Abstract
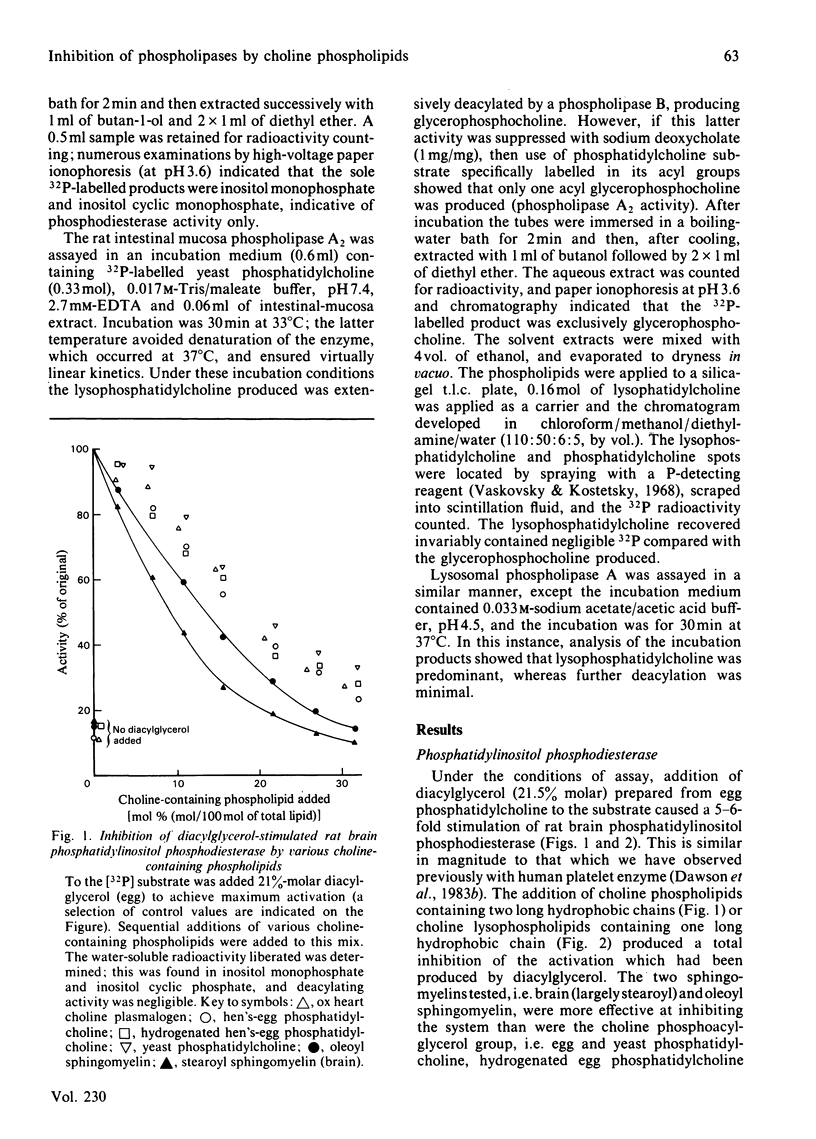
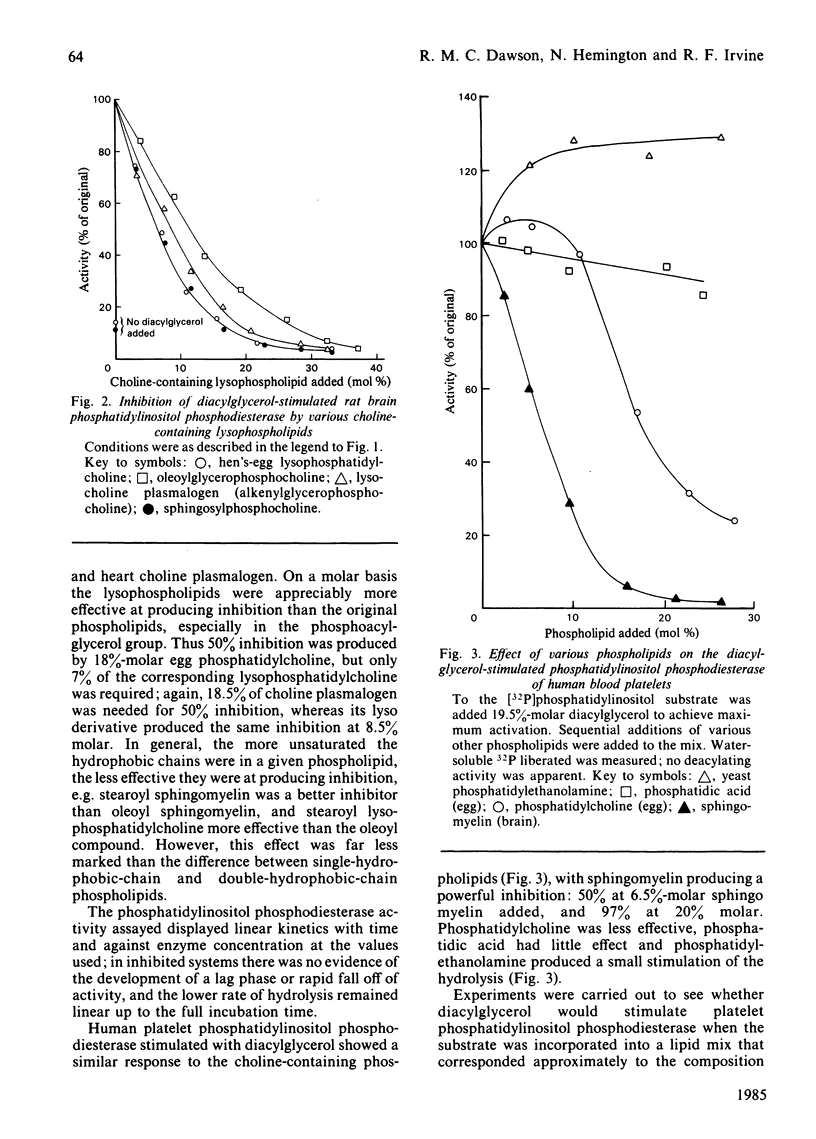
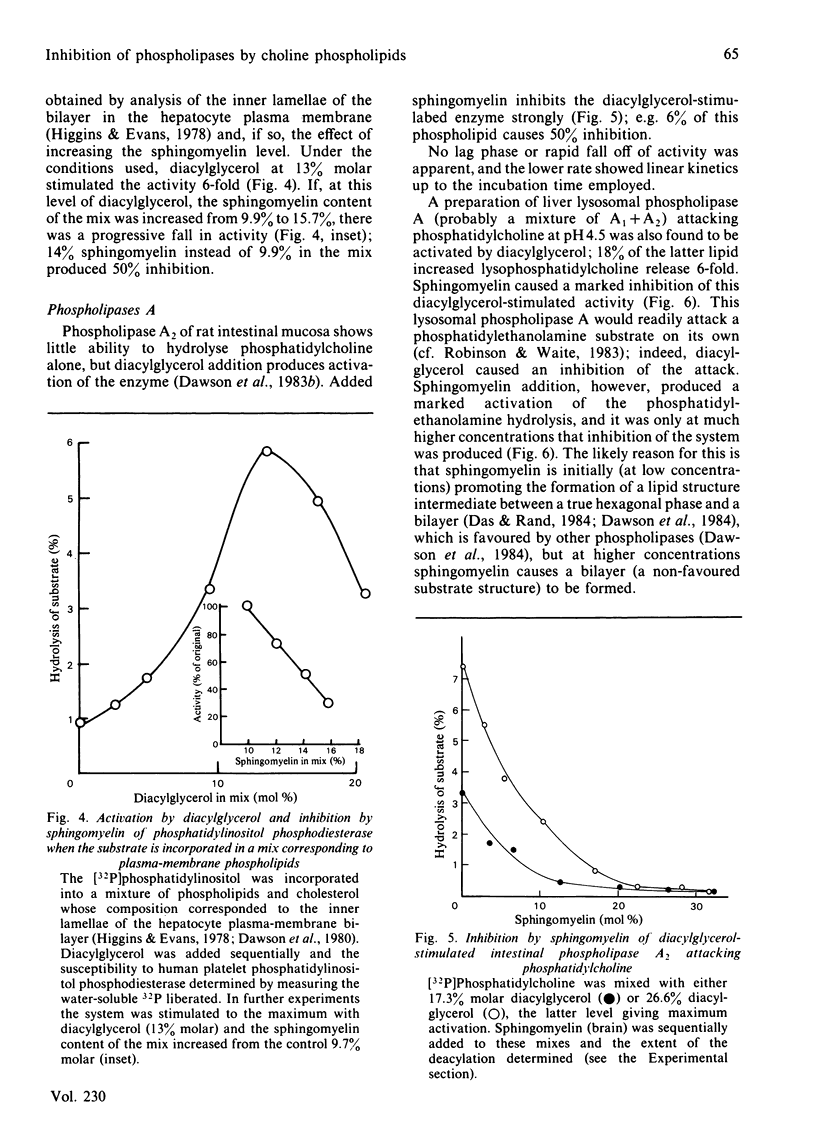
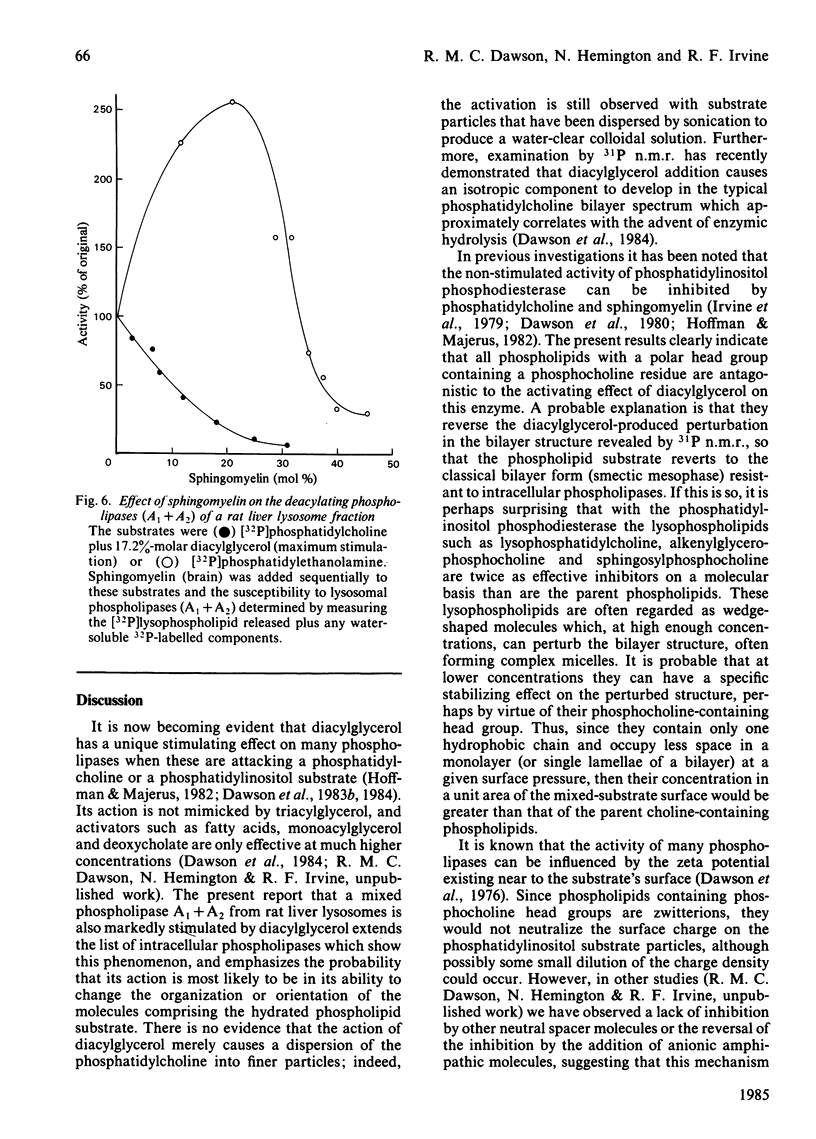
Phosphatidylinositol phosphodiesterase activated by diacylglycerol is substantially inhibited by all phospholipids containing a phosphocholine head group, including phosphatidylcholine, hydrogenated phosphatidylcholine, choline plasmalogen, lysophosphatidylcholine, lysocholine plasmalogen, sphingomyelin and sphingosylphosphocholine. The sphingosine-containing phospholipids are the most inhibitory. Phosphatidic acid does not inhibit, and phosphatidylethanolamine activates the hydrolysis still further. Sphingomyelin is highly inhibitory to a diacylglycerol-stimulated intestinal mucosal phospholipase A2, or a liver lysosomal phospholipase A1 + A2, both hydrolysing a phosphatidylcholine substrate. Sphingomyelin [20% molar (20 mol of sphingomyelin/80 mol of phosphatidylethanolamine)] activates phosphatidylethanolamine hydrolysis by intestinal mucosal phospholipase A2, and then at higher concentrations (40% molar) substantially inhibits the activity. The results are discussed in relation to possible molecular reorganizations brought about in the hydrated phospholipid substrate complex, and in particular the possible stabilizing role of sphingomyelin in the maintenance of membrane structure, and hence in the modulation of phospholipase activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell G. J., Carnuccio R., Di Rosa M., Flower R. J., Parente L., Persico P. Macrocortin: a polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 1980 Sep 11;287(5778):147–149. doi: 10.1038/287147a0. [DOI] [PubMed] [Google Scholar]
- Blackwell G. J., Flower R. J. Inhibition of phospholipase. Br Med Bull. 1983 Jul;39(3):260–264. doi: 10.1093/oxfordjournals.bmb.a071830. [DOI] [PubMed] [Google Scholar]
- Broekman M. J., Ward J. W., Marcus A. J. Phospholipid metabolism in stimulated human platelets. Changes in phosphatidylinositol, phosphatidic acid, and lysophospholipids. J Clin Invest. 1980 Aug;66(2):275–283. doi: 10.1172/JCI109854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke N. G., Dawson R. M. Alkaline O leads to N-transacylation. A new method for the quantitative deacylation of phospholipids. Biochem J. 1981 Apr 1;195(1):301–306. doi: 10.1042/bj1950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M. The identification of two lipid components in liver which enable Penicillium notatum extracts to hydrolyse lecithin. Biochem J. 1958 Feb;68(2):352–357. doi: 10.1042/bj0680352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Rand R. P. Diacylglycerol causes major structural transitions in phospholipid bilayer membranes. Biochem Biophys Res Commun. 1984 Oct 30;124(2):491–496. doi: 10.1016/0006-291x(84)91580-8. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Hemington N. L., Irvine R. F. Diacylglycerol potentiates phospholipase attack upon phospholipid bilayers: possible connection with cell stimulation. Biochem Biophys Res Commun. 1983 Nov 30;117(1):196–201. doi: 10.1016/0006-291x(83)91560-7. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Hemington N. L., Miller N. G., Bangham A. D. On the question of an electrokinetic requirement for phospholipase C action. J Membr Biol. 1976 Oct 20;29(1-2):179–184. doi: 10.1007/BF01868958. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Hemington N., Irvine R. F. The inhibition and activation of Ca2+-dependent phosphatidylinositol phosphodiesterase by phospholipids and blood plasma. Eur J Biochem. 1980 Nov;112(1):33–38. doi: 10.1111/j.1432-1033.1980.tb04983.x. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Hemington N. Some properties of purified phospholipase D and especially the effect of amphipathic substances. Biochem J. 1967 Jan;102(1):76–86. doi: 10.1042/bj1020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Irvine R. F., Bray J., Quinn P. J. Long-chain unsaturated diacylglycerols cause a perturbation in the structure of phospholipid bilayers rendering them susceptible to phospholipase attack. Biochem Biophys Res Commun. 1984 Dec 14;125(2):836–842. doi: 10.1016/0006-291x(84)90615-6. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Irvine R. F., Hemington N. L., Hirasawa K. The alkaline phospholipase A1 of rat liver cytosol. Biochem J. 1983 Mar 1;209(3):865–872. doi: 10.1042/bj2090865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt S., Bierman E. L. Sphingomyelin suppresses the binding and utilization of low density lipoproteins by skin fibroblasts. J Biol Chem. 1980 Apr 25;255(8):3371–3376. [PubMed] [Google Scholar]
- Gatt S., Dinur T., Kopolovic J. Niemann Pick disease: presence of the magnesium-dependent sphingomyelinase in brain of the infantile form of the disease. J Neurochem. 1978 Aug;31(2):547–550. doi: 10.1111/j.1471-4159.1978.tb02671.x. [DOI] [PubMed] [Google Scholar]
- Higgins J. A., Evans W. H. Transverse organization of phospholipids across the bilayer of plasma-membrane subfractions of rat hepatocytes. Biochem J. 1978 Aug 15;174(2):563–567. doi: 10.1042/bj1740563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F. The regulation of lipomodulin, a phospholipase inhibitory protein, in rabbit neutrophils by phosphorylation. J Biol Chem. 1981 Aug 10;256(15):7730–7733. [PubMed] [Google Scholar]
- Hofmann S. L., Majerus P. W. Modulation of phosphatidylinositol-specific phospholipase C activity by phospholipid interactions, diglycerides, and calcium ions. J Biol Chem. 1982 Dec 10;257(23):14359–14364. [PubMed] [Google Scholar]
- Irvine R. F., Hemington N., Dawson R. M. The hydrolysis of phosphatidylinositol by lysosomal enzymes of rat liver and brain. Biochem J. 1978 Nov 15;176(2):475–484. doi: 10.1042/bj1760475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F. How is the level of free arachidonic acid controlled in mammalian cells? Biochem J. 1982 Apr 15;204(1):3–16. doi: 10.1042/bj2040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Dawson R. M. Fatty acid stimulation of membrane phosphatidylinositol hydrolysis by brain phosphatidylinositol phosphodiesterase. Biochem J. 1979 Feb 15;178(2):497–500. doi: 10.1042/bj1780497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D., Matthews E. R., Melnykovych G. Glucocorticoid effects on lipid metabolism in HeLa cells: inhibition of cholesterol synthesis and increased sphingomyelin synthesis. Endocrinology. 1980 Nov;107(5):1482–1488. doi: 10.1210/endo-107-5-1482. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Cuatrecasas P. Stimulation of phosphatidic acid production in platelets precedes the formation of arachidonate and parallels the release of serotonin. Biochim Biophys Acta. 1979 May 25;573(2):394–402. doi: 10.1016/0005-2760(79)90072-9. [DOI] [PubMed] [Google Scholar]
- Murray D. K., Ruhmann-Wennhold A., Nelson D. H. Dexamethasone effect on the phospholipid content of isolated fat cell ghosts from adrenalectomized rats. Endocrinology. 1979 Sep;105(3):774–777. doi: 10.1210/endo-105-3-774. [DOI] [PubMed] [Google Scholar]
- Nelson D. H., Murray D. K., Brady R. O. Dexamethasone-induced change in the sphingomyelin content of human polymorphonuclear leukocytes in vitro. J Clin Endocrinol Metab. 1982 Feb;54(2):292–295. doi: 10.1210/jcem-54-2-292. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Calcium, phospholipid turnover and transmembrane signalling. Philos Trans R Soc Lond B Biol Sci. 1983 Jul 5;302(1108):101–112. doi: 10.1098/rstb.1983.0043. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Deykin D. Isolation of membranes from normal and thrombin-treated gel-filtered platelets using a lectin marker. Biochim Biophys Acta. 1976 Apr 5;426(4):688–696. doi: 10.1016/0005-2736(76)90133-4. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Differential activation of platelet phospholipases by thrombin and ionophore A23187. J Biol Chem. 1981 May 10;256(9):4153–4155. [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M., Waite M. Physical-chemical requirements for the catalysis of substrates by lysosomal phospholipase A1. J Biol Chem. 1983 Dec 10;258(23):14371–14378. [PubMed] [Google Scholar]
- Vaskovsky V. E., Kostetsky E. Y. Modified spray for the detection of phospholipids on thin-layer chromatograms. J Lipid Res. 1968 May;9(3):396–396. [PubMed] [Google Scholar]
- van den Bosch H. Intracellular phospholipases A. Biochim Biophys Acta. 1980 Sep 30;604(2):191–246. doi: 10.1016/0005-2736(80)90574-x. [DOI] [PubMed] [Google Scholar]


