020-322368. [DOI] [PubMed] [Google Scholar]
- 4.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 5.Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, Gasbarrini A, Hunt RH, Leja M, O'Morain C, Rugge M, Suerbaum S, Tilg H, Sugano K, El-Omar EM European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022 [Google Scholar]
- 7.Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut. 2020;69:2113–2121. doi: 10.1136/gutjnl-2020-320839. [DOI] [PubMed] [Google Scholar]
- 8.Zhou L, Lu H, Song Z, Lyu B, Chen Y, Wang J, Xia J, Zhao Z Helicobacter Pylori Study Group of Chinese Society of Gastroenterology. 2022 Chinese national clinical practice guideline on Helicobacter pylori eradication treatment. Chin Med J (Engl) 2022;135:2899–2910. doi: 10.1097/CM9.0000000000002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, Jones NL, Render C, Leontiadis GI, Moayyedi P, Marshall JK. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 2016;151:51–69.e14. doi: 10.1053/j.gastro.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 11.Furuta T, Yamade M, Kagami T, Uotani T, Suzuki T, Higuchi T, Tani S, Hamaya Y, Iwaizumi M, Miyajima H, Umemura K, Osawa S, Sugimoto K. Dual Therapy with Vonoprazan and Amoxicillin Is as Effective as Triple Therapy with Vonoprazan, Amoxicillin and Clarithromycin for Eradication of Helicobacter pylori. Digestion. 2020;101:743–751. doi: 10.1159/000502287. [DOI] [PubMed] [Google Scholar]
- 12.Wang YH, Lv ZF, Zhong Y, Liu DS, Chen SP, Xie Y. The internalization of Helicobacter pylori plays a role in the failure of H. pylori eradication. Helicobacter. 2017;22 doi: 10.1111/hel.12324. [DOI] [PubMed] [Google Scholar]
- 13.Shah SC, Iyer PG, Moss SF. AGA Clinical Practice Update on the Management of Refractory Helicobacter pylori Infection: Expert Review. Gastroenterology. 2021;160:1831–1841. doi: 10.1053/j.gastro.2020.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong TC, El-Omar EM, Kuo YT, Wu JY, Chen MJ, Chen CC, Fang YJ, Leow AHR, Lu H, Lin JT, Tu YK, Yamaoka Y, Wu MS, Liou JM Asian Pacific Alliance on Helicobacter and Microbiota. Primary antibiotic resistance of Helicobacter pylori in the Asia-Pacific region between 1990 and 2022: an updated systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2024;9:56–67. doi: 10.1016/S2468-1253(23)00281-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X. [Efficacy of amoxicillin combined with different antibiotic in anti Helicobacter pylori treatment] Nanjing Yike Daxue Xuebao (Ziran Kexue ban) 2013:255–256. [Google Scholar]
- 16.Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65:232–60. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bush LM, Johnson CC. Ureidopenicillins and beta-lactam/beta-lactamase inhibitor combinations. Infect Dis Clin North Am. 2000;14:409–433, ix. doi: 10.1016/s0891-5520(05)70255-5. [DOI] [PubMed] [Google Scholar]
- 18.Zhong Z, Zhang Z, Wang J, Hu Y, Mi Y, He B, Zhang Y, Zhang X, Xia X, Huang H, Lai Y, Lin M, Su C, Zhang Z, Wu Z, Lu L, Zhang B, Huang S, Zhong C, Zeng X, Peng Y, Chen G, Zhang H, Zhou G, Liu S, Yang C, Yan L, Chen A, Zhang G, Xu P, Wang S, Zheng P, Xu S, Gao H. A retrospective study of the antibiotic-resistant phenotypes and genotypes of Helicobacter pylori strains in China. Am J Cancer Res. 2021;11:5027–5037. [PMC free article] [PubMed] [Google Scholar]
- 19.Lv ZF, Wang FC, Zheng HL, Wang B, Xie Y, Zhou XJ, Lv NH. Meta-analysis: is combination of tetracycline and amoxicillin suitable for Helicobacter pylori infection? World J Gastroenterol. 2015;21:2522–2533. doi: 10.3748/wjg.v21.i8.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilaichone RK, Yamaoka Y, Shiota S, Ratanachu-ek T, Tshering L, Uchida T, Fujioka T, Mahachai V. Antibiotics resistance rate of Helicobacter pylori in Bhutan. World J Gastroenterol. 2013;19:5508–5512. doi: 10.3748/wjg.v19.i33.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vilaichone RK, Gumnarai P, Ratanachu-Ek T, Mahachai V. Nationwide survey of Helicobacter pylori antibiotic resistance in Thailand. Diagn Microbiol Infect Dis. 2013;77:346–349. doi: 10.1016/j.diagmicrobio.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Xie Y, Zhu Z, Wang J, Zhang L, Zhang Z, Lu H, Zeng Z, Chen S, Liu D, Lv N the Chinese Study Group on Helicobacter pylori, Chinese Society of Gastroenterology. Ten-Day Quadruple Therapy Comprising Low-Dose Rabeprazole, Bismuth, Amoxicillin, and Tetracycline Is an Effective and Safe First-Line Treatment for Helicobacter pylori Infection in a Population with High Antibiotic Resistance: a Prospective, Multicenter, Randomized, Parallel-Controlled Clinical Trial in China. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.00432-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun YC, Zhu MJ, Chen XQ, Yue L, Zhao YR, Wang XJ, Kim JJ, Du Q, Hu WL. Efficacy and safety of modified tetracycline dosing in a quadruple therapy for Helicobacter pylori: A retrospective single center study. World J Gastroenterol. 2023;29:3508–3518. doi: 10.3748/wjg.v29.i22.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q, Zhang W, Fu Q, Liang X, Liu W, Xiao S, Lu H. Rescue Therapy for Helicobacter pylori Eradication: A Randomized Non-Inferiority Trial of Amoxicillin or Tetracycline in Bismuth Quadruple Therapy. Am J Gastroenterol. 2016;111:1736–1742. doi: 10.1038/ajg.2016.443. [DOI] [PubMed] [Google Scholar]
- 25.Aumpan N, Issariyakulkarn N, Mahachai V, Graham D, Yamaoka Y, Vilaichone RK. Management of Helicobacter pylori treatment failures: A large population-based study (HP treatment failures trial) PLoS One. 2023;18:e0294403. doi: 10.1371/journal.pone.0294403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mégraud F. The challenge of Helicobacter pylori resistance to antibiotics: the comeback of bismuth-based quadruple therapy. Therap Adv Gastroenterol. 2012;5:103–109. doi: 10.1177/1756283X11432492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology. 2018;155:1372–1382.e17. doi: 10.1053/j.gastro.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie Y, Hu Y, Zhu Y, Wang H, Wang QZ, Li YQ, Wang JB, Zhang ZY, Zhang DK, Liu XW, Lu NH. Colloidal bismuth pectin-containing quadruple therapy as the first-line treatment of Helicobacter pylori infection: A multicenter, randomized, double-blind, non-inferiority clinical trial. Helicobacter. 2023;28:e12978. doi: 10.1111/hel.12978. [DOI] [PubMed] [Google Scholar]
- 29.Dore MP, Graham DY, Mele R, Marras L, Nieddu S, Manca A, Realdi G. Colloidal bismuth subcitrate-based twice-a-day quadruple therapy as primary or salvage therapy for Helicobacter pylori infection. Am J Gastroenterol. 2002;97:857–860. doi: 10.1111/j.1572-0241.2002.05600.x. [DOI] [PubMed] [Google Scholar]
- 30.Dore MP, Marras L, Maragkoudakis E, Nieddu S, Manca A, Graham DY, Realdi G. Salvage therapy after two or more prior Helicobacter pylori treatment failures: the super salvage regimen. Helicobacter. 2003;8:307–309. doi: 10.1046/j.1523-5378.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- 31.Dore MP, Saba F, Zanni L, Rocca A, Piroddu J, Gutierrez G, Pes GM. A Simplified Low-Dose 10-Day Quadruple Therapy with a Galenic Formulation of Bismuth Salicylate Is Highly Effective for Helicobacter pylori Eradication. J Clin Med. 2023;12 doi: 10.3390/jcm12020681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki T, Matsuo K, Ito H, Sawaki A, Hirose K, Wakai K, Sato S, Nakamura T, Yamao K, Ueda R, Tajima K. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. 2006;119:217–224. doi: 10.1016/j.amjmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Ojetti V, Pitocco D, Bartolozzi F, Danese S, Migneco A, Lupascu A, Pola P, Ghirlanda G, Gasbarrini G, Gasbarrini A. High rate of helicobacter pylori re-infection in patients affected by type 1 diabetes. Diabetes Care. 2002;25:1485. doi: 10.2337/diacare.25.8.1485. [DOI] [PubMed] [Google Scholar]
- 34.Horikawa C, Kodama S, Fujihara K, Hirasawa R, Yachi Y, Suzuki A, Hanyu O, Shimano H, Sone H. High risk of failing eradication of Helicobacter pylori in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract. 2014;106:81–87. doi: 10.1016/j.diabres.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Argueta EA, Moss SF. How We Approach Difficult to Eradicate Helicobacter pylori. Gastroenterology. 2022;162:32–37. doi: 10.1053/j.gastro.2021.10.048. [DOI] [PubMed] [Google Scholar]
- 36.Zhou L, Zhang J, Song Z, He L, Li Y, Qian J, Bai P, Xue Y, Wang Y, Lin S. Tailored versus Triple plus Bismuth or Concomitant Therapy as Initial Helicobacter pylori Treatment: A Randomized Trial. Helicobacter. 2016;21:91–99. doi: 10.1111/hel.12242. [DOI] [PubMed] [Google Scholar]
- 37.Song Z, Zhou L, Xue Y, Suo B, Tian X, Niu Z. A comparative study of 14-day dual therapy (esomeprazole and amoxicillin four times daily) and triple plus bismuth therapy for first-line Helicobacter pylori infection eradication: A randomized trial. Helicobacter. 2020;25:e12762. doi: 10.1111/hel.12762. [DOI] [PubMed] [Google Scholar]
- 38.Tian XL, Suo BJ, Zhang H, Lu HP, Li CL, Zhang YX, Ren XL, Yao XY, Zhou LY, Song ZQ. Bismuth, esomeprazole, metronidazole and amoxicillin or tetracycline as a first-line regimen for Helicobacter pylori eradication: A randomized controlled trial. Helicobacter. 2023;28:e12935. doi: 10.1111/hel.12935. [DOI] [PubMed] [Google Scholar]
- 39.Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. 2020;113:2019–2040. doi: 10.1007/s10482-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 40.Ji J, Jin W, Liu SJ, Jiao Z, Li X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm (2020) 2023;4:e420. doi: 10.1002/mco2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin LX, Fang YP, Xia CM, Cai TW, Li QQ, Wang YY, Yan HF, Chen X. Helicobacter pylori infection alters gastric microbiota structure and biological functions in patients with gastric ulcer or duodenal ulcer. World J Gastroenterol. 2024;30:3076–3085. doi: 10.3748/wjg.v30.i24.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lange K, Buerger M, Stallmach A, Bruns T. Effects of Antibiotics on Gut Microbiota. Dig Dis. 2016;34:260–268. doi: 10.1159/000443360. [DOI] [PubMed] [Google Scholar]
- 43.de la Fuente-Nunez C, Cesaro A, Hancock REW. Antibiotic failure: Beyond antimicrobial resistance. Drug Resist Updat. 2023;71:101012. doi: 10.1016/j.drup.2023.101012. [DOI] [PubMed] [Google Scholar]
- 44.Vich Vila A, Collij V, Sanna S, Sinha T, Imhann F, Bourgonje AR, Mujagic Z, Jonkers DMAE, Masclee AAM, Fu J, Kurilshikov A, Wijmenga C, Zhernakova A, Weersma RK. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun. 2020;11:362. doi: 10.1038/s41467-019-14177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du L, Chen B, Cheng F, Kim J, Kim JJ. Effects of Helicobacter pylori Therapy on Gut Microbiota: A Systematic Review and Meta-Analysis. Dig Dis. 2024;42:102–112. doi: 10.1159/000527047. [DOI] [PubMed] [Google Scholar]
- 46.Hu Y, Zhu Y, Lu NH. Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance. Front Cell Infect Microbiol. 2017;7:168. doi: 10.3389/fcimb.2017.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franceschi F, Cazzato A, Nista EC, Scarpellini E, Roccarina D, Gigante G, Gasbarrini G, Gasbarrini A. Role of probiotics in patients with Helicobacter pylori infection. Helicobacter. 2007;12 Suppl 2:59–63. doi: 10.1111/j.1523-5378.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- 48.Ruggiero P. Use of probiotics in the fight against Helicobacter pylori. World J Gastrointest Pathophysiol. 2014;5:384–391. doi: 10.4291/wjgp.v5.i4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.