Abstract
BACKGROUND
The incidence of oesophageal adenocarcinoma (OAC) has been reported to be increasing in many countries. Alongside this trend, an increase in incidence of early-onset OAC, defined as OAC in adults aged under 50 years, has been observed. It is unclear whether survival outcomes for early-onset OAC patients differ from older age groups.
AIM
To investigate survival outcomes in early-onset OAC patients.
METHODS
Ovid Medline and Embase were searched from inception to January 2022 for relevant studies relating to early-onset OAC and survival outcomes. Results regarding the overall five-year survival and risk of death of younger and older patients with OAC were extracted and pooled using meta-analyses to produce pooled estimates and 95%CIs where possible.
RESULTS
Eleven studies which compared survival of early-onset OAC, defined as age at diagnosis of < 50 years, with older patients were included. A narrative review of median and mean survival demonstrated conflicting results, with studies showing early-onset OAC patients having both better and worse outcomes compared to older age groups. A meta-analysis of five-year survival demonstrated similar outcomes across age groups, with 22%-25% of patients in the young, middle and older age groups alive after five years. A meta-analysis of four studies demonstrated that early-onset OAC patients did not have a significantly increased risk of death compared to middle-aged patients (hazard ratio 1.12, 95%CI: 0.85-1.47).
CONCLUSION
Results suggest that early-onset OAC patients do not have a significantly different survival compared to older patients, but further population-based research, taking into account stage and treatment, is required.
Keywords: Early-onset cancer, Early-onset oesophageal adenocarcinoma, Survival, Cancer epidemiology, Systematic review, Meta-analysis
Core Tip: In this systematic review, we investigated survival outcomes in early-onset oesophageal adenocarcinoma (OAC) (< 50 years) compared to older age groups. Eleven studies were included. A narrative review of median and mean survival demonstrated conflicting results. Meta-analyses of 5-year survival and risk of death demonstrated no significant difference in survival between younger and older OAC patients. Current evidence in this area has limitations, and up-to-date population-based research is required.
INTRODUCTION
There is concern that the incidence of oesophageal adenocarcinoma (OAC) in patients under 50, described as early-onset OAC, is increasing. However, data regarding survival of younger patients with OAC is sparse.
Globally, while increasing age remains a major non-modifiable risk factor for cancer, the incidence of early-onset cancers, largely accepted to be in adults aged under 50 years, is increasing[1]. This includes an observed increase in the incidence of gastrointestinal malignancies such as colorectal, oesophageal, gastric and hepatobiliary cancers[2-4].
Despite oesophageal squamous cell carcinoma (OSCC) being more common globally (88% of cases)[5], a striking increase in oesophageal OAC incidence has been reported in developed countries, such as the United States and Europe[6,7]. Worryingly, the United Kingdom has the highest incidence of OAC cases in the world[8]. In addition to the increase in OAC, an increase in incidence of early-onset OAC, defined as OAC in adults aged under 50 years, has been observed[9,10]. A population-based cohort in the Netherlands, consisting of 59584 patients, demonstrated the incidence of early-onset OAC to have tripled from 1989 to 2018, while OSCC cases declined in this age group[7].
OAC usually develops in the lower third of the oesophagus and the gastro-oesophageal junction, with risk factors including obesity and gastro-oesophageal reflux disease[11]. A poor prognosis is observed, with the overall five-year survival rate for oesophageal cancer between 15%-20%, even with treatment[12,13]. These low survival rates are likely due to a combination of late diagnosis, intrinsic resistance to systemic therapy and the limited efficacy of surgical resection.
Younger patients tend to present at a more advanced stage at diagnosis compared to those diagnosed later in life. A single centre, retrospective study found that 33.3% of patients in the younger age category (< 50 years old) presented with stage IV OAC, compared to the 20.6% of the oldest age category (> 70 years old)[14]. Another population-based study in the Netherlands observed that OAC patients under 50 years old also presented with distant metastasis more often in comparison to older patients (50.5% vs 44.7%), and that tumour differentiation also varied between age groups[15].
Reports of survival estimates in patients with early-onset OAC compared with older patients have resulted in contrasting findings to date. Some studies report that due to the advanced stage and aggressiveness of the tumours seen that the prognosis of these patients is almost always worse than their older counterparts[16]. In contrast, another study found that the overall survival, as well as stage-specific survival was higher in those who were younger[17]. A Dutch study which included only resectable cases found no difference in 5-year disease specific survival[18].
Given the conflicting evidence to date, the aim of this systematic review was to investigate survival in OAC patients according to age at diagnosis.
MATERIALS AND METHODS
A protocol was composed, and the reporting of this systematic review designed, using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines[19]. The protocol included: The review question, search strategy, inclusion criteria, type of quality assessment, the strategy for data analysis, and the ‘population, intervention, comparator, and outcome’ criteria. These are expanded below.
Study population
The population of interest was patients diagnosed with OAC aged less than 50 years. Survival outcomes were compared between older patients and those < 50 years with a diagnosis of OAC where possible, with older patients being considered the control group. Studies only reporting survival outcomes of those < 50 years were also included. In addition, studies were included if the cut off for early-onset OAC was less than 50 years.
Outcome
Overall survival estimates (e.g. those recorded as net, relative and observed) within patients aged < 50 years at the time of diagnosis of OAC.
Search strategy
The electronic databases Embase (Reed Elsevier PLC, Amsterdam, Netherlands) and Medline (United States National Library of Medicine, Bethesda, MD, United States) were searched from week of inception to 12 January 2022 for relevant studies relating to the age of diagnosis of OAC and the survival outcomes documented. The search strategy identified studies that contained at least one keyword or Medical Subject Heading term relating to young age, survival outcomes and oesophageal cancer. The full search terms are available in Supplementary Table 1. A rapid review of studies published up to mid-2024 did not identify any further relevant articles that met our inclusion criteria.
Inclusion and exclusion criteria
Population-based studies were included, along with population representative studies, for example, large database studies or using cancer registry data. Real-world studies considering consecutive patients were also included.
Clinical trial studies were excluded, in order to reduce the impact of selection biases or volunteer biases being present. Conference abstracts, case reports and review papers were also excluded. The exclusion criteria also removed any studies which did not specifically focus on the patients under the age of 50 years old. The search strategy was restricted to include English language articles and human studies only.
The reference lists of several studies, which included the correct age categories < 50 years were also searched for any relevant articles, but this did not identify any further studies.
Data extraction
Articles from the search were imported into Covidence, and duplicates were removed. Titles and abstracts were reviewed independently by at least two authors (Turkington RC, Coleman HC, and Mitchell S), to remove irrelevant studies. The full text of all selected articles was read independently by at least two authors, to determine whether they met the inclusion criteria. Any discrepancies were resolved by discussion among the reviewers of the text, with the third reviewer involved if required.
Methodological quality was evaluated using the Newcastle-Ottawa Scale (Supplementary Table 2). Studies were assessed on eight items, categorised into three groups: The selection of the study groups; the comparability of the groups; and the ascertainment of either the exposure or outcome of interest.
Statistical analysis
A narrative synthesis was assembled after a thorough and critical review of each study. Where it was deemed suitable, the association between the age of diagnosis of OAC and survival was summarised in meta-analyses by comparing the survival outcomes of those < 50 years old and those > 50 years old reported in the studies. Outcome measures were evaluated in three ways:
Firstly, the median and mean survival of patients < 50 years old and those > 50 years old diagnosed with OAC was extracted and summarised narratively since it was not possible to combine these in meta-analyses.
Secondly, the overall 5-year survival of early-onset OAC and older patients, i.e., the proportion of patients still alive after 5 years, was also extracted from relevant studies. A meta-analysis was performed on these results, using the Freeman-Tukey method. Meta-analyses were also performed using the Score (Wilson) method and exact CI method, with consistent results observed across all three methods, demonstrating the robustness of the results. I2 values were applied used to assess heterogeneity between study results.
Finally, the hazard ratios (HR) for risk of death, adjusted and unadjusted, and their 95%CI were extracted from relevant cohort studies. Random-effects meta-analyses were applied to combine adjusted HR and 95%CI from studies reporting on the risk of death in early-onset OAC patients, compared with older adults. The random effects model included study specific weights, which were calculated and then scaled to percentages. I2 values were used to assess heterogeneity between study findings.
All meta-analyses were conducted using Stata version 16 (StataCorp, College station, TC, United States).
RESULTS
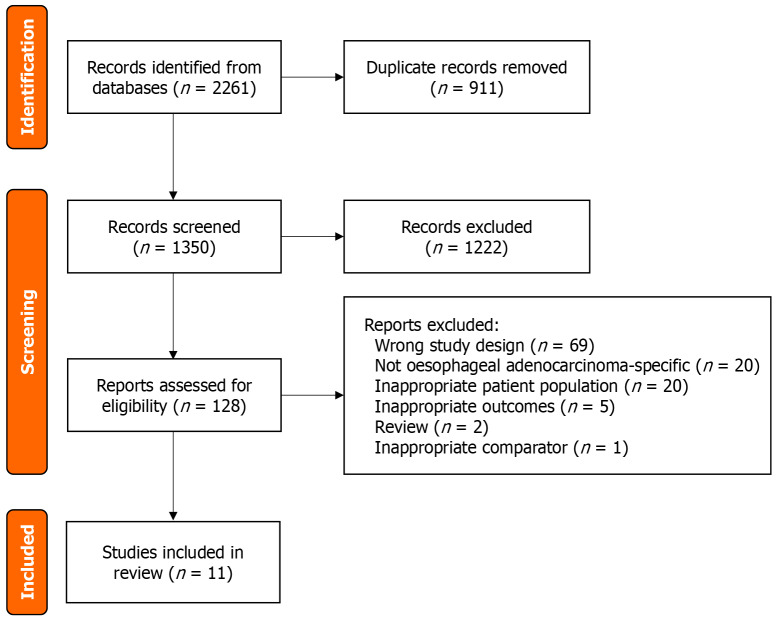
The search strategy identified 2261 papers, and 911 duplicates were removed, leaving 1350 articles for screening. Following title and abstract screening, 128 studies were eligible for full text review. Following full text review, 117 studies were excluded. Reasons for exclusion included the wrong patient population, wrong condition, inappropriate study design or outcomes, and are shown in Figure 1. Any conflicts which arose were resolved through discussion and received a final vote on whether or not to be included. After all screening processes had taken place, eleven studies were included in the review[14-17,20-26].
Figure 1.
Flow chart of the selected articles included in the review.
The characteristics of the included studies are summarised in Table 1. Six studies included population-based register cohorts[15,17,20-22,26], two were single centre retrospective cohorts[14,25], two were consecutive case studies[23,24], and one was a retrospective cohort[16]. Nine studies were conducted in the United States, four being from the Surveillance, Epidemiology, and End Results (SEER) database[17,20,22,26], while one was conducted in the Netherlands[15] and Sweden[21].
Table 1.
Characteristics of studies investigating survival outcomes in early-onset oesophageal adenocarcinoma patients
|
Ref.
|
Publication year
|
Country
|
Study design
|
No. of cases
|
No. of cases < 50 (%)
|
Recruitment period
|
Age categories (years)
|
Stage
|
Follow-up (Y/N)
|
Specific survival outcome
|
Confounders considered
|
| Boys et al[16] | 2015 | United States | Retrospective cohort | 772 | 42 (5) | 1990-2013 | < 40, > 40 | I-IV | Y | Median overall survival | Sex, race, staging, year of diagnosis |
| Kolb et al[17] | 2020 | United States | Population- based Register | 114123 | 10271 (9) | 2000-2017 | < 50, 50-69, > 70 | I-IV | Y | Median survival, HR of death | Treatment, staging, race |
| Codipilly et al[20] | 2021 | United States | Population based register | 25813 | 2183 (8) | 1975-2011 | < 50, 50-69, > 70 | I-IV | Y | Overall 5 year survival | Sex, race, staging, year of diagnosis |
| Xie et al[21] | 2017 | Sweden | Population- based cohort | 5140 | 212 (4.1) | 1961-2014 | < 50, 50-59, 60-69, 70-79, > 80 | Not recorded | Y | HR of death | Sex |
| Haiyu et al[22] | 2019 | United States | Population-based register | 16474 | Crude number not reported | 1984-2013 | 20-44, 45-54, 55-64, 65-74, > 75 | Not recorded | Y | 5-year relative survival | Sex, Socio-economic status, race |
| Hashemi et al[23] | 2009 | United States | Consecutive case series | 242 | 31 (12.8) | 1994-2004 | < 50, > 50 | 0-IV | N | Median survival | Sex, family history |
| Portale et al[24] | 2004 | United States | Consecutive case series | 263 | 32 (12.1) | 1992-2002 | < 50, > 50 | I-III | N | 5-year survival rates | |
| Sawas et al[14] | 2019 | United States | Single centre retrospective study |
682 | 105 (15.4) | 2009-2012 | < 50, 51-70, > 70 | I-IV | N | Mean survival, 5-year survival rates, HR of death | Sex, staging, intestinal metaplasia, Charlson comorbidity index |
| Strauss et al[25] | 2020 | United States | Single centre retrospective study |
630 | 65 (10.3) | 1991-2018 | < 50, > 50 | I-IV | Y | Overall survival, HR of death | Sex, race, pathology grade |
| van Nistelrooij et al[15] | 2014 | Netherlands | Population-based register | 13331 | 1466 (10.9) | 2000-2011 | < 50, > 50 | I-IV | Y | Median overall survival, 5-year overall survival | Sex, staging |
| Yang et al[26] | 2016 | United States | Population-based register | 2601 | 94 (3.6) | 1988-2011 | < 45, 45-59, 60-74, > 75 | I-III | Y | HR of death | Sex, race, lymph nodes examined |
HR: Hazard ratio; Y: Yes; N: No.
Median and mean survival
To compare the difference in median survival between those diagnosed with OAC < 50 years old and those > 50 years old we analysed three of the eleven studies[15-17,23]. One study compared the mean survival of these patients, and a summary of these results can be seen in Table 2[14]. Together these results show inconsistent differences in survival between age groups.
Table 2.
The median and mean survival of younger, middle-aged and older patients with oesophageal adenocarcinoma
|
Ref.
|
Young age
|
Middle age
|
Older age
|
Significance
|
| Median survival | ||||
| Boys et al[16]1 | 17 months | N/A | 30 months | P = 0.04 |
| Kolb et al[17]2 | 15.2 months | 15.1 months | 10.4 months | P < 0.1 |
| Hashemi et al[23]3 | 21.1 months | N/A | 22 months | Not significant |
| Mean survival | ||||
| Sawas et al[14]4 | 4 ± 4.2 years | 5 ± 3.9 years | 3.6 ± 3.2 years | P = 0.03 |
Young age < 40 years and old age ≥ 40 years.
Young age < 50 years, middle aged 50-69 and old age ≥ 70 years.
Young age ≤ 50 years and old age > 50 years.
Young age ≤ 50 years, middle age 51-70 years, old age > 70 years.
N/A: Not applicable.
There were three studies[14,16,17] which demonstrated a significant difference in survival between early onset OAC and patients diagnosed at an older age. However, these studies concluded in different directions on whether younger age at the time of diagnosis impacts survival. Boys et al[16] reported a significantly shorter median survival in younger patients (< 40 years old) at 17 months compared to older patients (> 40 years old) at 30 months. In contrast, Kolb et al[17] observed a lower median survival in older patients at 10.4 months with median survival in younger patients being 15.2 months. Sawas et al[14] reported that patients in the middle-aged (51-70 years old) category had the longest mean survival in comparison to shorter survival in the older (> 70 years old) and younger (< 50 years old) age categories. It is important to note that the age cut-offs and comparator older age groups differed in each study, limiting the interpretation of the results.
Overall five-year survival
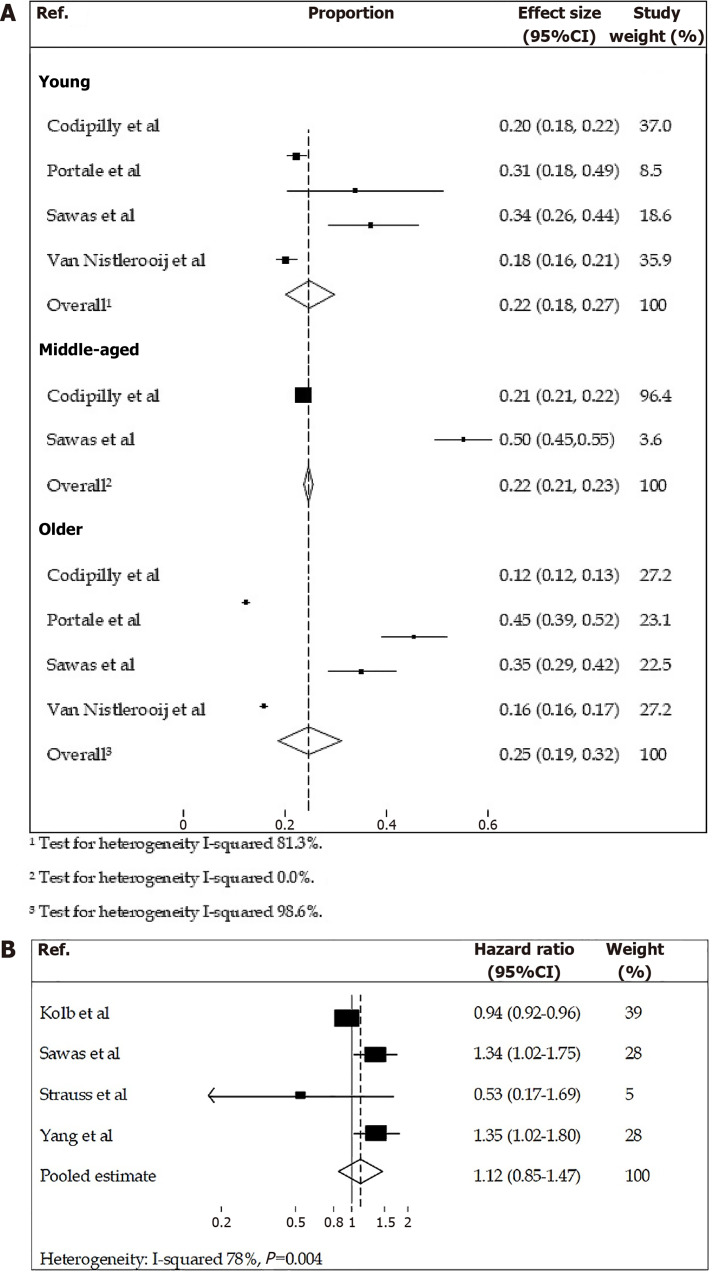
To compare the difference in overall 5-year survival between early-onset OAC patients and older age groups, we analysed five studies[14,15,20,22,24], with four being included in meta-analyses. The results demonstrated a similar proportion of patients still alive after 5 years in the young, middle and old age groups (Table 3 and Figure 2A). The study carried out by Haiyu et al[22] could not be included in the meta-analysis due to the use of the same database (SEER) and a similar time frame as Codipilly et al[20] which used the years 2000-2011 for the reported results.
Table 3.
The proportion of patients alive at five years in young, middle-aged and older patients diagnosed with oesophageal adenocarcinoma
| Ref. |
5-year survival (%) (proportion of patients still alive) |
|||
|
Young age
|
Middle age
|
Older age
|
Significance
|
|
| Codipilly et al[20]1 | 19.7 | 21.4 | 12.3 | P < 0.01 |
| Portale et al[24]2 | 32.6 | N/A | 45.5 | Survival similar |
| Sawas et al[14]3 | 34.3 | 49.9 | 33.3 | P < 0.01 |
| Van Nistelrooij et al[15]2 | 18.2 | N/A | 16.4 | P = 0.021 |
| Haiyu et al[22]4 | 19.4 | N/A | 22.6 | P < 0.05 |
Young age < 50 years, middle-age 50-69 years, old age ≥ 70 years.
Young age ≤ 50 years, old age > 50 years.
Young age ≤ 50 years, middle age 51-70 years, old age > 70 years.
Young age 20-44 years, old age 55-64 years.
N/A: Not applicable.
Figure 2.
Forest plots. A: Forest plots showing overall five-year survival of young, middle-aged, and older patients diagnosed with oesophageal adenocarcinoma (with effect size being the proportion of patients alive); B: Risk of death in adults diagnosed with early-onset oesophageal adenocarcinoma compared with older adults.
Meta-analyses results are shown in Figure 2A. The proportion of younger patients still alive five years after diagnosis was 22% (95%CI: 0.18-0.27) compared with 25% (95%CI: 0.19-0.31) in older patients, with high evidence of heterogeneity (83.49% and 98.56% respectively). Sawas et al[14] and Codipilly et al[20] considered older patients to be over 70 years old and this could not be separated in the statistical analysis. These studies also reported on the middle age category, those aged 50-70, separately and the overall effect size in this group was 22% (95%CI: 0.21-0.23); similar to the pooled analysis of the other two age groups.
Haiyu et al[22] compared the relative survival rates of younger patients and older patients from 1984-2013. For the purpose of this review, we extracted information from Haiyu et al[22] on survival rates in patients aged 20-44 and 55-64 years in the most recent time period analysed (2000-2013). The survival rates for both age categories in the first six months were similar, averaging at approximately 75%. However, the five-year survival differs between the age categories, with a lower proportion of younger patients still alive five years after diagnosis (19.4%) compared with older patients (22.6%).
Hazard ratios for risk of death over time
To compare the HRs between those diagnosed with OAC < 50 years old and those > 50 years old we analysed five studies (Table 4)[14,17,21,25,26], with four being included in a meta-analysis (Figure 2B). Together the results show an inconsistent risk of death between age groups. The study by Xie et al[21] was excluded from the meta-analysis due to the use of a different reference group (all other studies used middle aged patients as the reference group). Kolb et al[17] and Yang et al[26] both included data from SEER database, however each study covered different time periods with little overlap and so could be included in the meta-analysis.
Table 4.
Hazard ratios of risk of death and 95%CI for young, middle-aged, and older patients diagnosed with oesophageal adenocarcinoma
| Ref. |
Hazard ratios for risk of death and 95%CI |
|||||
|
Age categories (years)
|
Young age
|
Age categories (years)
|
Middle age
|
Age categories (years)
|
Older age
|
|
| Kolb et al[17] | < 50 | 0.94 (0.92-0.96) | 50-69 | Ref. (1.0) | ≥ 70 | 1.24 (1.21-1.26) |
| Xie et al[21] | < 50 | Ref. (1.0) | 50-59 | 1.19 (0.99-1.43) | 70-79 | 1.26 (1.06-1.50) |
| 60-69 | 1.09 (0.91-1.30) | ≥ 80 | 1.66 (1.38-1.99) | |||
| Sawas et al[14] | ≤ 50 | 1.34 (1.02-1.75) | 51-70 | Ref. (1.0) | > 70 | 1.70 (1.36-2.10) |
| Strauss et al[25] | < 50 | 0.53 (0.17-1.69) | > 50 | Ref. (1.0) | N/A | N/A |
| Yang et al[26] | < 45 | 1.35 (1.02-1.80) | 45-59 | Ref. (1.0) | ≥ 75 | 1.52 (1.28-1.81) |
| 60-74 | 1.11 (0.97–1.26) | |||||
N/A: Not applicable.
Three studies had significant results, with Kolb et al[17] finding that patients with early-onset OAC had a reduced risk of death compared to 50-69 year olds (adjusted HR 0.94, 95%CI: 0.92-0.96). In contrast, Sawas et al[14] and Yang et al[26] found that early-onset OAC patients had an increased risk of death compared to middle age groups (adjusted HR and 95%CI: 1.34 (1.02-1.75) and 1.35 (1.02-1.80) respectively). Strauss et al[25] reported no association between the age of diagnosis of OAC and overall survival. The one study that used early-onset OAC patients as the reference group, published by Xie et al[21] found that patients who were middle-aged (50-69 years old) or older-aged (> 70-80 years old) had an increased risk of death compared to younger (< 50 years old) OAC patients.
As shown in Figure 2B, the comparison of the pooled estimate for risk of death for younger patients in comparison to middle-aged patients was not statistically significant, HR 1.12 (95%CI: 0.85-1.47), with high evidence of heterogeneity (78%).
Quality assessment was carried out using the Newcastle-Ottawa Scale (Supplementary Table 2) with no studies excluded based on quality assessment.
DISCUSSION
We performed a systematic review of eleven studies which investigated the survival outcomes of younger patients (< 50 years old) and older patients diagnosed with OAC. A narrative review of median and mean survival demonstrated conflicting results, with studies showing early-onset OAC patients having both better and worse outcomes compared to older age groups. A meta-analysis of five-year survival demonstrated similar outcomes across age groups, with 22%-25% of patients in the young, middle and older age groups alive after five years. Finally, a meta-analysis of risk of death demonstrated that early-onset OAC patients did not have a significantly increased risk of death compared to middle-aged patients (HR 1.12, 95%CI: 0.85-1.47). Overall, our findings showed no clear difference in survival in OAC between age groups.
Despite Kolb et al[17] and Yang et al[26] using data from the same database, SEER in the United States, their HR for risk of death was different. It can be assumed these contradictions in findings were due to the differing time periods which data was taken from, with little overlap seen. The reduced risk of death in younger patients reported by Kolb et al[17] was from the more recent data set, which is likely due to improvements in cancer treatment over time. Encouragingly, it should be noted from Haiyu et al[22] that five-year survival rates improved over time from 1984 to 2013, almost doubling in the most recent decade studied, depending on age. This improvement in overall survival of patents reflects the developments made in diagnosis and treatment in the past decades, such as access to endoscopy, improvement in surgery and the introduction of neoadjuvant treatment[27].
Early-onset OAC has been observed to present at a more advanced stage than older patients, which was demonstrated in a number of studies included in this systematic review[17,20]. For example, Codipilly et al[20] found that 84.9% of early-onset cancer patients had regional/distant disease compared with 77.6% and 67.8% in middle age and older age groups respectively[20]. Despite this, our results demonstrate overall five-year survival was similar in younger and older patients. This may be because younger patients are more likely to receive aggressive cancer treatment in the presence of advanced stage of disease or have less comorbidities. This has been demonstrated in early-onset colorectal cancer, where young patients are more likely to receive chemotherapy and radiotherapy, as well as multimodality treatment, than older patients[28-30]. However, whether higher rates of treatment are given to early-onset OAC patients compared to older patients is yet to be determined.
The reason why younger patients present with a more advanced stage of OAC is still unknown and debated, but it may be that younger patients experience a delayed diagnosis[24]. This could be because young patients do not seek medical attention for their symptoms, or they do seek medical attention but physicians initially attribute their symptoms to benign conditions such as gastro-oesophageal reflux disease. There may also be a reluctancy to refer these younger patients for endoscopy and screening, which is needed to diagnose OAC, due to a lack of concern or a perception that oesophageal cancer is a disease of the elderly[31]. There is an urgent need to raise awareness of the observed increasing incidence of early-onset OAC, both in the general public and healthcare professionals.
Another reason why younger patients present at more advanced stage is the possibility of a more aggressive subtype, which should also be taken into consideration. The increase in incidence of early-onset OAC, along with differing clinical features compared to older patients, raises questions about the pathogenesis and molecular pathways that lead to the development of the disease. It has been shown that early-onset OAC has stronger associations with recurrent gastro-oesophageal reflux and obesity compared to later-onset OAC[32], suggesting age specific risk factor profiles. Furthermore, it may be that early-onset OAC carries distinct biological differences compared to late-onset OAC. Recently, a large, single-centre retrospective analysis of oesophageal, gastric and gastro-oesophageal junction tumours demonstrated that early-onset cases had a preponderance for gastric tumours but had a reduced frequency of genomic and microsatellite instability compared to average onset disease[33]. A study investigating the molecular profile of OAC found no overall difference in mutational load between early-onset OAC and late-onset OAC, but did demonstrate several additional mutations seen in early-onset OAC (including APC and CTNNB1) which were not seen in conventional OAC[34]. Further research is needed into the molecular profile of the disease in different age groups.
The majority of cases of early-onset OAC appear to be sporadic rather than hereditary, with germline mutations being demonstrated in 21% of cases of early-onset oesophagogastric cancer (< 50 years) compared to 13.8% in older patients[35]. This study also included gastric cancer cases and further research is needed into the role of germline mutations in OAC, in order to identify those at risk of a cancer diagnosis at a young age and to inform prevention strategies.
Since our literature search, two further papers have been published which include survival outcomes in early-onset OAC, although neither would have met our inclusion criteria. Firstly, a large United States-based single centre study published in 2023 investigated oesophagogastric cancers (n = 218 early-onset cases), and did not find a significant difference in stage distribution or survival between early-onset and older patients[33]. Of note, this study also included gastric cancer, which comprised 64% of the early-onset group, and included non-adenocarcinoma pathology (although these numbers were small). As such, further studies are needed on presenting symptoms, time to diagnosis and pathology of early-onset OAC specifically. Secondly, a Swedish study using a population-based cancer register (n = 470 early-onset OAC, defined as < 55 years) found that 5-year relative survival was 20% in early-onset OAC compared to 16% in later-onset OAC, a similar proportion to our meta-analyses results[36].
In summary, there are a number of potential reasons for the observed variation in early-onset OAC survival. This includes biological differences in OAC across age groups, such as stage at diagnosis and molecular characteristics. Predictors of poor survival observed include ethnicity, treatment modality, tumour differentiation and comorbidity[17]. Different time periods covered by included studies will reflect developments in treatment over the decades, with more favourable outcomes being observed in later studies. Disparities in healthcare access may also be contributing–most of the studies included used data from the United States, where adults aged over 65 years are eligible for Medicare, leaving younger patients at a disadvantage. However, many factors such as ethnicity and socioeconomic status also affect access to healthcare and so firm conclusions cannot be drawn about this in our study.
This systematic review had several strengths. To our knowledge, this is the first systematic review undertaken to investigate survival in early-onset OAC. Excluding studies set within a trial setting which investigated age and outcomes in cancer patients, reduced the impact of selection biases and any possible volunteer biases. An evaluation of the quality of the studies was also carried out using the Newcastle-Ottawa Scale quality assessment, a robust and recognised tool.
This systematic review has some limitations. The search strategy which was conducted was limited to English language only, and findings may not have been representative of all the evidence available. Several of the studies defined ‘young age’ and ‘older age’ at different thresholds. Studies which consider a middle-aged category overlap with older age categories in other studies. These differences could not be separated in the statistical analysis and so could have affected the results seen. Several of the studies could not be included in the meta-analyses for various reasons; including the use of the same database or not reporting outcomes in a consistent manner.
CONCLUSION
In summary, our results suggest that early-onset OAC does not have a significantly different survival compared to older patients, but interpretation is limited due to a small number of studies over a long time period. Other factors, such as pathological characteristics and treatment receipt, also influence OAC outcomes and need to be taken into account. The inconsistency of the findings from the retrieved studies suggests the need for a more thorough population-based study with patients of all ages receiving similar treatment types. There is an urgent need for research to better understand the complex factors affecting survival such as inherited vs sporadic cases, stage of disease and pathology, as well as decision making by clinicians and patients and their influence on treatment uptake and survival for a cancer that, until now, has been mostly studied in older age groups.
ACKNOWLEDGEMENTS
The authors wish to thank Professor Gary Falk and Dr. Alexandra Strauss (University of Pennsylvania, United States) for helpful correspondence and clarification on the results for their study which was included in meta-analysis.
Footnotes
Conflict-of-interest statement: Payment or honoraria for lectures, presentations, or educational events: Dr. Ashleigh Russell (née Hamilton): Bristol-Myers Squibb (independent Speaker - paid directly by BMS).
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2020 Checklist, and the manuscript was prepared and revised according to the PRISMA 2020 Checklist.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade A
P-Reviewer: Mohammadi S S-Editor: Li L L-Editor: A P-Editor: Zhang L
Contributor Information
Ashleigh Russell, Centre for Public Health, Queen's University Belfast, Belfast BT12 6BA, United Kingdom. a.russell@qub.ac.uk.
Shauna Mitchell, Patrick G Johnston Centre for Cancer Research, Queen's University Belfast, Belfast BT7 1NN, United Kingdom.
Richard C Turkington, Patrick G Johnston Centre for Cancer Research, Queen's University Belfast, Belfast BT7 1NN, United Kingdom.
Helen G Coleman, Centre for Public Health, Queen's University Belfast, Belfast BT12 6BA, United Kingdom.
References
- 1.Zhao J, Xu L, Sun J, Song M, Wang L, Yuan S, Zhu Y, Wan Z, Larsson S, Tsilidis K, Dunlop M, Campbell H, Rudan I, Song P, Theodoratou E, Ding K, Li X. Global trends in incidence, death, burden and risk factors of early-onset cancer from 1990 to 2019. BMJ Oncol. 2023;2:e000049. [Google Scholar]
- 2.Ben-Aharon I, van Laarhoven HWM, Fontana E, Obermannova R, Nilsson M, Lordick F. Early-Onset Cancer in the Gastrointestinal Tract Is on the Rise-Evidence and Implications. Cancer Discov. 2023;13:538–551. doi: 10.1158/2159-8290.CD-22-1038. [DOI] [PubMed] [Google Scholar]
- 3.Islami F, DeSantis CE, Jemal A. Incidence Trends of Esophageal and Gastric Cancer Subtypes by Race, Ethnicity, and Age in the United States, 1997-2014. Clin Gastroenterol Hepatol. 2019;17:429–439. doi: 10.1016/j.cgh.2018.05.044. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, Jemal A. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68:2179–2185. doi: 10.1136/gutjnl-2019-319511. [DOI] [PubMed] [Google Scholar]
- 5.Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2018;154:360–373. doi: 10.1053/j.gastro.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rumgay H, Arnold M, Laversanne M, Whiteman DC, Thrift AP, Wei W, Lemmens VEPP, Soerjomataram I. International Trends in Esophageal Squamous Cell Carcinoma and Adenocarcinoma Incidence. Am J Gastroenterol. 2021;116:1072–1076. doi: 10.14309/ajg.0000000000001121. [DOI] [PubMed] [Google Scholar]
- 7.Al-Kaabi A, Baranov NS, van der Post RS, Schoon EJ, Rosman C, van Laarhoven HWM, Verheij M, Verhoeven RHA, Siersema PD. Age-specific incidence, treatment, and survival trends in esophageal cancer: a Dutch population-based cohort study. Acta Oncol. 2022;61:545–552. doi: 10.1080/0284186X.2021.2024878. [DOI] [PubMed] [Google Scholar]
- 8.Morgan E, Soerjomataram I, Gavin AT, Rutherford MJ, Gatenby P, Bardot A, Ferlay J, Bucher O, De P, Engholm G, Jackson C, Kozie S, Little A, Møller B, Shack L, Tervonen H, Thursfield V, Vernon S, Walsh PM, Woods RR, Finley C, Merrett N, O'Connell DL, Reynolds JV, Bray F, Arnold M. International trends in oesophageal cancer survival by histological subtype between 1995 and 2014. Gut. 2021;70:234–242. doi: 10.1136/gutjnl-2020-321089. [DOI] [PubMed] [Google Scholar]
- 9.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathieu LN, Kanarek NF, Tsai HL, Rudin CM, Brock MV. Age and sex differences in the incidence of esophageal adenocarcinoma: results from the Surveillance, Epidemiology, and End Results (SEER) Registry (1973-2008) Dis Esophagus. 2014;27:757–763. doi: 10.1111/dote.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson LA, Watson RG, Murphy SJ, Johnston BT, Comber H, Mc Guigan J, Reynolds JV, Murray LJ. Risk factors for Barrett's oesophagus and oesophageal adenocarcinoma: results from the FINBAR study. World J Gastroenterol. 2007;13:1585–1594. doi: 10.3748/wjg.v13.i10.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He H, Chen N, Hou Y, Wang Z, Zhang Y, Zhang G, Fu J. Trends in the incidence and survival of patients with esophageal cancer: A SEER database analysis. Thorac Cancer. 2020;11:1121–1128. doi: 10.1111/1759-7714.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Research UK. Cancer Statistics for the UK. [cited 3 September 2024]. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk .
- 14.Sawas T, Manrique GC, Iyer PG, Wang KK, Katzka DA. Young Adults With Esophageal Adenocarcinoma Present With More Advanced Stage Tumors and Have Shorter Survival Times. Clin Gastroenterol Hepatol. 2019;17:1756–1762. doi: 10.1016/j.cgh.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 15.van Nistelrooij AM, van Steenbergen LN, Spaander MC, Tilanus HW, van Lanschot JJ, Lemmens VE, Wijnhoven BP. Treatment and outcome of young patients with esophageal cancer in the Netherlands. J Surg Oncol. 2014;109:561–566. doi: 10.1002/jso.23533. [DOI] [PubMed] [Google Scholar]
- 16.Boys JA, Oh DS, Lewis JS, DeMeester SR, Hagen JA. Esophageal Adenocarcinoma in Patients Younger than 40 Years: A Two-Decade Experience at a Public and Private Hospital. Am Surg. 2015;81:974–978. [PubMed] [Google Scholar]
- 17.Kolb JM, Han S, Scott FI, Murphy CC, Hosokawa P, Wani S Early Onset Esophageal Adenocarcinoma Study Group. Early-Onset Esophageal Adenocarcinoma Presents With Advanced-Stage Disease But Has Improved Survival Compared With Older Individuals. Gastroenterology. 2020;159:2238–2240.e4. doi: 10.1053/j.gastro.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Nistelrooij AM, Andrinopoulou ER, van Lanschot JJ, Tilanus HW, Wijnhoven BP. Influence of young age on outcome after esophagectomy for cancer. World J Surg. 2012;36:2612–2621. doi: 10.1007/s00268-012-1718-6. [DOI] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Codipilly DC, Sawas T, Dhaliwal L, Johnson ML, Lansing R, Wang KK, Leggett CL, Katzka DA, Iyer PG. Epidemiology and Outcomes of Young-Onset Esophageal Adenocarcinoma: An Analysis from a Population-Based Database. Cancer Epidemiol Biomarkers Prev. 2021;30:142–149. doi: 10.1158/1055-9965.EPI-20-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie SH, Wahlin K, Lagergren J. Cause of death in patients diagnosed with esophageal cancer in Sweden: a population-based study. Oncotarget. 2017;8:51800–51809. doi: 10.18632/oncotarget.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haiyu Z, Xiaofeng P, Xiangqiong M, Junlan Q, Xiaobin Z, Shuncong W, Huanhuan S, Haiqing M. Incidence and Survival Changes in Patients with Esophageal Adenocarcinoma during 1984-2013. Biomed Res Int. 2019;2019:7431850. doi: 10.1155/2019/7431850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashemi N, Loren D, DiMarino AJ, Cohen S. Presentation and prognosis of esophageal adenocarcinoma in patients below age 50. Dig Dis Sci. 2009;54:1708–1712. doi: 10.1007/s10620-008-0565-7. [DOI] [PubMed] [Google Scholar]
- 24.Portale G, Peters JH, Hsieh CC, Tamhankar AP, Almogy G, Hagen JA, Demeester SR, Bremner CG, Demeester TR. Esophageal adenocarcinoma in patients < or = 50 years old: delayed diagnosis and advanced disease at presentation. Am Surg. 2004;70:954–958. [PubMed] [Google Scholar]
- 25.Strauss A, Min EJ, Long Q, Gabriel P, Yang YX, Falk GW. Is the age of diagnosis of esophageal adenocarcinoma getting younger? Analysis at a tertiary care center. Dis Esophagus. 2020;33 doi: 10.1093/dote/doz112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S, Li H, Jia C, Ma X, Guo W, Li H. Clinicopathological features and prognosis of patients <45 years old with esophageal adenocarcinoma comparing to other age groups. J Thorac Dis. 2016;8:2724–2729. doi: 10.21037/jtd.2016.09.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Putten M, de Vos-Geelen J, Nieuwenhuijzen GAP, Siersema PD, Lemmens VEPP, Rosman C, van der Sangen MJC, Verhoeven RHA. Long-term survival improvement in oesophageal cancer in the Netherlands. Eur J Cancer. 2018;94:138–147. doi: 10.1016/j.ejca.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 28.Burnett-Hartman AN, Powers JD, Chubak J, Corley DA, Ghai NR, McMullen CK, Pawloski PA, Sterrett AT, Feigelson HS. Treatment patterns and survival differ between early-onset and late-onset colorectal cancer patients: the patient outcomes to advance learning network. Cancer Causes Control. 2019;30:747–755. doi: 10.1007/s10552-019-01181-3. [DOI] [PubMed] [Google Scholar]
- 29.Manjelievskaia J, Brown D, McGlynn KA, Anderson W, Shriver CD, Zhu K. Chemotherapy Use and Survival Among Young and Middle-Aged Patients With Colon Cancer. JAMA Surg. 2017;152:452–459. doi: 10.1001/jamasurg.2016.5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilleron S, Charvat H, Araghi M, Arnold M, Fidler-Benaoudia MM, Bardot A, Grønlie Guren M, Tervonen H, Little A, O'Connell DL, Gavin A, De P, Aagard Thomsen L, Møller B, Jackson C, Bucher O, Walsh PM, Vernon S, Bray F, Soerjomataram I. Age disparities in stage-specific colon cancer survival across seven countries: An International Cancer Benchmarking Partnership SURVMARK-2 population-based study. Int J Cancer. 2021;148:1575–1585. doi: 10.1002/ijc.33326. [DOI] [PubMed] [Google Scholar]
- 31.Oezcelik A, Ayazi S, DeMeester SR, Zehetner J, Abate E, Dunn J, Grant KS, Lipham JC, Hagen JA, DeMeester TR. Adenocarcinoma of the esophagus in the young. J Gastrointest Surg. 2013;17:1032–1035. doi: 10.1007/s11605-013-2177-6. [DOI] [PubMed] [Google Scholar]
- 32.Drahos J, Xiao Q, Risch HA, Freedman ND, Abnet CC, Anderson LA, Bernstein L, Brown L, Chow WH, Gammon MD, Kamangar F, Liao LM, Murray LJ, Ward MH, Ye W, Wu AH, Vaughan TL, Whiteman DC, Cook MB. Age-specific risk factor profiles of adenocarcinomas of the esophagus: A pooled analysis from the international BEACON consortium. Int J Cancer. 2016;138:55–64. doi: 10.1002/ijc.29688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lumish MA, Walch H, Maron SB, Chatila W, Kemel Y, Maio A, Ku GY, Ilson DH, Won E, Li J, Joshi SS, Gu P, Schattner MA, Laszkowska M, Gerdes H, Jones DR, Sihag S, Coit DG, Tang LH, Strong VE, Molena D, Stadler ZK, Schultz N, Janjigian YY, Cercek A. Clinical and molecular characteristics of early-onset vs average-onset esophagogastric cancer. J Natl Cancer Inst. 2024;116:299–308. doi: 10.1093/jnci/djad186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Nistelrooij AM, van Marion R PALGA-group, Biermann K, Spaander MC, van Lanschot JJ, Wijnhoven BP, Dinjens WN. Early onset esophageal adenocarcinoma: a distinct molecular entity? Oncoscience. 2016;3:42–48. doi: 10.18632/oncoscience.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ku GY, Kemel Y, Maron SB, Chou JF, Ravichandran V, Shameer Z, Maio A, Won ES, Kelsen DP, Ilson DH, Capanu M, Strong VE, Molena D, Sihag S, Jones DR, Coit DG, Tuvy Y, Cowie K, Solit DB, Schultz N, Hechtman JF, Offit K, Joseph V, Mandelker D, Janjigian YY, Stadler ZK. Prevalence of Germline Alterations on Targeted Tumor-Normal Sequencing of Esophagogastric Cancer. JAMA Netw Open. 2021;4:e2114753. doi: 10.1001/jamanetworkopen.2021.14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radkiewicz C, Asplund J, Lagergren J. Incidence Trends and Survival in Early-Onset Esophagogastric Adenocarcinoma: A Swedish Population-Based Cohort Study. Cancer Epidemiol Biomarkers Prev. 2023;32:919–926. doi: 10.1158/1055-9965.EPI-23-0169. [DOI] [PubMed] [Google Scholar]